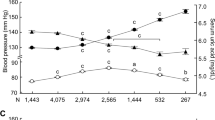
Abstract
The association between uric acid (UA) and hyperuricemia with 5-year hypertension incidence using different blood pressure (BP) diagnostic references in men and women without cardiometabolic diseases is unknown. We used the checkup data from Kagoshima Kouseiren Hospital. All participants with hypertension or on BP medication, diabetes, dyslipidemia, obesity, estimated glomerular filtration rate<60 ml/min/1.73m2, metabolic syndrome, history of gout, and UA-lowering medication were excluded. UA was categorized into sex-specific quartiles and hyperuricemia was defined as UA > 7 mg/dl in men and UA > 6 mg/dl in women. We performed multivariate logistic regression to assess the effects of UA on hypertension development. The 5-year hypertension incidence was defined as subsets of BP ≥ 140/90 mmHg in cohort 1 and BP ≥ 130/80 mmHg in cohort 2. The study enrolled 21,443 participants (39.8%, men) in cohort 1 and 15,245 participants (36.5%, men) in cohort 2. The incidence of hypertension in cohorts 1 and 2 over 5 years was 16.3% and 29.7% in men and 10.9% and 21.4% in women, respectively. When comparing the fourth to the first UA quartile, there was an association with hypertension in men in cohort 1, with odds ratio (OR): 1.36 (95% confidence interval [CI], 1.13–1.63, p < 0.01) and cohort 2, OR: 1.31 (95%CI, 1.09–1.57, p < 0.01), respectively, but not in women. Additionally, an association between hyperuricemia and hypertension was observed in men only in cohort 1, with OR: 1.23 (95%CI, 1.07–1.42, p = 0.02), and in women in cohort 2, OR: 1.57 (95%CI, 1.14–2.16, p < 0.01). The effect of UA on the development of hypertension is influenced by sex and incidence differs with the BP reference used.

Uric acid effect on the development of hypertension is affected by sex and incidence differs with the BP reference used.
Similar content being viewed by others
Introduction
Hypertension is responsible for the growing burden of cardiovascular diseases (CVD) and is the leading cause of death globally [1,2,3], yet it remains an inexpensive and easy condition to diagnose. Family history of hypertension, aging, obesity, high cholesterol, unhealthy diet, physical inactivity, tobacco smoking, alcohol consumption, as well as factors including diabetes and chronic kidney disease are substantial risks associated with hypertension incidence, making it a complex lifestyle disease. The diagnostic reference value of high blood pressure (BP) for hypertension was lowered by the American College of Cardiology and American Heart Association (ACC/AHA) in 2017 to systolic BP (SBP) of ≥130 mmHg or diastolic BP (DBP) of ≥80 mmHg [4]. However, guidelines from the International Society of Hypertension, European Society of Cardiology, and Japanese Hypertension Society retained the traditional definition of SBP ≥ 140 mmHg and/or DBP ≥ 90 mmHg [5,6,7].
Serum uric acid (UA), a product of purine metabolism, is increasingly becoming a common risk factor associated with cardiometabolic diseases, with evidence suggesting a causal role of UA in hypertension development [8,9,10,11]. In human and experimental studies, a higher UA level was associated with hypertension [12,13,14]. Recent studies reported how UA-lowering medications could lower the BP among hypertensives with hyperuricemia [15,16,17]. Despite these efforts, it is inconclusive how UA is associated with hypertension due to the close associations between UA and other cardiometabolic risks. Furthermore, the effects of UA levels on cardiometabolic health appear different for women and men and may have different outcomes throughout their lifespans [18,19,20]. Whether UA levels have other effects between men and women, using different reference values of hypertension, remains unknown. Serum UA levels are generally higher in men than in women in early life. The associated incidence of conditions such as obesity [21], metabolic syndrome [22], atrial fibrillation [23, 24], diabetes [20, 25], CVD mortality, and all-cause mortality [26, 27] gradually increases with an increase in age and the consequent diminishing uricosuric effects of estrogen [28, 29] making women with high or even lower UA thresholds vulnerable. Previous reports present inconsistent findings: for instance, in studies that assessed hyperuricemia and hypertension, the association appears to be in both sexes but is stronger in women compared with men [30,31,32,33]. Another study suggests that men are more prone to develop hypertension than women with hyperuricemia [34]. Other findings suggest the association between hyperuricemia and hypertension is present only in women [35], or in men [36]. These findings could be affected by the reference value for hyperuricemia, ethnicity, lifestyle, and baseline characteristics of a particular population under study.
This study focused on contributing to the sex-specific risk factors of hypertension and the associations between UA quartiles, hyperuricemia, and hypertension incidence using the traditional hypertension diagnostic reference value of ≥140/90 mmHg in comparison with the 2017 ACC/AHA guideline definition of ≥130/80 mmHg, in a population without cardiometabolic risk factors at baseline.
Methods
Study population
This retrospective cohort study recruited healthy participants from 198,292 individuals who participated in an annual health checkup at the Kagoshima Kouseiren Hospital between October 2008 and March 2019 and had baseline and 5-year follow-up data. We grouped participants in cohort 1 and cohort 2 (a subset of cohort 1) (Fig. 1). Individuals with the following cardiometabolic conditions were excluded at baseline: (1) Hypertension, defined as SBP ≥ 140 mmHg and/or DBP ≥ 90 mmHg or on antihypertensive medication in cohort 1 and SBP ≥ 130 mmHg and/or DBP ≥ 80 mmHg or on antihypertensive medication in cohort 2; (2) Diabetes defined as fasting plasma glucose (FPG) of ≥126 mg/dl or the use of anti-diabetic medication; Dyslipidemia defined as elevated serum low-density lipoprotein cholesterol (LDL-C) ≥ 140 mg/dl or serum high-density lipoprotein cholesterol (HDL-C) < 40 mg/dl or elevated serum triglycerides (TG) ≥ 150 mg/dl or use of lipid-lowering medications; and (3) Obesity defined as a body mass index (BMI) ≥ 25 kg/m2; Reduced kidney function, with estimated glomeruli filtration rate (eGFR) < 60 ml/min/1.73m2; Metabolic syndrome defined as per Japanese diagnostic criteria [37] and participants with a history of gout and/or history of UA-lowering medication. The data were anonymized, and the participants were allowed to opt out of the study. The study was conducted according to the Declaration of Helsinki and approved by the Institutional Ethics Committee of the Kagoshima University, Graduate School of Medical and Dental Sciences (IRB Approval number: 520).
Data collection
A self-administered questionnaire was used to collect information on medications for hypertension, T2DM, dyslipidemia, gout, and lifestyle factors during annual checkups. Anthropometric measurements, including height, weight, and waist circumference (WC), were obtained using standard operating procedures established by the WHO [38]. The BMI was calculated as body weight in kilograms divided by the height squared in meters. Participants were categorized into tobacco smokers (those who are currently smoking), non-tobacco smokers (those with no history or smoked in the past), alcohol consumers (those who had daily alcohol intake), and non-alcohol consumers (those who never, rarely, or sometimes drank alcohol). An exercise habit was defined as having a 30-min exercise regimen at least once a week. BP measurements were taken during enrollment and subsequent visits. Brachial BP was measured in a seated position after 3–5 min rest in a quiet room, with an appropriately sized cuff on the right arm; the elbow was rested on a desk with the mid-arm at heart level. A well-trained staff member recorded the readings using a calibrated automated BP machine. During the annual health checkup, the BP reading was recorded only once. The blood samples were obtained after overnight fasting. After that, we measured the serum TG, LDL-C, HDL-C, FPG, creatinine, and UA levels using standard laboratory procedures. The eGFR was determined according to the new Japanese coefficient for the modified isotope dilution mass spectrometry-traceable Modification of Diet in Renal Disease study equation:
For women, eGFR was multiplied by a correction factor of 0.739 [39].
Uric acid quartiles, hyperuricemia, and outcome definitions
Initially, we grouped all study participants according to the serum UA quartiles as follows: 1st quartile (Q1); UA ≤ 3.7 mg/dl, 2nd quartile (Q2); 3.8–4.6 mg/dl, 3rd quartile (Q3); 4.7–5.4 mg/dl, and 4th quartile (Q4); UA ≥ 5.5 mg/dl. Later we grouped study participants according to UA quartiles and by sex, as follows; in men: Q1; UA ≤ 4.8 mg/dl, Q2; 4.9–5.6 mg/dl, Q3; 5.7–6.4 mg/dl, and Q4; UA ≥ 6.5 mg/dl and in women: Q1; UA ≤ 3.4 mg/dl, Q2; 3.5–4.0 mg/dl, Q3; 4.1–4.7 mg/dl, and Q4; UA ≥ 4.8 mg/dl. Similar UA quartiles were used in both cohorts for analysis. Furthermore, we defined hyperuricemia as UA > 7.0 mg/dl in men and > 6.0 mg/dl in women, a commonly suggested threshold, as described previously [12]. A subanalysis without hypouricemia individuals was performed by excluding individuals with hypouricemia defined as UA < 2 mg/dL. Considering the influence of age and hormones, we also performed a subanalysis by excluding postmenopausal women defined as age ≥55 years. The 5-year incidence of hypertension was defined as the development of SBP ≥ 140 mmHg and/or DBP ≥ 90 mmHg or newly prescribed antihypertensive medication after a 5-year follow-up in cohort 1. Moreover, we used the ACC/AHA 2017 guidelines to define hypertension incidence as the development of SBP ≥ 130 mmHg or DBP ≥ 80 mmHg or newly prescribed antihypertensive medication after 5 years of follow-up in cohort 2 [4].
Statistical analysis
All statistical analyses were performed using JMP Pro Version 17 (SAS Institute, Inc., Cary, NC, USA). Statistical significance was set at p < 0.05. For baseline characteristics, we calculated mean ± standard deviation (SD) or median with interquartile range for continuous variables, and categorical variables were expressed as frequencies and proportions. We compared the baseline characteristics between men and women, using the chi-square test for categorical variables, the unpaired student t-test for normally distributed continuous variables, and the Wilcoxon rank-sum test for skewed-continuous distribution variables. The associations between UA quartiles, hyperuricemia, and 5 Year hypertension incidence in all participants and by sex were estimated using multivariate logistic regression analysis; the odds ratio (OR) and 95% confidence interval (CI) were adjusted using three different models as follows: Model 1: age, BMI and UA quartiles; Model 2: age, BMI, SBP, and UA quartiles; and Model 3: age, BMI, SBP, DBP, tobacco smoking, alcohol consumption, exercise habit, triglycerides, LDL-C, HDL-C, FPG, eGFR, and UA quartiles. For all participants’ analyses, the sex variable was added as a confounder in models 2 and 3. Considering the possibility of multicollinearity, we performed Pearson’s correlation coefficient and variance inflation factor (VIF) for SBP and DBP variables. A similar analysis was performed in both cohorts.
Results
Baseline characteristics
A total of 21,443 (mean [±SD] age, 52.9 ± 10.9 years; men, 39.7%) participants, with a 5-year follow-up period, were included in cohort 1. The baseline characteristics of all participants and by sex are presented in Table 1. We observed no difference in age between men and women, whereas all other variables significantly differed between the sexes in this study population. The mean BMI was 21.6 ± 2.0 kg/m2 in men and 20.9 ± 2.1 kg/m2 in women, and the mean WC was 79.0 ± 6.2 cm in men and 77.1 ± 7.3 cm in women, respectively. Compared with women, men had higher baseline SBP, DBP, FPG, TG, and serum UA and had a lower measure of HDL-C, LDL-C, and eGFR. Men were more likely to be tobacco smokers and alcohol consumers and had frequent exercise habits compared to women. After 5 years of follow-up, 1 389 (16.3%) men and 1 407 (10.9%) women developed hypertension, respectively. In cohort 2, 15 245 participants (age 51.3 ± 10.9 years; men, 36.5%) were included (Table 1). Compared with women, men had higher BMI, WC, SBP, and DBP; had more frequent tobacco smoking, alcohol consumption, and exercise habits; had lower levels of LDL-C, HDL-C, and eGFR; had higher levels of triglycerides, FPG, and serum UA. After 5 years of follow-up, 1652 (29.7%) men and 2072 (21.4%) women developed hypertension, respectively.
Uric acid and other risk factors associated with hypertension
The risk factors associated with 5-year hypertension incidence using the reference of BP ≥ 140/90 mmHg are shown in Table 2. In univariate analysis, age, BMI, SBP, DBP, tobacco smoking, alcohol consumption, triglycerides, as well as HDL-C, FPG, eGFR, and serum UA levels were associated with hypertension in both sexes. In a multivariate analysis, age, SBP, DBP, tobacco smoking, alcohol consumption, and serum UA levels (odds ratio [OR] 1.10, 95% confidence interval [CI] 1.05–1.16, p < 0.01) were significantly associated with hypertension in men. In contrast, age, BMI, SBP, DBP, alcohol consumption, and FPG were associated with hypertension but not serum UA (OR 1.04, 95% CI 0.97–1.11, p = 0.23) in women. The risk factors associated with a 5-year hypertension incident using the reference of BP ≥ 130/80 mmHg are shown in Table 3. In univariate analysis, age, BMI, SBP, DBP, alcohol consumption, triglycerides, LDL-C, HDL-C, FPG, eGFR, and serum UA were associated with hypertension in both sexes. In a multivariate analysis, age, BMI, SBP, DBP, and alcohol consumption were associated with hypertension in both sexes. Serum UA (OR 1.07, 95% CI 1.01–1.12, p = 0.02) and tobacco smoking (OR 1.22, 95% CI 1.04–1.29, p < 0.01) were associated with hypertension incidence in men but not in women. In contrast, serum FPG (OR 1.01, 95% CI 1.00–1.02, p < 0.01) and HDL-C (OR 1.00, 95% CI 0.99–1.00, p = 0.04) were associated with hypertension in women, but not in men. The Pearson’s correlation coefficient was 0.65 in cohort 1 and 0.56 in cohort 2. The VIF for SBP and DBP in cohort 1 was 1.25 and 1.13 and in cohort 2 was 1.26 and 1.35 respectively (Supplementary Fig. 1).
Uric acid quartiles, hyperuricemia, and incidence of hypertension
We assessed the associations between UA quartiles and 5-year hypertension incidence defined as ≥140/90 mmHg in all study participants and by sex (Table 4). In cohort 1, all participant analyses that compared the UA fourth quartiles with the first quartiles (reference group) showed UA as an independent risk factor for hypertension development (OR 1.23, 95% CI 1.06–1.43, p < 0.01). In the sex-specific, univariate analysis, UA was associated with hypertension defined as ≥140/90 mmHg in both sexes after comparing the UA fourth and first quartiles. In men, the OR was 1.48 (95% CI 1.26–1.74, p < 0.01), and in women, 1.68 (95% CI 1.42–1.97, p < 0.01). Analysis in Model 1, which was controlled for age and BMI, indicated an association between UA and incidence of hypertension in both sexes: In men, the OR was 1.51 (95% CI 1.28–1.78, p < 0.01), and in women 1.27 (95% CI 1.05–1.45, p < 0.01). In Model 2, after adding SBP to Model 1, the association remained in both sexes: In men, OR was 1.42 (95% CI 1.19–1.69, p < 0.01), and in women the OR was 1.17 (95% CI 0.98–1.39, p = 0.03), respectively. Further adjustment with DBP, tobacco smoking, alcohol consumption, exercise habit, triglycerides, LDL-C, HDL-C, FPG, and eGFR (Model 3), retained the significant association between the fourth quartile of UA and incidence of hypertension in men OR 1.36 (95% CI 1.13–1.63, p < 0.01) and no association was observed in women, OR 1.18 (95% CI 0.95–1.36, p = 0.08), respectively. A similar analysis was performed for the association between UA quartiles and 5-year hypertension incidence defined as ≥130/80 mmHg by sex. All participants were associated with hypertension development, defined as BP ≥ 130/80 mmHg after adjustments (OR 1.22, 95% CI 1.06–1.40, p < 0.01). In univariate analysis, comparing the UA fourth and first quartiles, both sexes were significantly associated with the incidence of hypertension: In men, OR 1.45 (95% CI 1.24–1.71, p < 0.01) and in women OR 1.50 (95% CI 1.30–1.73, p < 0.01). In multivariate analysis, the UA fourth quartiles were significantly associated with hypertension in all models compared with the first quartile (Model 1: OR 1.43, 95% CI 1.21–1.70, p < 0.01; Model 2: OR 1.37, 95% CI 1.15–1.63, p < 0.01; Model 3: OR 1.31, 95% CI 1.09–1.57, p < 0.01). In women, only Model 1 displayed that the uric acid quartile was significantly associated with the development of hypertension compared with the first quartile (OR 1.17, 95% CI 1.01–1.36, p = 0.04). A subanalysis that excluded hypouricemic individuals yielded similar results (Supplementary Table 1). In a subanalysis without postmenopausal women (Supplementary Table 2), there was a significant association between the 4th UA quartile and hypertension incidence in cohort 2.
Hyperuricemia was associated with hypertension incidence in all participant analyses in both cohorts (Table 5). The OR were 1.21 (95% CI 1.03–1.42, p = 0.02) in cohort 1 and 1.26 (95% CI 1.07–1.48, p < 0.01) in cohort 2, respectively. For sex-specific analysis, after full adjustments, in cohort 1, men with hyperuricemia were significantly associated with hypertension development (OR 1.23, 95% CI 1.07–1.42, p = 0.02), but not in women. In contrast, in cohort 2, a reverse finding was observed, with women having a stronger association for hyperuricemia and 5-year hypertension incidence after adjustments in Model 3, with the OR of 1.57 (95% CI 1.14–2.16, p < 0.01) and no associations in men (OR 1.15, 95% CI 0.95–1.40, p = 0.15). We observed a simultaneous increase in the 5-year hypertension incidence with increasing levels of UA in both cohorts, across both sexes (Supplementary Fig. 2). The hypertension incidence proportions appeared higher in cohort 2 than in cohort 1.
Discussion
In this cardiometabolic risk-free study population of Japanese adults, we found that when compared with the first quartiles, the fourth quartiles of UA (men; UA ≤ 4.8 mg/dl vs. UA ≥ 6.5 mg/dl and women; UA ≤ 3.4 mg/dl vs. UA ≥ 4.8 mg/dl) were associated with a 5-year hypertension incidence only in men when the outcome BP references were either ≥140/90 mmHg or ≥130/80 mmHg. However, women had low UA values with an insignificant positive trend observed toward hypertension incidence. Furthermore, hyperuricemia of >7 mg/dl in men and >6 mg/dl in women was only associated with 5-year hypertension incidence defined as BP of ≥140/90 mmHg and BP of ≥130/80 mmHg, respectively.
Despite hypertension being the leading cause of death for non-communicable diseases, most hypertensive individuals are either unaware of their BP, remain untreated, or have poor control of hypertension [40, 41]. Understanding the risks associated with hypertension is vital in improving hypertension detection and lifestyle modification. The definition of hypertension and the BP reference values used are essential for screening, diagnosing, and managing patients. The ACC/AHA lowered the reference values to SBP of ≥130 mmHg and DBP of ≥80 mmHg in 2017 [4]. According to these new references, the hypertension incidence is higher than the traditional references of ≥140/90 mmHg [42]. In this study, the 5-year hypertension incidence according to ACC/AHA definition reference was 29.7% in men and 21.4% in women; however, considering the definition reference of ≥140/90 mmHg, the incidence was 16.3% in men and 10.9% in women, respectively. Interestingly, after multivariable analysis, the risk factors associated with a 5-year incidence of hypertension defined as BP of ≥140/90 mmHg were age, SBP, DBP, and alcohol consumption in both sexes. UA and tobacco smoking were only significant in men, while BMI and FPG were only significant in women. These differences may be attributed to higher serum levels of UA and smoking habits in men at baseline. Obesity and insulin resistance are closely associated with hypertension, and obesity has been reportedly a more substantial risk for hypertension incidence in women compared with men in previous studies [43,44,45]. These could explain the similar association between BMI, FPG, and hypertension among women in this study population. When the hypertension outcome was defined as ≥130/80 mmHg, the risk factors were age, BMI, SBP, DBP, and alcohol consumption in both sexes. UA and tobacco smoking were significantly associated with hypertension incidence only in men and FPG and HDL-C only in women. Furthermore, we observed a risk reduction with exercise habits regardless of the hypertension definition in men. This could be explained as men with more risk factors and higher BP values tend to try to exercise more to achieve a healthier lifestyle.
A few studies have reported the similarities and differences between risk factors associated with hypertension reference values [46, 47]; however, the sex-related risk associations for UA and different hypertension definitions observed in our study are novel. UA is a product of purine mononucleotide metabolism. Purines are produced endogenously by the body or accumulated from food that we consume daily, with vast beneficial and non-beneficial functions to the body. In brief, under circumstances where UA is overproduced or under-excreted, prolonged elevated levels cause damage to the endothelial wall through chronic inflammation [14]. To date, several studies have demonstrated the association of hyperuricemia with increased cardiovascular risk, including the risk of developing hypertension [30, 31, 48, 49]. However, metabolically, the UA effect on hypertension is strongly confounded with other risk factors such as age, obesity, dyslipidemia, smoking, and sex. Therefore, to address this, we strictly excluded all individuals with cardiometabolic risks at baseline, including higher age, obesity, dyslipidemia, hypertension, diabetes, metabolic syndrome, eGFR<60 ml/min/1.73 m2, and the use of UA-lowering medication. In multivariate logistic regression, when comparing fourth to first UA quartiles, the association between UA and hypertension was observed only in men, for the BP reference of ≥140/90 mmHg, and even after lowering the BP reference to ≥130/80 mmHg. In a study by Yokokawa et al., UA quartiles or hyperuricemia was associated with hypertension in both sexes, however, it was a cross-sectional design study and had pre-existing individuals with diabetes, obesity, and dyslipidemia which may compound UA effect on hypertension incidence [50]. Another study by Lin et al. observed a higher prevalence of hyperuricemia and hypertension in men compared to women. Men with hyperuricemia, especially in middle age, exhibited a significantly heightened susceptibility to hypertension, which wasn’t observed in women [36]. Furthermore, despite differences in some of the results, the insignificant association observed in women has been similarly reported in another study by Nagahama et al. [51]. Interestingly, Kuwabara et al. demonstrated a relationship between hyperuricemia and hypertension, CKD, and dyslipidemia after adjustment. Notably, in categories of eGFR ≥60 mL/min per 1.73 m2 and eGFR ≥75 mL/min per 1.73 m2, hyperuricemia was associated with hypertension in both sexes upon introducing essential covariates, such as age, sex, BMI, SBP, and DBP [12]. However, the risk for moderate hypertension was not considered in this study. These differences in study designs are important to note, as our study used two different definitions of hypertension and adjusted for more covariates in a single model than most previous studies. In addition, the average UA level in this study was comparatively lower, potentially indicating a lower or negligible risk of hypertension. This observation is particularly relevant to women, as their upper limit of UA was lower than that of men. It is also possible that it was not associated with hypertension incidence when a BP of 140/90 mmHg is considered. Therefore, setting a higher criterion for UA values may increase the risk of increased BP. Furthermore, our study observed that women over 55 years of age who are considered to be menopausal accounted for 48% of the female population. This age distribution could have influenced the absence of an association between UA quartiles and hypertension in women, consistent with findings from Ohyama et al., where women below 40 years were not linked to hypertension incidence [52]. Moreover, the absence of hormonal influence in men may contribute to higher levels of UA and a higher risk of hypertension [28]. In our analysis, hyperuricemia defined as UA > 7 mg/dl in men and >6 mg/dl in women showed a significant increase in risk in women, compared with men when the BP outcome is ≥130/80 mmHg; however, this was not the case when the BP outcome is ≥140/90 mmHg. The number of women with hyperuricemia in this study was small resulting in low statistical power; nevertheless, with elevated UA, these results indicate that women may have an increased incidence of hypertension than men when lower BP references are considered. These consistencies and differences highlight the complex interplay of gender, UA levels, and hypertension risk, warranting further investigation. In a nutshell, the findings in our study do not invalidate previous studies, however, add new knowledge that this relationship may not be present specifically in women without cardiometabolic diseases and that the risk may increase if the UA is >6 mg/dl for moderate hypertension defined as ≥130/80 mmHg, while men with UA ≥ 6.5 mg/dL are associated with hypertension regardless of the definition used.
The present study had several limitations. Initially, the study population comprised individuals who participated in health examinations at a single hospital in Japan, thereby limiting the generalizability of the findings to other ethnic groups. Nonetheless, the large sample size of our study provides statistical power. Secondly, the study’s retrospective design cannot establish a causal relationship between UA and hypertension. However, the study results possess high validity by excluding participants with cardiometabolic risk and adjusting for relatively potential confounders. Thirdly, most participants missed their annual health checkup visits, which constrained the statistical techniques employed in the study, such as time-to-event analysis. Fourthly, our study used general population health checkup data, which is limited to time for data collection. Consequently, a single BP reading was recorded. As a standard, future studies should examine the average of two BP readings to assess sustained BP. Lastly, although high fructose intake is known to raise intracellular urate levels, our study did not analyze the impact of fructose consumption due to data limitations. Future research should explore dietary aspects, including fructose for a more complete understanding.
Perspectives in Asia
Hypertension is a widespread disease in Asia, with a variation in prevalence between regions. In several epidemiological studies, elevated UA levels have been linked to hypertension incidence. Despite changes in the criteria used to diagnose hypertension in the United States (i.e., BP ≥130/80 mmHg), most guidelines in Asia emphasize using the traditional definition of hypertension like in Europe (i.e., BP ≥140/90 mmHg) [53]. Furthermore, the cut-off points of serum UA associated with hypertension incidence are lacking. For instance, in Japan, the current cut-off value of UA > 7 mg/dl regardless of sex is based on the saturation point of UA defined in gout guidelines [9]. Considering that this study used Japanese data, which may be a better reference for the cut-off point of UA levels that could be used especially for the Asian population. We suggest using a reference value of ≥6.5 mg/dL in men and >6 mg/dL in women respectively for screening hypertension among healthy populations. These findings emphasize the importance of considering sex-specific factors in hypertension research and highlight the need for further investigation to better understand the complexities of this association and if there is a need to treat individuals with asymptomatic hyperuricemia.
In conclusion, our findings suggest that the relationship between UA and hypertension development is influenced by sex and the reference values used for high BP assessment. Importantly, it is novel to note that even in healthy individuals, higher serum UA can impose a risk of propagating hypertension development. These results underscore the importance of early screening for UA levels in individuals free of cardiometabolic conditions, as it may aid in preventing hypertension. Further research is required to fully elucidate the mechanism underlying these sex-specific effects and refine the optimal UA screening approach in this context.
References
Roth GA, Mensah GA, Johnson CO, Addolorato G, Ammirati E, Baddour LM, et al. Global Burden of cardiovascular diseases and risk factors, 1990–2019: update from the GBD 2019 study. J Am Coll Cardiol. 2020;76:2982–3021.
Mills KT, Bundy JD, Kelly TN, Reed JE, Kearney PM, Reynolds K, et al. Global disparities of hypertension prevalence and control: a systematic analysis of population-based studies from 90 countries. Circulation. 2016;134:441–50.
NCD Risk Factor Collaboration (NCD-RisC). Worldwide trends in blood pressure from 1975 to 2015: a pooled analysis of 1479 population-based measurement studies with 19·1 million participants. Lancet. 2017;389:37–55.
Whelton PK, Carey RM, Aronow WS, Casey DE, Collins KJ, Dennison Himmelfarb C, et al. 2017 ACC/AHA/AAPA/ABC/ACPM/AGS/APhA/ASH/ASPC/NMA/PCNA guideline for the prevention, detection, evaluation, and management of high blood pressure in adults: executive summary: a report of the American college of cardiology/american heart association task force on clinical practice guidelines. Hypertension. 2018;71:1269–324.
Unger T, Borghi C, Charchar F, Khan NA, Poulter NR, Prabhakaran D, et al. 2020 International Society of Hypertension Global Hypertension Practice guidelines. Hypertension. 2020;75:1334–57.
Mancia G, Kreutz R, Brunström M, Burnier M, Grassi G, Januszewicz A, et al. 2023 ESH guidelines for the management of arterial hypertension The Task Force for the management of arterial hypertension of the European Society of Hypertension Endorsed by the European Renal Association (ERA) and the International Society of Hypertension (ISH). J Hypertens. 2023;41:1874–2071.
Umemura S, Arima H, Arima S, Asayama K, Dohi Y, Hirooka Y, et al. The Japanese Society of Hypertension Guidelines for the Management of Hypertension (JSH 2019). Hypertens Res. 2019;42:1235–481.
Feig DI, Kang DH, Johnson RJ. Uric acid and cardiovascular risk. N Engl J Med. 2008;359:1811–21.
Hisatome I, Li P, Miake J, Taufiq F, Mahati E, Maharani N, et al. Uric acid as a risk factor for chronic kidney disease and cardiovascular disease—Japanese guideline on the management of asymptomatic hyperuricemia. Circ J. 2021;85:130–8.
Yu W, Cheng JD. Uric acid and cardiovascular disease: an update from molecular mechanism to clinical perspective. Front Pharm. 2020;11:582680.
Lanaspa MA, Andres-Hernando A, Kuwabara M. Uric acid and hypertension. Hypertens Res. 2020;43:832–4.
Kuwabara M, Niwa K, Hisatome I, Nakagawa T, Roncal-Jimenez CA, Andres-Hernando A, et al. Asymptomatic hyperuricemia without comorbidities predicts cardiometabolic diseases: 5-year Japanese cohort study. Hypertension. 2017;69:1036–44.
Stewart DJ, Langlois V, Noone D. Hyperuricemia and Hypertension: Links and Risks. Integr Blood Press Control. 2019;12:43–62.
Kanellis J, Kang DH. Uric acid as a mediator of endothelial dysfunction, inflammation, and vascular disease. Semin Nephrol. 2005;25:39–42.
Qu LH, Jiang H, Chen JH. Effect of uric acid-lowering therapy on blood pressure: systematic review and meta-analysis. Ann Med. 2017;49:142–56.
Gunawardhana L, McLean L, Punzi HA, Hunt B, Palmer RN, Whelton A, et al. Effect of febuxostat on ambulatory blood pressure in subjects with hyperuricemia and hypertension: a phase 2 randomized placebo‐controlled study. J Am Heart Assoc. 2017;6:e006683.
Feig DI, Soletsky B, Johnson RJ. Effect of allopurinol on blood pressure of adolescents with newly diagnosed essential hypertension: a randomized trial. JAMA. 2008;300:924.
Engel B, Hoffmann F, Freitag MH, Jacobs H. Should we be more aware of gender aspects in hyperuricemia? Analysis of the population-based German health interview and examination survey for adults (DEGS1). Maturitas. 2021;153:33–40.
Kuwabara M, Hisatome I, Niwa K, Bjornstad P, Roncal-Jimenez CA, Andres-Hernando A, et al. The optimal range of serum uric acid for cardiometabolic diseases: a 5-year Japanese cohort study. J Clin Med. 2020;9:942.
Cheng F, Li Y, Zheng H, Tian L, Jia H. Mediating effect of body mass index and dyslipidemia on the relation of uric acid and type 2 diabetes: results from China health and retirement longitudinal study. Front Public Health. 2021;9:823739.
Yang T, Chu CH, Bai CH, You SL, Chou YC, Chou WY, et al. Uric acid level as a risk marker for metabolic syndrome: a Chinese cohort study. Atherosclerosis. 2012;220:525–31.
Sumiyoshi H, Ohyama Y, Imai K, Kurabayashi M, Saito Y, Nakamura T. Association of uric acid with incident metabolic syndrome in a Japanese general population. Int Heart J. 2019;60:830–5.
Kawasoe S, Kubozono T, Yoshifuku S, Ojima S, Miyata M, Miyahara H, et al. Uric acid level and new-onset atrial fibrillation in the Japanese General population- longitudinal study. Circ J. 2018;83:156–63.
Nyrnes A, Toft I, Njølstad I, Mathiesen EB, Wilsgaard T, Hansen JB, et al. Uric acid is associated with future atrial fibrillation: an 11-year follow-up of 6308 men and women-the Tromso Study. Europace. 2014;16:320–6.
Shani M, Vinker S, Dinour D, Leiba M, Twig G, Holtzman EJ, et al. High normal uric acid levels are associated with an increased risk of diabetes in lean, normoglycemic healthy women. J Clin Endocrinol Metab. 2016;101:3772–8.
Kawai T, Ohishi M, Takeya Y, Onishi M, Ito N, Yamamoto K, et al. Serum uric acid is an independent risk factor for cardiovascular disease and mortality in hypertensive patients. Hypertens Res. 2012;35:1087–92.
Kikuchi A, Kawamoto R, Ninomiya D, Kumagi T. Hyperuricemia is associated with all-cause mortality among males and females: findings from a study on Japanese community-dwelling individuals. Metab Open. 2022;14:100186.
Antón FM, García Puig J, Ramos T, González P, Ordás J. Sex differences in uric acid metabolism in adults: evidence for a lack of influence of estradiol-17 beta (E2) on the renal handling of urate. Metabolism. 1986;35:343–8.
Guan S, Tang Z, Fang X, Wu X, Liu H, Wang C, et al. Prevalence of hyperuricemia among Beijing post-menopausal women in 10 years. Arch Gerontol Geriatr. 2016;64:162–6.
Grayson PC, Kim SY, LaValley M, Choi HK. Hyperuricemia and incident hypertension: a systematic review and meta-analysis. Arthritis Care Res. 2011;63:102–10.
Nagahama K, Inoue T, Iseki K, Touma T, Kinjo K, Ohya Y, et al. Hyperuricemia as a predictor of hypertension in a screened cohort in Okinawa, Japan. Hypertens Res. 2004;27:835–41.
Nishio S, Maruyama Y, Sugano N, Hosoya T, Yokoo T, Kuriyama S. Gender interaction of uric acid in the development of hypertension. Clin Exp Hypertens. 2018;40:446–51.
Kim W, Go TH, Kang DO, Lee J, Choi JY, Roh SY, et al. Age and sex-dependent association of uric acid and incident hypertension. Nutr Metab Cardiovasc Dis. 2021;31:1200–8.
Wang SF, Shu L, Wang S, Wang XQ, Mu M, Hu CQ, et al. Gender difference in the association of hyperuricemia with hypertension in a middle-aged Chinese population. Blood Press. 2014;23:339–44.
Mori K, Furuhashi M, Tanaka M, Higashiura Y, Koyama M, Hanawa N, et al. Serum uric acid level is associated with an increase in systolic blood pressure over time in female subjects: linear mixed-effects model analyses. Hypertens Res. 2022;45:344–53.
Lin X, Wang X, Li X, Song L, Meng Z, Yang Q, et al. Gender- and age-specific differences in the association of hyperuricemia and hypertension: a cross-sectional study. Int J Endocrinol. 2019;2019:7545137.
Teramoto T, Sasaki J, Ueshima H, Egusa G, Kinoshita M, Shimamoto K, et al. Metabolic syndrome. J Atheroscler Thromb. 2008;15:1–5.
World Health Organization: Waist circumference and waist-hip ratio: report of a WHO expert consultation [Internet]. WHO Press, Geneva, Switzerland, 2011; 2008. Available from: https://www.who.int/publications/i/item/9789241501491.
Matsuo S, Imai E, Horio M, Yasuda Y, Tomita K, Nitta K, et al. Revised equations for estimated GFR from serum creatinine in Japan. Am J Kidney Dis. 2009;53:982–92.
Risk NCD. Factor Collaboration (NCD-RisC). Worldwide trends in hypertension prevalence and progress in treatment and control from 1990 to 2019: a pooled analysis of 1201 population-representative studies with 104 million participants. Lancet 2021;398:957–80.
Mills KT, Stefanescu A, He J. The global epidemiology of hypertension. Nat Rev Nephrol. 2020;16:223–37.
Bundy JD, Mills KT, He J. Comparison of the 2017 ACC/AHA hypertension guideline with earlier guidelines on estimated reductions in cardiovascular disease. Curr Hypertens Rep. 2019;21:76.
Wilson PWF, D’Agostino RB, Sullivan L, Parise H, Kannel WB. Overweight and obesity as determinants of cardiovascular risk: the Framingham experience. Arch Intern Med. 2002;162:1867–72.
Aronow WS. Association of obesity with hypertension. Ann Transl Med. 2017;5:350.
Salim AA, Kawasoe S, Kubozono T, Ojima S, Kawabata T, Ikeda Y, et al. Assessment of future hypertension risk by sex using combined body mass index and waist-to-height ratio. Circ Rep. 2022;4:9–16.
Rahman MA, Halder HR, Yadav UN, Mistry SK. Prevalence of and factors associated with hypertension according to JNC 7 and ACC/AHA 2017 guidelines in Bangladesh. Sci Rep. 2021;11:15420.
Liu C-W, Ke S-R, Tseng G-S, Wu Y-W, Hwang J-J. Elevated serum uric acid is associated with incident hypertension in the health according to various contemporary blood pressure guidelines. Nutr, Metab Cardiovasc Dis. 2021;31:1209–18.
Wang J, Qin T, Chen J, Li Y, Wang L, Huang H, et al. Hyperuricemia and risk of incident hypertension: a systematic review and meta-analysis of observational studies. PLoS One. 2014;9:e114259.
Borghi C, Agnoletti D, Cicero AFG, Lurbe E, Virdis A. Uric acid and hypertension: a review of evidence and future perspectives for the management of cardiovascular risk. Hypertension. 2022;79:1927–36.
Yokokawa H, Fukuda H, Suzuki A, Fujibayashi K, Naito T, Uehara Y, et al. Association between serum uric acid levels/hyperuricemia and hypertension among 85,286 Japanese workers. J Clin Hypertens. 2016;18:53–59.
Nagahama K, Inoue T, Kohagura K, Kinjo K, Ohya Y. Associations between serum uric acid levels and the incidence of hypertension and metabolic syndrome: a 4-year follow-up study of a large screened cohort in Okinawa, Japan. Hypertens Res. 2015;38:213–8.
Ohyama Y, Imai K, Obokata M, Araki M, Sumiyoshi H, Koitabashi N, et al. Impact of uric acid on incident hypertension: sex-specific analysis in different age groups. Int J Cardiol Hypertens. 2019;2:100009.
Hoshide S, Yamamoto K, Katsurada K, Yano Y, Nishiyama A, Wang JG, et al. Agreement regarding overcoming hypertension in the Asian hypertension society network 2022. Hypertens Res. 2023;46:3–8.
Acknowledgements
We thank the medical staff at Kagoshima Kouseiren Hospital for their support with data collection.
Author information
Authors and Affiliations
Contributions
All authors made substantial contribution to this work.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Salim, A.A., Kawasoe, S., Kubozono, T. et al. Sex-specific associations between serum uric acid levels and risk of hypertension for different diagnostic reference values of high blood pressure. Hypertens Res 47, 1120–1132 (2024). https://doi.org/10.1038/s41440-023-01535-0
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41440-023-01535-0
Keywords
This article is cited by
-
Original article and review highlighted in this month of Hypertension Research
Hypertension Research (2024)