Abstract
Expanded porphyrins have received considerable attention due to their unique optical, electrochemical and coordination properties. Here, we report benzene- and pyridine-incorporated octaphyrins(1.1.0.0.1.1.0.0), which are synthesized through Suzuki-Miyaura coupling of α,α′-diboryltripyrrane with m-dibromobenzene and 2,6-dibromopyridine, respectively, and subsequent oxidation with 2,3-dicyano-5,6-dichlorobenzoquinone. Both octaphyrins are nonaromatic and take on dumbbell structures. Upon treatment with Pd(OOCCH3)2, the benzene-incorporated one gives a Ci symmetric NNNC coordinated bis-PdII complex but the pyridine incorporated one gives Ci and Cs symmetric NNNC coordinated bis-PdII complexes along with an NNNN coordinated bis-PdII complex bearing a transannular C–C bond between the pyrrole α-positions. In addition, these two pyridine-containing NNNC PdII complexes undergo trifluoroacetic acid-induced clean interconversion.
Similar content being viewed by others
Introduction
In recent years, considerable attention has been focused on expanded porphyrins in light of their attractive optical, electrochemical, and coordination properties1,2,3,4,5,6,7,8,9,10,11,12. Among these, pyridine-incorporated expanded porphyrins possess a unique position, since they showed interesting chemical behaviors arising from the basic pyridine subunits. More than two decades ago Corriu et al. reported the synthesis of benzene-incorporated and pyridine-incorporated amethyrin analogs 1 and 2 via a rational route13. Later, several analogous molecules possessing unique functions have been reported. As representative examples, cryptand-like molecule 3 exhibited unique properties such as binding three ethanol molecules and positive cooperativity in binding carboxylic acids14. Cyclo[2]pyridine[4]pyrrole 4 and cyclo[3]pyridine[3]pyrrole 5 showed protonation-induced realization of global conjugated networks15 and supramolecular assembling with dicarboxylic acids16. Pyridine-modified rubyrin 6 underwent protonation-induced flipping of the pyridine subunits17, and cyclo[6]pyridine[6]pyrrole 7 was synthesized as the largest pyridine-incorporating expanded porphyrin that showed characteristic conformational flexibility (Fig. 1)14,15,16,17,18,19,20,21,22,23.
Recently we explored various porphyrinoids by using Suzuki–Miyaura coupling. Reported examples include cyclic porphyrin rings, BODIPY-porphyrin hybrids, and earring porphyrins24,25,26,27,28. Despite of these studies, we thought that this coupling strategy could be applied to the synthesis of pyridine-incorporated expanded porphyrins. [36]Octaphyrins(1.1.1.1.1.1.1.1) 8 have been known to bind two metal ions within two semi-porphyrin-like pockets and their bis-CuII and bis-CoII complexes were demonstrated to undergo quantitative splitting reactions to give the corresponding two metaloporphyrins almost quantitatively29,30.
Here, we report the synthesis of phenylene-incorporated and pyridine-incorporated octaphyrins that have remained largely unexplored. The presence of pyridine units into the octaphyrin scaffold leads to unexpected metalation modes and reactivity of the resultant metal complexes. Since the pyridine is quite basic it enables preferential protonation and metal coordination. The pyridine-incorporated octaphyrin coordinates PdII to form Ci and Cs symmetric NNNC bis-PdII complexes, and an NNNN coordinated bis-PdII complex bearing a transannular C–C bond between the pyrrole α-positions. The former two complexes can interconvert upon addition of trifluoroacetic acid (TFA).
Results
Synthesis and structural characterization of 12 and 14
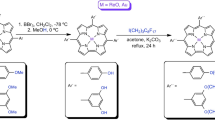
1,3-Phenylene-incorporated and 2,6-pyridylene-incorporated octaphyrins(1.1.0.0.1.1.0.0) 12 and 13 are the target molecules in the present study. The key precursor α,α′-Diboryltripyrrane 9 was prepared by regioselective Ir-catalyzed borylation of 5,10-dimesityltripyrrane (Fig. 2)28. Firstly, we tried the synthesis of 1,3-phenylene-incorporating octaphyrin 12 by Suzuki-Miyaura coupling of 9 with m-dibromobenzene and subsequent oxidation with 2,3-dichloro-5,6-dicyano-1,4-benzoquinone (DDQ) but this reaction sequence gave linear precursor 10 in 53% yield. We then examined the cyclization of 10 with 9 under similar coupling and oxidation conditions. The equimolecular reaction of 9 and 10 yielded 12 in 3.8% yield but the yield of 12 could be improved to 8.0% when 1.2 equivalent of 9 was applied. The parent ion peak of 12 was detected at m/z = 1062.5479 (calcd for [C76H66N6]+ = 1062.5343 [M]+). The 1H nuclear magnetic resonance (NMR) spectrum shows a symmetric feature displaying a broad singlet at 13.00 ppm due to the pyrrolic NH protons, two doublets at 7.39 and 7.03 ppm and a singlet at 6.33 ppm due to the pyrrolic β-protons, and only three singlet signals due to the methyl protons of the mesityl groups. Collectively, these chemical shifts indicate a nonaromatic character for 12 (see Supplementary Fig. 4 and Supplementary Table 8). Fortunately, we obtained single crystals of 12 by slow diffusion of isopropanol vapor into its chlorobenzene solution. As shown in Fig. 3a, b, 12 takes a planar dumbbell structure with Ci symmetry, in which the two 1,3-phenylene units are pointing inward, being close to overlapping, and the two tripyrrin units are also pointing inward, forming two hemiporphyrin-like pockets. There is no particular bond-length alteration in the 1,3-phenylene units.
Reaction conditions: i) excess m-dibromobenzene or 2,6-dibromopyridine, Pd2(dba)3, PPh3, Cs2CO3, CsF, p-xylene or toluene/DMF, reflux, 48 h. ii) DDQ, CH2Cl2, r.t. iii) α,α′-diboryltripyrrane Pd2(dba)3, PPh3, Cs2CO3, CsF, p-xylene or toluene/DMF, reflux, 48 h. iv) DDQ, CH2Cl2, r.t. v) m-dibromobenzene or 2,6-dibromopyridine, Pd2(dba)3, PPh3 or XPhos, Cs2CO3, CsF, toluene/DMF, reflux, 48 h. vi) DDQ, CH2Cl2, r.t. vii) Pd(OAc)2, NaOAc, CHCl3/CH3OH, reflux. dba = dibenzylideneacetone, Mes = 2,4,6-trimethylphenyl, Bpin = pinacolatoboryl.
a Top view and b side view of 12. c top view, and d side view of 14. The thermal ellipsoids are on 30% probability level. Hydrogen atoms except those connected to N atoms are omitted for clarity. Carbon atom, black ellipsoid; nitrogen atom, blue; palladium atom, orange; hydrogen atom small black ball.
Encouraged by the hemiporphyrin-like pockets, we examined PdII metalation of 12. A solution containing 12, Pd(OAc)2, and NaOAc in a mixture of chloroform and methanol was refluxed for 24 h, which yielded 14 in 74% yield as a single product. The parent ion peak was detected at m/z = 1270.3018 (calcd for [C76H62N6Pd2]+ = 1270.3134 [M]+). The structure of 14 was also determined by single crystal X-ray diffraction analysis (Fig. 3c,d). Complex 14 shows a Ci structure, in which the two PdII ions take square planar coordination bound with the three pyrrolic nitrogen atoms and one carbon atom in the 1,3-phenylene unit.
Synthesis and structural characterization of 13, 15, 16, and 17
By following the stepwise synthetic protocol for 10, α,α′-di(6-bromopyrid-2-yl)tripyrrin 11 was prepared by the reaction of 9 with 2,6-dibromopyridine in 48% yield, and octaphyrin 13 was synthesized by the reaction of 9 with 11 in 9.0% yield. The direct one-pot method gave 13 in 8.1% yield. The parent ion peak of 13 was observed at m/z = 1064.5239 (calcd for [C74H64N8]+ 1064.5248 [M]+) and the 1H NMR spectrum of 13 is similar to that of 12, indicating its symmetric structure and a nonaromatic character. Octaphyrin 13 shows a planar dumbbell structure quite similar to that of 12, in which the two hemiporphyrin-like cavities are secured (Fig. 4a, b).
Considering the high coordination ability of pyridine, it was thought that metalation behaviors of 13 might be different from those of 12. PdII metalation of 13 under the same conditions afforded three PdII complexes 15, 16, and 17 in 20%, 60%, and 12% yield, respectively. The structures of 15 and 16 are analogous to that of 14 but that of 17 is substantially different. The parent ion peaks of 15 and 16 were observed at m/z = 1272.2944 (calcd for [C74H60N8Pd2]+ 1272.3038 [M]+) and m/z = 1272.3063 (calcd for [C74H60N8Pd2]+ 1272.3038 [M]+), respectively.
Structures of 15 and 16 were unambiguously determined by X-ray analysis as shown in Fig. 4c–f. Both complexes show the similar planar dumbbell structures but differ in PdII coordination modes. Namely, in 15 the PdII metals are respectively bound to different pyridine units, while the two PdII metals in 16 are bound to the same pyridine unit in a Cs symmetric manner. The Pd–N bond lengths are 1.969(5), 1.978(6), 2.053(6), 2.069(6), 2.071(5), and 2.085(6) Å, and Pd–C bond lengths are 2.077(7) and 2.102(7) Å in 16. The sum of the bond angles around the Pd ions are 361.4(3)° and 361.1(3)° for 16, being closer to the ideal square-planar coordination geometry. In line with these structures, the 1H NMR spectrum of 15 shows a set of doublets at 5.46 and 4.07 ppm due to the 2,6-pyridinylene units and that of 16 displays a singlet at 3.69 ppm and a set of mutually coupled doublet at 7.67 ppm and a triplet at 7.03 ppm. These chemical shifts can be explained by the local aromaticity of 1,3-phenylene and 2,6-pyridylene, which meet well with the NICS calculation. DFT calculation reveals that either in 15 or 16 d orbitals of Pd atoms are clearly involved in the HOMO and LUMO orbitals, which indicates a strong electron interaction between Pd atoms and octaphyrin scaffolds.
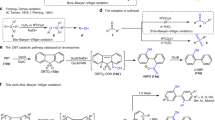
The structure of 17 is shown in Fig. 5. The Pd1 atom is coordinated with three pyrroles B, C, D and pyridine A in a square planar manner with bond lengths of 1.922(3), 1.962(6), 1.942(4), and 2.024(5) Å. The Pd2 atom is bounded to the three nitrogen atoms of pyrrole rings F, G, H and pyridine E with distances of 1.926(4), 1.971(5), 1.947(4), and 2.023(4) Å. This hemi-porphyrin-like unit is relatively planar with a small mean plane deviation of 0.037(6) Å. Surprisingly, a new C–C bond is formed between the α-positions of the pyrroles D and H, causing a disruption of the macrocyclic conjugated network. This transannular bond length is rather large, being 1.568(8) Å. As a consequence, complex 17 exhibits a roughly perpendicular arrangement of two hemiporphyrin-like units. The parent ion peak was detected at m/z = 1274.3141 (calcd for [C74H62N8Pd2]+ 1274.3195 [M]+), which is two units larger than those of 15 and 16. The 1H NMR and 13C NMR spectra are consistent with the structure. Characteristically, the 13C NMR spectrum shows a signal at 91.44 ppm due to the sp3-hybridized quaternary carbon atoms. The detailed reaction mechanism is not clear but may involve the bis-PdII complex 18, which may be highly distorted and undergo a transannular C–C bond forming reaction to give 17 (Fig. 6). This process differs from the previous transannular reactions of expanded porphyrins (Fig. 7)30,31,32,33,34,35.
Electrochemical and optical properties of 12, 13, 14, 15, 16, and 17
Cyclic voltammetry (CV) and differential-pulse voltammetry (DPV) experiments were conducted and the redox potentials are summarized in Table 1.
In all cases, the oxidation waves are irreversible, indicating certain instabilities under oxidative conditions. On the other hands, the first and second reduction waves are reversible, showing a stepwise 1e– reduction at –1.31 and –1.48 V for 12, at –1.07 and –1.29 V for 14. The PdII complexation result in a definitive anodic shift by about 0.2 V. It is also the case for 13, the reduction peaks were observed at –0.92 and –1.18 V for 15 and at –0.97 and –1.20 V for 16, both of which were anodically shifted from those of 13. Interestingly, the first oxidation potential of 16 (0.47 V) is more negatively shifted than that of 15 (0.62 V), resulting in the smallest gap (ΔEHL) of 1.44 eV. The ΔEHL values of 14 and 15 are almost identical, which is consistent with the orders of absorption red-shifts. The first oxidation potential of 17 was observed at –0.02 V, indicating its electron rich nature. This may be accounted for the presence of two 2-hydropyrrole units in 17.
The UV-Vis absorption spectra of 12 and 13 are quite similar, exhibiting bands at 402, 551, and 598 nm and at 406, 555, and 610 nm, respectively. The absorption spectrum of 14 is broader and red-shifted, showing bands at 422, 599, and 700 nm. The absorption spectra of 15 and 16 are even broader and red-shifted. The spectral profiles of 15 and 16 are similar in the range of 350–500 nm but are different in the low energy region. Namely, 15 shows a tail up to 850 nm but 16 shows a tail reaching around 1000 nm. The absorption spectrum of 17 is also broader and red-shifted, exhibiting absorption bands at 444, 651, and 697 nm. Upon addition of acid, the absorption spectrum of 13 was largely changed probably due to the protonation of the pyridine moieties, since the addition of acid to CH2Cl2 solution of 12 did not lead to any changes in its UV–Vis spectrum (Supplementary Fig. 27).
Mutual interconversion between 15 and 16
In the meanwhile, we found TFA-triggered clean mutual interconversion between 15 and 16. In neutral or basic solutions, the mutual interconversion was not detected even at high temperature. Small amounts of TFA (0.05 or 0.2 equiv) accelerated the interconversion with molar ratios of 15/16 at the saturation point: 3.2 or 1.1 (see Supplementary Figs. 40 and 41). On the other hand, in the presence of 1.0 or 2.0 equiv TFA, the interconversion was rapid but gave 16 predominantly (see Supplementary Figs. 42 and 43). After certain reaction time, the interconversion showed the same saturation feature starting from either 15 or 16, suggesting thermodynamic control. We thought that the relative stability of neutral 15 to 16 determined the equilibrium at low concentration of TFA and that of monoprotonated 15 to 16 determined the equilibrium at high concentration of TFA. TFA-d was then applied to explore the process of proton transferring process. Spectra of the product 16-d indicated that C–H activation and the following C–Pd bonds deuteration underwent in the isomerization process. To better understand this process DFT calculations of the relative energies of 15 and 16 in neutral and monoprotonated forms were conducted (see Supplementary Figs. 56 and 57). In the monoprotonated forms, the most stable form was 16 protonated at the Pd-coordinated pyridine site, which is 9.0 kcal/mol more stable than 15, in line with the experimental results. In contrast, the Gibbs free energy difference between neutral 15 and 16 is subtle (~2.7 kcal/mol), so that the equilibrium may be influenced by solvent effects or other experimental conditions.
Discussion
In summary, the benzene and pyridine incorporating octaphyrins 12 and 13 were synthesized through Suzuki–Miyaura coupling reaction of the simple starting materials and subsequent oxidation with DDQ. These octaphyrins are nonaromatic and show dumbbell structures. The incorporation of pyridine units into the octaphyrin framework has strong influences such as the formation of the isomeric bis-PdII complexes 15 and 16 along with the rearranged complex 17. Further, the complexes 15 and 16 underwent the TFA-induced mutual interconversion depending upon the concentration of TFA. Further exploration of core-modified expanded porphyrins is underway in our laboratories.
Methods
Materials, characterizations, and theoretical calculations
1H NMR (500 MHz) spectra were measured by a Bruker AVANCE-500 spectrometer, and chemical shifts were reported on the delta scale in ppm relative to CHCl3 as an internal reference (δ = 7.260 ppm). Assignments of 1H NMR were based on HH COSY spectra and D2O exchange experiments. UV/Vis absorption spectra were recorded on a Shimadzu UV-3600 spectrometer. X-ray crystallographic data were taken on an Agilent SuperNova X-ray diffractometer equipped with a large area CCD detector. Using Olex2, structures of compounds 12–17 were solved with the ShelXS structure solution program using Direct Methods and refined with the ShelXL refinement package using Least Squares minimization. Disordered solvent molecules were treated by SQUEEZE program of Platon. Redox potentials were measured by the cyclic voltammetry and differential pulse voltammetry method on an ALS660 electrochemical analyzed model (Solvent: PhCN, electrolyte: 0.1 M n-Bu4NPF6, working electrode: glassy carbon, reference electrode: Ag/AgNO3, Counter electrode: Pt wire, scan rate: 0.05 V/s, external reference: ferrocene/ferrocenium cation). Benzonitrile passed through alumina column was used for electrochemical analysis. Unless otherwise noted, materials obtained from commercial suppliers were used without further purification. All calculations were carried out using the Gaussian 09 program36. Initial geometries for 12–17 were obtained from X-ray structures. The structures were fully optimized without any symmetry restriction. Geometry optimizations in the ground state (S0) were performed by the density functional theory (DFT) method with restricted B3LYP (Becke’s three-parameter hybrid exchange functionals and the Lee–Yang–Parr correlation functional)37,38 level employing basis sets and pseudopotentials; 6–311G(d,p) for C, H, N39, and SDD for Pd40. NICS(0) values were calculated with GIAO method at the B3LYP level employing the same basis sets and pseudopotentials for geometry optimizations. Calculated chemical shifts were estimated relative to the magnetic shielding of a proton of chloroform (24.95 ppm) calculated at the same level.
Synthesis of 10
A toluene-DMF solution (3/1.5 mL) of 9 (178.4 mg, 0.25 mmol), 1,3-dibromobenzene (301.7 μL, 589.8 mg, 2.5 mmol), Pd2(dba)3 (22.9 mg, 0.025 mmol), PPh3 (26.2 mg, 0.1 mmol), Cs2CO3 (172.7 mg, 0.53 mmol), and CsF (77.5 mg, 0.51 mmol) was degassed through three freeze-pump-thaw cycles, and the reaction Schlenk tube was purged with argon. The resulting mixture was stirred at reflux for 48 h. The reaction mixture was diluted with CHCl3, washed with water, and dried over anhydrous sodium sulfate. After the solvent was removed, 2,3-dichloro-5,6-dicyano-1,4-benzoquinone (DDQ) (136.2 mg, 0.6 mmol) was added to the resulting mixture in CH2Cl2 and this reaction mixture was stirred for another 4 h. Evaporation of the solvent followed by silica-gel column chromatography (CH2Cl2/n-hexane as an eluent) and recrystallization with CH3OH to afford 10 as dark green solids (101.7 mg, 0.13 mmol, 53% yield). 1H NMR (500 MHz) (CDCl3) δ = 13.60 (br, 1H), 8.00 (s, 2H), 7.68 (d, 2H, J = 7.7 Hz), 7.20 (d, 2H, J = 8.0 Hz), 7.14 (d, 2H, J = 4.5 Hz), 6.97 (s, 4H), 6.88 (d, 2H, J = 4.5 Hz), 6.75 (t, 2H, J = 7.6 Hz), 6.19 (s, 2H), 2.38 (s, 6H), 2.18 (s, 12H) ppm; 13C NMR (126 MHz) (CDCl3): δ = 168.2, 152.7, 139.0, 137.7, 137.3, 137.0, 135.7, 133.6, 132.1, 131.0, 128.8, 127.9, 126.8, 124.1, 122.1, 121.0, 21.1, and 20.5 ppm; HRMS (m/z): [M]+ calcd. for C44H37Br2N3, 765.1349; found 765.1291.
Synthesis of 11
A toluene-DMF solution (3/1.5 mL) of 9 (178.4 mg, 0.25 mmol), 2,6-dibromopyridine (592.2 mg, 2.5 mmol), Pd2(dba)3 (22.9 mg, 0.025 mmol), PPh3 (26.2 mg, 0.1 mmol), Cs2CO3 (172.7 mg, 0.53 mmol), and CsF (77.5 mg, 0.51 mmol) was degassed through three freeze-pump-thaw cycles, and the reaction Schlenk tube was purged with argon. The resulting mixture was stirred at reflux for 48 h. The reaction mixture was diluted with CHCl3, washed with water, and dried over anhydrous sodium sulfate. After the solvent was removed, DDQ (136.2 mg, 0.6 mmol) was added to the resulting mixture in CH2Cl2 and this reaction mixture was stirred for another 4 h. The reaction mixture was passed through a short alumina column (CH2Cl2 as an eluent) and recrystallization with CH3OH to afford 11 as dark green solids (91.9 mg, 0.119 mmol, 47.8% yield). 1H NMR (500 MHz) (CDCl3): δ = 13.31 (br, 1H), 7.47 (m, 4H), 7.27 (d, 2H), 6.97 (s, 4H), 6.89 (d, 2H, J = 4.5 Hz), 6.66 (t, 2H, J = 7.6 Hz), 6.28 (s, 2H), 2.38 (s, 6H), 2.16 (s, 12H) ppm. 13C NMR (126 MHz) (CDCl3): δ = 168.4, 153.2, 153.0, 140.8, 139.4, 139.0, 138.0, 137.5, 137.1, 135.5, 133.4, 128.0, 127.5, 125.9, 121.9, 121.8, 21.1, 20.5 ppm; HRMS (m/z): [M+H]+ calcd. for C42H36Br2N5, 768.1332; found 768.1354.
Synthesis of 12
Route 1: A p-xylene-DMF solution (3/1.5 mL) of 9 (84 mg, 0.12 mmol), 10 (77.7 mg, 0.1 mmol), Pd2(dba)3 (18.5 mg, 0.02 mmol), PPh3 (21.2 mg, 0.08 mmol), Cs2CO3 (71.0 mg, 0.2 mmol), and CsF (33.4 mg, 0.2 mmol) was degassed through three freeze-pump-thaw cycles, and the reaction Schlenk tube was purged with argon. The resulting mixture was stirred at reflux for 48 h. The reaction mixture was diluted with CHCl3, washed with water, and dried over anhydrous sodium sulfate. After the solvent was removed, DDQ (65.8 mg, 0.29 mmol) was added to the resulting mixture in CH2Cl2, and this reaction mixture was stirred for another 8 h. The reaction mixture was passed through a short alumina column (CH2Cl2 as an eluent). Evaporation of the solvent followed by silica-gel column chromatography (CH2Cl2/n-hexane as an eluent) and recrystallization with CH2Cl2/MeOH afforded 12 as violet solids (8.4 mg, 0.008 mmol, 8.0% yield).
Route 2: A toluene-DMF solution (3/1.5 mL) of 9 (180 mg, 0.25 mmol), 1,3-dibromobenzene (25.6 μL, 49.5 mg, 0.21 mmol), Pd2(dba)3 (23 mg, 0.025 mmol), X-Phos (48 mg, 0.10 mmol), Cs2CO3 (136.0 mg, 0.42 mmol), and CsF (67.0 mg, 0.44 mmol) was degassed through three freeze-pump-thaw cycles, and the reaction flask was purged with argon. The resulting mixture was stirred for 48 h at reflux. The reaction mixture was diluted with CHCl3, washed with water, and dried over anhydrous sodium sulfate. After the solvent was removed, DDQ (136.2 mg, 0.6 mmol) was added to the resulting mixture in CH2Cl2 and this reaction mixture was stirred for another 8 h. The reaction mixture was passed through a short alumina column (CH2Cl2 as an eluent). Evaporation of the solvent followed by column chromatography on silica gel (CH2Cl2/n-hexane as an eluent) and recrystallization from n-hexane gave 12 as violet solids (7.9 mg, 0.007 mmol, 7.0% yield) 1H NMR (500 MHz) (CDCl3): δ = 13.0 (br, 2H, N–H), 8.34 (br, 2H, m-phenylene-H), 7.39 (d, 4H, J = 4.5 Hz, pyrrole-H), 7.25 (dd, 4H, J = 7.8, 1.5 Hz, m-phenylene-H), 7.03–7.00 (m, 12H, pyrrole-H and Ar-m-H), 6.33 (s, 4H, pyrrole-H), 5.63 (t, 2H, J = 7.7 Hz, m-phenylene-H), 2.40 (s, 12H, Me–H), 2.25 (s, 12H, Me–H), 2.18 (s, 12H, Me–H) ppm; 13C NMR (126 MHz) (CDCl3): δ = 169.2, 152.8, 138.9, 137.7, 137.7, 137.5, 135.8, 134.2, 132.9, 129.5, 128.1, 128.0, 127.3, 126.9, 124.7, 121.0, 21.3, 20.8, and 20.7 ppm; UV/Vis (CH2Cl2): λmax (ε[M–1 cm–1]) = 402 (132,023), 551 (48,426), 598 (20,366) nm; HRMS (m/z): [M]+ calcd. for C42H36Br2N5, 1062.53; found 1062.49.
Synthesis of 14
12 (20 mg, 0.019 mmol) was added to a round-bottomed 100 mL flask containing a magnetic bar and dissolved in CHCl3/MeOH (15/6 mL). Pd(OAc)2 (42.6 mg, 0.19 mmol) and NaOAc (18.7 mg, 0.22 mmol) was added, after being refluxed for 24 h, the solvent was evaporated in vacuo. The product was purified by column chromatography on silica-gel (CH2Cl2/n-hexane as an eluent). Recrystallization with n-hexane gave 14 (17.8 mg, 0.014 mmol, 73.6% yield) as green solids. 1H NMR (500 MHz) (CDCl3): δ = 8.67 (d, 2H, J = 1.8 Hz, C6H3-H), 7.59 (d, 2H, J = 4.8 Hz, pyrrole-H), 7.31 (d, 2H, J = 4.7 Hz, pyrrole-H), 7.25 (s, 2H, Ar-m-H), 7.03–7.01 (m, 6H, pyrrole-H and Ar-m-H), 6.98 (s, 2H, Ar-m-H), 6.94 (d, 2H, J = 4.3 Hz, pyrrole-H), 6.67 (d, 2H, J = 4.3 Hz, pyrrole-H), 6.59 (d, 2H, J = 4.3 Hz, pyrrole-H), 5.32 (dd, 2H, J = 7.6, 1.8 Hz, C6H3-H), 3.95 (d, 2H, J = 7.7 Hz, C6H3-H), 2.44 (s, 6H, Me–H), 2.41 (s, 6H, Me–H), 2.31 (s, 6H, Me–H), 2.17 (s, 6H, Me–H), 2.16 (s, 6H, Me–H), 2.07 (s, 6H, Me–H); 13C NMR (126 MHz) (CDCl3): δ = 172.4, 170.7, 170.6, 145.0, 144.2, 144.1, 140.5, 139.4, 139.2, 138.9, 137.8, 137.6, 137.6, 137.5, 137.4, 137.2, 136.7, 135.7, 133.9, 133.2, 132.6, 129.5, 128.0, 127.8, 127.7, 126.3, 126.2, 124.9, 123.7, 119.0, 21.2, 20.8, 20.7, 20.7, and 20.6 ppm; UV/Vis (CH2Cl2): λmax (ε[M–1 cm–1]) = 422 (73,384), 599 (17,949), 700 (21,503) nm; HRMS (m/z): [M]+ calcd. for C76H62N6Pd2, 1270.3134; found 1270.3018.
Synthesis of 13
Route 1: A p-xylene-DMF solution (3/1.5 mL) of 9 (84.0 mg, 0.12 mmol), 11 (77.7 mg, 0.1 mmol), Pd2(dba)3 (18.5 mg, 0.02 mmol), PPh3 (21.2 mg, 0.08 mmol), Cs2CO3 (71.0 mg, 0.2 mmol), and CsF (33.4 mg, 0.2 mmol) was degassed through three freeze-pump-thaw cycles, and the reaction Schlenk tube was purged with argon. The resulting mixture was stirred at reflux for 48 h. The reaction mixture was diluted with CHCl3, washed with water, and dried over anhydrous sodium sulfate. After the solvent was removed, DDQ (65.8 mg, 0.29 mmol) was added to the resulting mixture in CH2Cl2 stirring for another 8 h. The reaction mixture was passed through a short alumina column (CH2Cl2 as an eluent). Evaporation of the solvent followed by silica-gel column chromatography (CH2Cl2/n-hexane as an eluent) and recrystallization with CH2Cl2/MeOH afforded 13 as violet solids (9.6 mg, 0.009 mmol, 9.0% yield).
Route 2: A toluene-DMF solution (3/1.5 mL) of 9 (180 mg, 0.25 mmol), 2,6-dibromopyridine (50.0 mg, 0.21 mmol), Pd2(dba)3 (38.8 mg, 0.042 mmol), PPh3 (44.2 mg, 0.17 mmol), Cs2CO3 (138.0 mg, 0.42 mmol), and CsF (69.5 mg, 0.45 mmol) was degassed through three freeze-pump-thaw cycles, and the reaction Schlenk tube was purged with argon. The resulting mixture was stirred at reflux for 48 h. The reaction mixture was diluted with CHCl3, washed with water, and dried over anhydrous sodium sulfate. After the solvent was removed, DDQ (136.2 mg, 0.6 mmol) was added to the resulting mixture in CH2Cl2 and this reaction mixture was stirred for another 8 h. The reaction mixture was passed through a short alumina column (CH2Cl2 as an eluent). Evaporation of the solvent followed by silica-gel column chromatography (CH2Cl2/n-hexane as an eluent) and recrystallization with CH2Cl2/MeOH afforded 13 as violet solids (9.1 mg, 0.009 mmol, 8.1% yield). 1H NMR (500 MHz) (CDCl3): δ = 13.43 (br, 2H, N–H), 7.63 (d, 4H, J = 4.5 Hz, pyrrole-H), 7.41 (d, 4H, J = 8.0 Hz, pyridine-H), 7.01-6.98 (m, 12H, pyrrole-H and Ar-m-H), 6.35 (s, 4H, pyrrole-H), 6.05 (t, 2H, J = 8.0 Hz, pyridine-H), 2.40 (s, 12H, Me–H), 2.26 (s, 12H, Me–H), 2.18 (s, 12H, Me–H) ppm; 13C NMR (126 MHz) (CDCl3): δ = 169.2, 153.1, 150.8, 139.3, 137.9, 137.6, 137.5, 137.4, 135.2, 134.2, 133.8, 128.2, 128.1, 125.8, 123.5, 121.3, 21.3, 20.8, and 20.7 ppm; UV/Vis (CH2Cl2): λmax (ε[M–1 cm–1]) = 344 (40,195), 406 (128,444), 555 (46,048), 610 (16,662) nm; HRMS (m/z): [M]+ calcd. for C74H64N8, 1064.5248; found 1064.5239.
Synthesis of 15, 16, and 17
13 (19.1 mg, 0.018 mmol) was added to a round-bottomed 100 mL flask containing a magnetic bar, and dissolved in CHCl3/MeOH (15/6 mL). Pd(OAc)2 (40.5 mg, 0.18 mmol) and NaOAc (17.8 mg, 0.21 mmol) was added, after being refluxed for 24 h, the solvent was evaporated in vacuo. The residue was purified by column chromatography on silica-gel (CH2Cl2/n-hexane as an eluent), three fractions were obtained. The first fraction was recrystallized with CH2Cl2/CH3OH to afford 17 (2.8 mg, 2.2 μmol, 12.0% yield) as green solids. The second fraction was recrystallized with CH2Cl2/n-hexane to afford 15 (4.58 mg, 3.6 μmol, 20.0% yield) as dark green solids. The third fraction was recrystallized with CH2Cl2/CH3OH to afford 16 (13.7 mg, 10.8 μmol, 60.0% yield) as navy blue solids.
15: 1H NMR (500 MHz) (CDCl3): δ = 8.00 (d, 2H, J = 4.7 Hz, pyrrole-H), 7.43 (d, 2H, J = 4.7 Hz, pyrrole-H), 7.34 (d, 2H, J = 4.7 Hz, pyrrole-H), 7.06–7.02 (m, 6H, Ar-m-H), 6.98 (s, 2H, Ar-m-H), 6.94 (d, 2H, J = 4.7 Hz, pyrrole-H), 6.74 (d, 2H, J = 4.5 Hz, pyrrole-H), 6.65 (d, 2H, J = 4.3 Hz, pyrrole-H), 5.46 (d, 2H, J = 7.6 Hz, pyridine-H), 4.07 (d, 2H, J = 7.6 Hz, pyridine-H), 2.44 (s, 6H, Me–H), 2.42 (s, 6H, Me–H), 2.31 (s, 6H, Me–H), 2.18 (s, 6H, Me–H), 2.13 (s, 6H, Me–H), 2.09 (s, 6H, Me–H) ppm; 13C NMR (126 MHz) (CDCl3): δ = 171.8, 170.7, 162.4, 157.4, 146.2, 144.9, 144.7, 144.1, 141.7, 140.6, 139.6, 138.4, 138.1, 137.7, 137.6, 137.6, 137.3, 136.8, 136.4, 135.5, 134.0, 133.4, 129.2, 128.2, 128.2, 127.0, 125.6, 121.9, 120.5, 21.5, 21.1, 21.0, and 21.0 ppm; UV/Vis (CH2Cl2): λmax (ε[M–1 cm–1]) = 309 (33,094), 405 (55,309), 461 (38,258), 597 (23,944), 660 (20,273), 723 (22,148) nm; HRMS (m/z): [M]+ calcd. for C74H60N8Pd2, 1272.3038; found 1272.2944.
16: 1H NMR (500 MHz) (CDCl3): δ = 7.67 (d, 2H, J = 8.0 Hz, pyridine-H), 7.14 (d, 2H, J = 4.5 Hz, pyrrole-H), 7.03 (t, 1H, J = 7.7 Hz, pyridine-H), 6.95–6.92 (m, 12H, Ar-m-H and pyrrole-H), 6.52 (d, 2H, J = 4.9 Hz, pyrrole-H), 6.25 (d, 2H, J = 4.3 Hz, pyrrole-H), 6.22 (d, 2H, J = 4.5 Hz, pyrrole-H), 3.69 (s, 1H, pyridine-H), 2.37 (s, 6H, Me–H), 2.35 (s, 6H, Me–H), 2.21 (s, 6H, Me–H), 2.15 (s, 6H, Me–H), and 2.12 (s, 12H, Me–H) ppm; 13C NMR (126 MHz) (CDCl3): δ = 175.9, 169.9, 160.9, 155.2, 149.3, 147.4, 146.6, 144.7, 143.0, 140.5, 139.9, 138.0, 137.8, 137.3, 137.2, 137.2, 137.1, 136.8, 135.0, 134.4, 134.2, 133.4, 128.1, 128.0, 127.9, 127.4, 127.0 126.9, 123.2, 121.7, 21.3, 20.8, 20.6, 20.6, and 20.5 ppm; UV/Vis (CH2Cl2): λmax (ε[M–1 cm–1]) = 403 (51,227), 556 (10,987), 664 (15,863), 777 (11,779), 925 (4759) nm; HRMS (m/z): [M]+ calcd. for C74H60N8Pd2, 1272.3038; found 1272.3063.
17: 1H NMR (500 MHz) (CDCl3): δ = 7.74 (d, 2H, J = 5.3 Hz, pyrrole-H), 7.44 (t, 2H, J = 7.9 Hz, pyridine-H), 7.31–7.29 (m, 4H, pyridine-H), 6.98–6.93 (m, 10H, Ar-m-H and pyrrole-H), 6.71 (d, 2H, J = 5.0 Hz, pyrrole-H), 6.26–6.24 (m, 4H, pyrrole-H), 6.01 (d, 2H, J = 5.3 Hz, pyrrole-H), 2.38 (s, 6H, Me–H), 2.31 (s, 6H, Me–H), 2.15 (s, 6H, Me–H), 2.09 (s, 6H, Me–H), 2.07 (s, 6H, Me–H), 2.03 (s, 6H, Me–H); 13C NMR (126 MHz) (CDCl3): δ = 161.2, 157.1, 156.2, 154.7, 147.0, 140.4, 139.9, 139.4, 138.9, 138.0, 137.8, 136.8, 136.7, 134.8, 134.6, 134.5, 133.5, 131.8, 128.1, 128.0, 127.8, 127.7, 125.2, 124.6, 117.0, 116.9, 113.2, 112.1, 100.3, 90.4, 21.2, 21.1, 21.1, 21.0, 20.7, and 20.6 ppm; UV/Vis (CH2Cl2): λmax (ε[M–1 cm–1]) = 332 (38,615), 444 (51,873), 651 (15,961), 697 (27,095) nm; HR-MS (MALDI-TOF-MS): HRMS (m/z): [M]+ calcd. for C74H62N8Pd2, 1274.3195; found 1274.3141.
Data availability
The X-ray crystallographic coordinates for structures reported in this study have been deposited at the Cambridge Crystallographic Data Centre (CCDC), under deposition numbers 1959823, 1959827, 1959825, 1959828, 1959830, and 1975890 (12, 13, 14, 15, 16, and 17). These data can be obtained free of charge from The Cambridge Crystallographic Data Centre via www.ccdc.cam.ac.uk/data_request/cif. The authors declare that all other data supporting the findings of this study are available within the paper and its supplementary information files.
References
Sessler, J. L. & Seidel, D. Synthetic expanded porphyrin chemistry. Angew. Chem. Int. Ed. 42, 5134–5175 (2003).
Stępień, M., Sprutta, N. & Latos-Grażyński, L. Figure eights, möbius bands, and more: conformation and aromaticity of porphyrinoids. Angew. Chem. Int. Ed. 50, 4288–4340 (2011).
Saito, S. & Osuka, A. Expanded porphyrins: intriguing structures, electronic properties, and reactivities. Angew. Chem. Int. Ed. 50, 4342–4373 (2011).
Osuka, A. & Saito, S. Expanded porphyrins and aromaticity. Chem. Commun. 47, 4330–4339 (2011).
Tanaka, T. & Osuka, A. Chemistry of meso-aryl-substituted expanded porphyrins: aromaticity and molecular twist. Chem. Rev. 117, 2584–2640 (2017).
Lash, T. D. Carbaporphyrins, porphyrin isomers and the legacy of Emanuel Vogel. J. Porphyr. Phthalocyanines 16, 423–433 (2012).
Lash, T. D. Metal complexes of carbaporphyrinoid systems. Chem. Asian J. 9, 682–705 (2014).
Lash, T. D. Benziporphyrins, a unique platform for exploring the aromatic characteristics of porphyrinoid systems. Org. Biomol. Chem. 13, 7846–7878 (2015).
Pawlicki, M. & Latos-Grażyński, L. Aromaticity switching in porphyrinoids. Chem. Asian J. 10, 1438–1451 (2015).
Lash, T. D. Carbaporphyrinoid systems. Chem. Rev. 117, 2313–2446 (2017).
Szyszko, B., Białek, M. J., Pacholska-Dudziak, E. & Latos-Grażyński, L. Flexible porphyrinoids. Chem. Rev. 117, 2839–2909 (2017).
Setsune, J.-i 2, 2′-Bipyrrole-based porphyrinoids. Chem. Rev. 117, 3044–3101 (2017).
Carré, F. H., Corriu, R. J. P., Bolin, P. G., Moreau, J. J. E. & Vernhet, C. Aminosilanes in organic synthesis. Preparation of new expanded porphyrin ligands and bimetallic transition-metal complexes. Crystal structure of a tetrapyrrole macrocycle dirhodium complex. Organometallics 12, 2478–2486 (1993).
Setsune, J. & Watanabe, K. Cryptand-like porphyrinoid assembled with three dipyrrylpyridine chains: synthesis, structure, and homotropic positive allosteric binding of carboxylic acids. J. Am. Chem. Soc. 130, 2404–2405 (2008).
Zhang, Z. et al. Cyclo[m]pyridine[n]pyrroles: hybrid macrocycles that display expanded π-conjugation upon protonation. J. Am. Chem. Soc. 134, 4076–4079 (2012).
Zhang, Z. et al. Expanded porphyrin-anion supramolecular assemblies: environmentally responsive sensors for organic solvents and anions. J. Am. Chem. Soc. 137, 7769–7774 (2015).
Myśliborski, R., Hurej, K., Pawlicki, M. & Latos-Grażyński, L. Inversion triggered by protonation-a rubyrin with embedded α, β′-pyridine moieties. Angew. Chem. Int. Ed. 57, 16866–16870 (2018).
Zhang, Z. et al. Cyclo[6]pyridine[6]pyrrole: a dynamic, twisted macrocycle with no meso bridges. J. Am. Chem. Soc. 136, 7591–7594 (2014).
Stępień, M. & Latos-Grażyński, L. Benziporphyrins: exploring arene chemistry in a macrocyclic environment. Acc. Chem. Res. 38, 88–98 (2005).
Myśliborski, R., Latos-Grażyński, L. & Szterenberg, L. Pyriporphyrin-a porphyrin homologue containing a built-in pyridine moiety. Eur. J. Org. Chem. 2006, 3064–3068 (2006).
Myśliborski, R., Latos-Grażyński, L., Szterenberg & Lis, L. Subpyriporphyrin-a [14]triphyrin(1.1.1) homologue with an embedded pyridine moiety. Angew. Chem. Int. Ed. 45, 3670–3674 (2006).
Setsune, J. & Yamato, K. Highly reactive meso-like positions of dipyrihexaphyrin. Chem. Commun. 48, 4447–4449 (2012).
Setsune, J., Kawama, M. & Nishinaka, T. Helical binuclear CoII complexes of pyriporphyrin analogue for sensing homochiral carboxylic acids. Tetrahedron Lett. 52, 1773–1777 (2011).
Aratani, N. & Osuka, A. Exploration of giant functional porphyrin arrays. Bull. Chem. Soc. Jpn. 88, 1–27 (2015).
Cai, H. et al. Synthesis of direct β-to-β linked porphyrin arrays with large electronic interactions: branched and cyclic oligomers. Angew. Chem. Int. Ed. 53, 11088–11091 (2014).
Rao, Y. et al. β-to‐β 2,5-Pyrrolylene-linked cyclic porphyrin oligomers. Chem. Eur. J. 22, 8801–8804 (2016).
Xu, L. et al. Strategic construction of directly linked porphyrin-BODIPY hybrids. Angew. Chem. Int. Ed. 56, 12322–12326 (2017).
Rao, Y. et al. π-Extended “earring” porphyrins with multiple cavities and near-infrared absorption. Angew. Chem. Int. Ed. 55, 6438–6442 (2016).
Tanaka, Y. et al. Thermal splitting of bis-Cu(II) octaphyrin(1.1.1.1.1.1.1.1) into two Cu(II) porphyrins. J. Am. Chem. Soc. 126, 3046–3047 (2004).
Tanaka, Y., Shinokubo, H., Yoshimura, Y. & Osuka, A. Metathesis-like splitting reactions of metallated [36]octaphyrins(1.1.1.1.1.1.1.1): experimental and computational investigations. Chem. Eur. J. 15, 5674–5685 (2009).
Suzuki, M. & Osuka, A. Cross-bridging reaction of 5,20-diethynyl substituted hexaphyrins to vinylene-bridged hexaphyrins. J. Am. Chem. Soc. 129, 464–465 (2007).
Suzuki, M. & Osuka, A. Intramolecular [3+2] annulation of 5-aryl-, 20-ethynyl-substituted [26]hexaphyrin(1.1.1.1.1.1) triggered by molecular compression through a dynamic conformational change. Angew. Chem. Int. Ed. 46, 5171–5174 (2007).
Vogel, E. et al. N Spirodiporphyrins-as binuclear metal complexes. Angew. Chem. Int. Ed. 42, 2857–2862 (2003).
Latos-Grażyński, L. Bimetallic figure-eight octaphyrins split into four-pyrrolic macrocycles. Angew. Chem. Int. Ed. 43, 5124–5128 (2004).
Tanaka, Y. et al. Metalation of expanded porphyrins: a chemical trigger used to produce molecular twisting and möbius aromaticity. Angew. Chem. Int. Ed. 47, 681–684 (2008).
Frisch, M. J. et al. Gaussian 09, Revision C.01 (Gaussian, Inc., Wallingford, CT, 2009).
Becke, A. D. A new mixing of Hartree–Fock and local density-functional theories. J. Chem. Phys. 98, 1372–1377 (1993).
Lee, C., Yang, W. & Parr, R. G. Development of the Colle-Salvetti correlation-energy formula into a functional of the electron density. Phys. Rev. B 37, 785–789 (1998).
Krishnan, R., Binkley, J. S., Seeger, R. & Pople, J. A. Self‐consistent molecular orbital methods. XX. A basis set for correlated wave functions. J. Chem. Phys. 72, 650–654 (1980).
Dolg, M., Wedig, U., Stoll, H. & Preuss, H. Energy-adjusted ab initio pseudopotentials for the first row transition elements. J. Chem. Phys. 86, 866–872 (1987).
Acknowledgements
The work at Hunan Normal University was supported by the National Natural Science Foundation of China. (Grant Nos. 21772036, 21602058, and 21702057), Science and Technology Planning Project of Hunan Province (2018TP1017), Scientific Research Fund of Hunan Provincial Education Department (19A331). We thank Dr. Prof. Hiromitsu Maeda in Ritsumeikan University for MALDI-TOF MS Analysis.
Author information
Authors and Affiliations
Contributions
J.S. designed and conducted the project. L.L., Z.H., F.Z., Y.L., and T. T performed the synthesis and characterization, and measured the optical and electrochemical properties. L.X., M.Z., and T.T performed X-ray diffraction analysis and DFT calculations. A.O. and J.S. prepared the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Peer review information Nature Communications thanks Martin Breza and the other, anonymous reviewers for their contribution to the peer review of this work. Peer reviewer reports are available.
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Liu, L., Hu, Z., Zhang, F. et al. Benzene- and pyridine-incorporated octaphyrins with different coordination modes toward two PdII centers. Nat Commun 11, 6206 (2020). https://doi.org/10.1038/s41467-020-20072-9
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41467-020-20072-9
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.