Abstract
Light harvesting is fundamental for production of ATP and reducing equivalents for CO2 fixation during photosynthesis. However, electronic energy transfer (EET) through a photosystem can harm the photosynthetic apparatus when not balanced with CO2. Here, we show that CO2 binding to the light-harvesting complex modulates EET in photosynthetic cyanobacteria. More specifically, CO2 binding to the allophycocyanin alpha subunit of the light-harvesting complex regulates EET and its fluorescence quantum yield in the cyanobacterium Synechocystis sp. PCC 6803. CO2 binding decreases the inter-chromophore distance in the allophycocyanin trimer. The result is enhanced EET in vitro and in live cells. Our work identifies a direct target for CO2 in the cyanobacterial light-harvesting apparatus and provides insights into photosynthesis regulation.
Similar content being viewed by others
Introduction
Oxygenic photosynthesis evolved in the cyanobacteria about 2.5 Gyr ago and provided much of our current atmospheric O21. Cyanobacteria gather photons using a harvesting antenna known as the phycobilisome (PBS). Phycobiliproteins (PBPs) constitute PBSs to produce one of the largest protein complexes known. The PBPs include phycocyanin, phycoerythrin and allophycocyanin, together with their associated linker proteins2. PBS light absorption is achieved by open-chain tetrapyrrole (bilin) chromophores covalently attached to the PBPs and their associated linker proteins3. Two subunits of a PBP, an α- and a β-subunit, form an αβ heterodimer known as an (αβ) monomer, which is assembled into an (αβ)3 trimer. Electronic energy transfer (EET) within the (αβ)3 trimer is fast and efficient (on the sub-ps to ps timescales)4. The PBP (αβ)3 trimers form the ordered supramolecular light-harvesting complex via the linker proteins5. A typical cyanobacterial hemidiscoidal PBS consists of a central core surrounded by peripheral chromophore-containing rods that capture photons6. The PBS core transfers excitation energy via a terminal emitter to the photosynthetic reaction centres with the subsequent synthesis of ATP and NADPH7.
CO2 assimilation into sugars requires NADPH and ATP. There is evidence from non-cyanobacterial model systems that CO2 fixation and availability are coupled to light-harvesting8,9. For example, CO2-deficient conditions in algae reduce photosystem (PS) II antenna size, and PSI/PSII fluorescence intensity increases10,11. A light-harvesting chlorophyll–protein complex II migrated from PSII to PSI under CO2-deficient conditions in Chlamydomonas12. Insufficient CO2 can also result in over-reduction of the plastoquinone pool, resulting in singlet oxygen species and photoinhibition13. The addition of a suitable carbon source under CO2-deficient conditions restored photosynthetic activity14.
The mechanism(s) that couples inorganic carbon availability to light-harvesting is uncertain. In higher plants, bicarbonate binding to PSII provides a redox tuning mechanism regulating and protecting PSII15. However, questions remain as HCO3− is also proposed to be tightly bound to PSII under physiologically relevant environmental CO2, meaning this binding site’s role as an authentic regulatory site at limiting CO2 awaits further experimentation16. Nevertheless, there is considerable evidence that CO2/HCO3− availability can regulate light-harvesting and subsequent EET.
How might CO2 regulate light-harvesting? CO2 and protein can interact through carbamates on neutral N-terminal α-amino- or lysine ε-amino groups. For example, carbamylation regulates the activities of Rubisco17, haemoglobin (Hb)18, and ubiquitin19. In addition, several proteins carry a stable carbamate required for catalysis, e.g., urease, alanine racemase, transcarboxylase 5 S, class D β-lactamase and phosphotriesterase20. Therefore, we and others have hypothesized that reversible carbamylation of neutral N-terminal α-amino groups and/or lysine ε-amino groups could form a widespread mechanism for protein regulation by CO217,20. We developed triethyloxonium tetrafluoroborate (TEO) as a chemical proteomics tool to identify carbamate post-translational modifications (PTMs)20. We hypothesised the TEO chemical proteomics tool could be used to discover CO2-binding proteins in the cyanobacterial PBS to determine the mechanistic basis for coupling CO2 availability to EET. Here we describe the discovery of a CO2-binding site in the PBS and its role in regulating EET.
Results
Synechocystis sp. PCC 6803 allophycocyanin A binds CO2
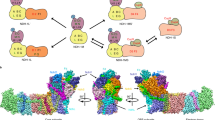
An extract of Synechocystis sp. PCC 6803 soluble protein was incubated with 20 mM NaH14CO3 and subjected to TEO-trapping (Fig. 1a). An insignificant level of 14CO2 was incorporated into the protein extract in the absence of TEO. The inability to observe protein-bound 14CO2 in the absence of TEO is likely due to the ready reversibility of carbamylation that leads to sample degassing during preparation for analysis. However, the TEO-trapped protein lysate contained significant protein-bound 14CO2, even when accounting for 50% of the total protein sample being Rubisco. Thus, we conclude that the Synechocystis proteome contains CO2-interacting proteins carbamylated at labile sites exchangeable with the environment. We proceeded to identify carbamate PTMs to investigate proteins that couple CO2-binding to EET.
a Demonstration of exchangeable CO2-binding sites on Synechocystis protein extract. CO2 trapped onto protein extract of Synechocystis sp. PCC 6803 (*p = 0.002, two-tailed t-test, n = 5 independent replicates for 14CO2 + TEO and n = 4 independent replicates for 14CO2 only, t = 4.794, df = 7, mean ± 95% CI). b–e Demonstration of exchangeable CO2-binding sites on ApcA by MS/MS. Plots of relative fragment intensity versus mass/charge ratio (m/z) for fragmentation data from MS/MS identifying ethyl-trapped carbamate on whole Synechocystis sp. PCC 6803 (b), isolated phycobilisomes from Synechocystis sp. PCC 6803 (c) or recombinant Synechococcus sp. PCC 7002 ApcABWT (αβ)3 trimers (d, e) in the presence of 12CO2 (b–d) or 13CO2 (e). Peptide sequences indicate predominant +1 y (red) +1b (blue) ions identified by MS/MS shown in the plot. Other observed ions, not labelled on the figure panels for clarity, are shown in grey and identified in Supplementary Table 1 (whole Synechocystis sp. PCC 6803) and Supplementary Table 3 (isolated phycobilisomes from Synechocystis sp. PCC 6803). The modified residue is indicated in bold. Kcarb.Et indicates the molecular weight difference between ions diagnostic of the modified Lys. f 13C-NMR spectrum demonstrating the formation of a carbamate on recombinant Synechococcus sp. PCC 7002 ApcABWT (αβ)3, but not the mutant ApcABK6A (αβ)3, trimers by the appearance of a peak at 165 ppm. Source data are provided as a Source Data file.
Soluble Synechocystis protein lysate was equilibrated with 20 mM CO2/HCO3− at pH 7.4, and TEO was added to trap carbamate PTMs. The trapping reaction mixture was digested with trypsin, and samples were analysed by ESI-MS/MS (Electrospray Ionisation Tandem Mass Spectrometry). The data were interrogated for variable PTMs on lysine with masses of 72.0211 Da (trapped carbamate) and 28.0313 Da (O-ethylation on glutamate and aspartate side chains). A lysine carbamylation site was identified on open reading frame Slr2067 (MS/MS peptide amino acids 2–16 SIVTKSIVNADAEAR, proposed carbamylation on K6) (Fig. 1b; Supplementary Fig. 1a; Supplementary Table 1). The MSMS spectrum lacked detectable b ions crossing the modification. Therefore, independent experiments were performed and identified the same carbamylation site with either both y and b ions or only b ions crossing the modification (Supplementary Fig. 2; Supplementary Table 2). In all cases, the K6 carbamate was identified on the internal lysine residue of the peptide, a so-called missed cleavage. Carbamylation removes the positive charge on the lysine that is essential for cleavage site recognition by trypsin. This missed cleavage supports the Slr2067 K6 carbamate as genuine as a missed cleavage is an otherwise rare event, despite the suboptimal spectra quality. The slr2067 gene encodes the allophycocyanin α-subunit (ApcA). ApcA is the α-subunit of the allophycocyanin (αβ)3 trimer located within the PBS core. It has a critical role in EET from the PBS antennal proteins to the photosystem via the terminal pigment. We identified the same carbamate PTM on ApcA residue K6 in TEO-trapping experiments performed on isolated PBS (Fig. 1c; Supplementary Table 3) and purified recombinant ApcAB (αβ)3 trimer (Fig. 1d; Supplementary Fig. 3). We performed the CO2 trapping experiments on ApcA with 13CO2 to corroborate the carbamate PTM by interrogating the MS/MS data for a 73.0211 Da modification. The expected +1 Da m/z increase (from 72.0211 Da) was observed for the carbamylation site at K6 (Fig. 1e). We performed a control experiment to identify the reason for the relative difficulty in observing multiple ions that encompass the K6 carbamate. Synthetic SIVTKSIVNADAEAR peptide was equilibrated with 50 mM CO2/HCO3− at pH 8.5, TEO was added, and the peptide analysed by MS/MS (Supplementary Fig. 4; Supplementary Tables 4–6)20. MS/MS fragment ions that cross K6 were observed to be lower intensity for the carboxyethylated peptide compared to the unmodified or ethylated peptide (note how the intensity of the labelled ions is almost indistinguishable from background non-specific ions in Supplementary Fig. 4b compared to Supplementary Fig. 4a, c). Therefore, the relative difficulty of observing the carbamate modification on K6 by LC-MS/MS is an intrinsic property of the carboxyethylated peptide.
To compensate for the relative difficulty in using MS/MS to observe a carbamate at K6, we used 13C-NMR as an orthologous method to confirm the ApcA CO2-binding site at K6. Carbamate formation on wild type recombinant ApcA (αβ)3 trimer (ApcABWT (αβ)3) was confirmed using 13C-NMR spectroscopy by observing a signal at ~165 ppm, which matched literature values for carbamate formation (Fig. 1f)21. We generated a K6A mutant recombinant protein (ApcABK6A (αβ)3) to investigate whether the observed 13C-NMR signal was due to the carbamate forming at K6. We also generated a K6E mutant recombinant protein (ApcABK6E (αβ)3) as we hypothesised that mutation of K6 to glutamate would represent the local carbamate charge state at 100% occupancy. Circular dichroism spectra for ApcABWT (αβ)3 versus ApcABK6A (αβ)3 and ApcABK6E proteins demonstrated that the three proteins possessed similar secondary structures (Supplementary Fig. 5). Therefore, there is no gross alteration in the ApcABK6A (αβ)3 structure in the mutant protein. No signal corresponding to a carbamate was observed in ApcABK6A (αβ)3 trimers. ApcA is, consequently, a CO2-binding protein via carbamylation of K6 at photosynthetically appropriate gas levels22.
Carbamylated ApcA peptide detection was not sufficiently reliable to quantify carbamylation at varying CO2/HCO3− by either labelling or label-free methods and 13C-NMR was insufficiently sensitive. However, we hypothesised that increased ApcA carbamylation could be indirectly monitored through a decreased observation of the unmodified peptide. ApcABWT (αβ)3was equilibrated with varying CO2/HCO3− at pH 7.4, TEO was added to trap carbamate PTMs, and samples were analysed by ESI-MS/MS. However, the unmodified K6-containing peptide was more reliably detected by MS/MS than the modified peptide. Therefore, we measured the peak area of the unmodified K6-containing peptide and calculated it as a ratio of the peak area of four other control peptides. At varying CO2/HCO3−, this ratio was compared to the absence of CO2/HCO3− (Supplementary Fig. 6). The control peptides were also compared to one another. CO2/HCO3− caused an immediate decrease in the relative amount of the unmodified peptide, which we infer is due to an increase in the modified peptide. This decrease did not occur for the control peptides. The equivalent drop in the K6-containing peptide at all [CO2/HCO3−] suggested that the carbamate might be acting as an on/off switch in the presence or absence of CO2.
Allophycocyanin A CO2-binding regulates EET in vitro
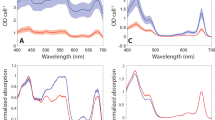
We analysed the spectroscopic properties of ApcAB (αβ)3 trimers to gain insight into the influence of CO2 on EET. We first compared the excitation (Supplementary Fig. 7) and emission (Supplementary Fig. 8) spectra of ApcABWT (αβ)3 and ApcABK6A (αβ)3 trimers. CO2/HCO3−, but not NaCl (as a control for the Na+ cation), enhanced the excitation and emission spectra in wild type but not K6A mutant (αβ)3 trimers. Calculation of the fluorescence quantum yield (QY) provides information on the efficiency of EET through the PBS to the photosynthetic reaction centre. We measured absolute fluorescence QY using an integrating sphere fibre-coupled to a fluorimeter23 (λex = 610 nm; λabs = 600–620 nm; λem = 620–750 nm). Inorganic carbon (CO2/HCO3−) enhanced the QY for ApcABWT (αβ)3 trimers by 22.6 ± 0.1% (S.D.) compared to NaCl. We next asked whether ApcA K6 carbamylation was responsible for the increase in QY in ApcAB (αβ)3 trimers in response to CO2/HCO3−. Therefore, we measured fluorescence QY for recombinant ApcABWT (αβ)3 versus ApcABK6A (αβ)3 and ApcABK6E proteins in the presence of CO2/HCO3− or the corresponding NaCl concentration. Only the QY for ApcABWT (αβ)3 trimer was enhanced by CO2/HCO3− compared to NaCl (Fig. 2a). Photosynthetically relevant CO2/HCO3− levels, therefore, regulate ApcAB EET dependent on carbamylation at K6.
a Ratio of the measured fluorescence QY with 20 mM CO2/HCO3− or NaCl for recombinant Synechococcus sp. PCC 7002 ApcABWT (αβ)3, ApcABK6A (αβ)3, or ApcABK6E (αβ)3 trimers (*p = 0.0036, one sample t-test, ratio is derived from n = 11 independent replicates for WT and n = 7 independent replicates for K6A, mean ± 95% CI). b Basal fluorescence QY for recombinant Synechococcus sp. PCC 7002 ApcABWT (αβ)3, ApcABK6A (αβ)3, or ApcABK6E (αβ)3 trimers (Kruskal-Wallis test, n = 20, 17, 16 independent replicates for WT, K6A, and K6E respectively, mean ± 95% CI). Source data are provided as a Source Data file.
We next compared the ps to ns fluorescence lifetimes for ApcABWT (αβ)3 and ApcABK6A (αβ)3 for emission after any internal energy transfer (Supplementary Table 7). We observed a predominant fluorescence lifetime (in a bi-exponential decay) for ApcABWT (αβ)3 of 1.87 ± 0.04 ns (96.8 ± 2.8 % yield; SD) and for ApcABK6A (αβ)3 of 1.96 ± 0.10 ns (97.0 ± 3.3 % yield; S.D.). These observations reflect the fluorescence lifetime from the emitting state of the APC antennae24. Therefore, the basal fluorescence properties for ApcABWT (αβ)3 and ApcABK6A (αβ)3 trimers are broadly similar.
As we hypothesised that mutation of K6 to glutamate would represent the local carbamate charge state at 100% occupancy, we compared the basal fluorescence QY for recombinant ApcABWT, ApcABK6A, and ApcABK6E (αβ)3 trimers. The basal fluorescence QY for ApcABK6E (αβ)3 trimer was significantly higher than both ApcABWT and ApcABK6A (αβ)3 trimers (Fig. 2b). Of note, the basal fluorescence QY for ApcABK6A (αβ)3 trimer was also significantly higher than ApcABWT (αβ)3 trimer but lower than the ApcABK6E (αβ)3 trimer. These data further support the hypothesis that CO2/HCO3− alters EET dependent on carbamylation at K6.
CO2 alters allophycocyanin A inter-chromophore distance
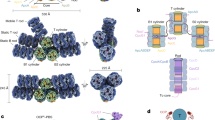
We used molecular dynamics (MD) simulations to understand the mechanism underlying the increase in QY in ApcABWT (αβ)3 trimer. To this end, we performed two 1 μs simulations of Thermosynechococcus vulcanus (PDB: 3DBJ; ref. 25) ApcABWT (αβ)3 trimer (apo and CO2 bound). The ApcAB (αβ)3 trimer has six chromophore-binding pockets that we defined as either monomeric (enclosed by residues of a monomer alone) or interface (enclosed by residues of adjacent monomers) (Fig. 3a). Analysis of the root mean square deviation (RMSD) with reference to the initial state demonstrated that both simulations stabilised after 600 ns (Supplementary Fig. 9). We examined the rotamer states for K6, whether apo or CO2-bound (Supplementary Fig. 10, Supplementary Table 8). We observed an overall change in the occupancy of rotamer states and noted the loss of a major rotamer when CO2-bound corresponding to χ1 ~75°. Contact analysis demonstrated the disruption of a salt bridge between K6 and D100 in the CO2-bound state (Fig. 3b, Supplementary Fig. 11) where D100 resides on an α-helix that couples K6 to the chromophore-binding site. Two helices in the (αβ)3 trimer showed a higher RMSD in the CO2-bound state (Supplementary Fig. 12). This higher RMSD reflected a change in the contact frequency between the chromophore and binding-pocket residues for both the monomer (Supplementary Fig. 13a) and interface (Supplementary Fig. 13b) pockets as observed during the last equilibrated 300 ns of the simulations.
a Ribbon diagram of the ApcAB (αβ)3 trimer demonstrating the monomeric and interface chromophore-binding pockets. ApcA is shown in red and ApcB in blue. b K6 forms a salt bridge with D100. D100 is sited on an α-helix that connects to the chromophore-binding pocket. c Plot of the frequency of distance against inter-chromophore distance for three monomer-interface chromophore pairs in the apo state. d Plot of the frequency of distance against inter-chromophore distance for three monomer-interface chromophore pairs in the CO2-bound state. Source data are provided as a Source Data file.
We asked whether the alterations in (αβ)3 trimer contact frequency could result in changes that explained the difference in QY with CO2/HCO3−. Therefore, we measured the change in distance of each chromophore pair (i.e., one chromophore bound to an interface pocket of an αβ heterodimer and one bound to a monomer pocket of another heterodimer) (Fig. 3c, d). We observed that in the last 300 ns of the simulation, distances are comparable between all three chromophore pairs (24–25 Å) without CO2 (Fig. 3c), but the distance of one pair reduces to 22 Å when CO2-bound (Fig. 3d). Therefore, the implication is that the reduced inter-chromophore separation enhances EET to the final emitting site in the system. These data would accordingly support the observations of the basal fluorescent QY for ApcABWT, ApcABK6A, ApcABK6E (αβ)3 trimers (Fig. 2c). ApcABK6E resembles a fully carbamylated ApcAB protein and thus shows the most significant increase in QY. The ApcABK6A protein also has an ablated salt-bridge between K6 and D100, so basal QY is increased relative to ApcABWT but not as much as ApcABK6E presumably because the new contacts made by the anionic carbamate are not present.
The monomer- and interface-pocket chromophores have been proposed to form exciton-coupled pairs in (αβ)3 trimers26. However, a weaker coupling regime allowing for incoherent EET according to the Förster mechanism has also been proposed4,24. When two chromophores with similar excitation energies are located close to one another and chirally disposed, the excited state can delocalise over both chromophores to become an exciton. Exciton interactions are important in photosynthetic light-harvesting complexes by expanding the spectral cross-section to permit faster and more robust EET27. However, in a weaker coupling regime, EET, according to the Förster mechanism, is applicable, and the rate of EET can be obtained for a given pairwise interaction and separation. The rate of Förster EET is provided by
Where Rof and RDA is the Förster radius and distance in the donor-acceptor pair, respectively. κ2 is a mutual orientation factor, and τD is the donor fluorescence lifetime. Using a Förster radius of 68.0 Å23, a fluorescence lifetime of 1.5 ns24, and a mutual orientation factor of 1.0, we obtain an EET time of 3.3 ps for a distance of 24.5 Å (without CO2) and 1.7 ps for a distance of 22.0 Å (in the presence of CO2). These values are similar to previously measured EET rates in related systems4,24. The orientation factor κ2 has a range; 0 ≤ κ2 ≤ 4. For κ2 = 1.0 the interaction dipoles adopt a “sandwich” configuration. If the orientation factor substantially impacted the rate of electronic energy transfer on CO2 binding, there would have to be a change in mutual dipole orientation from the “sandwich” towards an “in line” configuration. No structural change consistent with this configuration change was evidenced from the molecular dynamics simulations. Therefore, altered change in distance between interacting chromophores is the significant contributor to the modulation of the electronic energy transfer. The increased EET rate calculated from distances measured in our MD simulations correlates with the higher QY measured for ApcAB (αβ)3 trimers in the presence of CO2 and corroborates our hypothesis.
We employed ultrafast transient absorption spectroscopy to validate the calculated EET rates experimentally. The ApcAB (αβ)3 trimers of Synechococcus sp. PCC 7002 used for in vitro biochemistry are very similar in structure to the ApcAB (αβ)3 trimers of Thermosynechococcus vulcanus used for molecular dynamics (Supplementary Fig. 14). Synechoccus ApcAB (αβ)3 trimers were, therefore, used for ultrafast transient absorption spectroscopy. ApcAB (αβ)3 trimers were monitored over a 2.5 ps time window after excitation by a ~200 fs optical pulse at 515 nm (Fig. 4, Supplementary Fig. 15). Probing the absorption recovery at 610–630 nm revealed a fast initial stimulated emission component of 200–400 fs, observed in both the absence and presence of CO2 in both ApcABWT (αβ)3 and ApcABK6A (αβ)3 trimers. We attribute this fast initial stimulated emission component to relaxation within the strongly coupled αβ dimer, consistent with earlier work28.
PCC 7002 ApcAB EET rate in vitro. A plot of the change in transmission versus time for ApcABWT (αβ)3 (a, b) or ApcABK6A (αβ)3 (c, d) trimers in the presence of NaCl (a, c) or CO2/HCO3−. The sample was excited at 0 ps with a pulse temporal width of 200 fs at 515 nm, and transmission was analysed over the range 610–630 nm. The red line indicates the data fit for calculating EET rates. Source data are provided as a Source Data file.
The 200–400 fs component is followed by a stimulated emission component in the ps timescale (the EET rate according to the Förster mechanism) which is modulated by CO2 in ApcABWT (αβ)3. In the absence of CO2, we observed a long lifetime component significantly longer than could be fit with the period of data acquisition. The lifetime was significantly reduced in the presence of CO2 and could be fit to give a value of 2.9 ps (τ1 = 200 fs (60% of the amplitude), τ2 = 2.9 ps (40% of the amplitude)). Crucially, in ApcABK6A (αβ)3, the stimulated emission component was too long to be fit by the data and was also not observed to be reduced by CO2 within the sensitivity of the experiment. We conclude that this stimulated emission component is due to weaker coupling Förster EET within ApcAB (αβ)3. The modulation of this component with CO2 is consistent with the distance variations predicted in the MD simulations. Further, the Förster EET rates measured here are on a ps timescale and therefore compatible with the values calculated from MD simulations. In conclusion, ultrafast transient absorption measurements show an enhanced rate of Förster EET in ApcABWT (αβ)3 in the presence of CO2. Consequently, we propose that the CO2 enhanced EET rate reduces the yield of non-radiative decay early in the allophycocyanin EET sequence. Thus, the excitation energy is more effectively transferred to the final emitting state, resulting in a higher fluorescence QY.
Allophycocyanin A CO2-binding regulates EET in the cell
We next assessed whether CO2/HCO3− enhanced EET in the context of the whole organism. QY measurements from the PBS in vivo are typically an order of magnitude lower than for the isolated recombinant proteins. This decreased QY is due to efficient EET from the PBS to the photosystem via the terminal pigment and subsequent photochemistry (charge separation and electron transfer) at the reaction centres. Therefore, the observed QY in vivo reflects energy lost from the PBS that is not transmitted to the photosystem. However, we reasoned that an increased observed QY would be reflective of increased EET to the photosystem due to reduced non-radiative decay at the initially excited chromophores, as previously discussed.
We used CRISPR-Cpf1 to generate Synechocystis sp. PCC 6803 with single amino acid mutations at K6 in ApcA. Absorption spectra were measured for wild type, K6A and K6E cells and were broadly similar (Supplementary Fig. 16). The relative peak heights for the PBS were similar in all strains (Supplementary Fig. 16 inset). The peak height for P680 for K6E cells was increased. As this absorption at the photosynthetic reaction centre is downstream of the effects studies at the PBS, this strain was still investigated further. We depleted Synechocystis sp. PCC 6803 wild type and K6A cells of CO2/HCO3− under cool white light and measured the fluorescence QY when exciting chlorophyll a and PBS at 485 nm and measuring emission at 660 nm (Fig. 5a). Although 485 nm will also excite carotenoids, their low QY and efficient electronic energy transfer to chromophores makes it very unlikely they will impact on the observations. We observed a slight but significant enhancement of QY for wild type cells but not K6A cells when exposed to CO2/HCO3−. The observed effect is small, as expected, as energy transfer from the PBS to the photosystem is extremely efficient. However, both the small size of the effect and the indirect measure of EET limited the utility of this approach. We, therefore, measured whole-cell fluorescence lifetimes at 660 nm for wild type, K6A and K6E cells excited at 635 nm (Table 1; Fig. 5b). Two predominant fluorescent lifetimes on the ps timescale were observed. No difference in lifetime or fluorescence amplitude was observed between cells in the presence of NaCl or CO2/HCO3− within the measurement error for the experiment. However, the τ2 fluorescence lifetime for both K6A and K6E cells was significantly longer than for wild type cells, indicating increased energy transfer to the emitting species (Fig. 4b). This observation is consistent with the increased basal fluorescent QY for ApcABK6A, ApcABK6E (αβ)3 trimers compared to wild type (Fig. 2c) and decreased inter-chromophore distance (Fig. 3d). The 635 nm excitation wavelength can also excite phycocyanin. However, as the effect on the τ2 fluorescence lifetime is dependent on allophyocyanin genotype (Table 1), the impact of phycocyanin is likely minimal but cannot be ruled out.
a The measured fluorescence QY with 1 mM NaHCO3 (CO2/HCO3−) or NaCl for Synechocystis sp. PCC 6803 wild type or K6A cells (*q = 0.0058, one-way ANOVA with post hoc two-stage linear set-up procedure of Benjamini, Krieger and Yekutieli, n = 6 independent replicates for WT + NaCl, K6A + NaCl and K6A + CO2, n = 5 independent replicates for WT + CO2, t = 2.382, df = 19, nd no discovery, mean ± 95% CI). b The second fluorescent lifetime component (Table 1) with 1 mM NaHCO3 (-CO2) or NaCl (-Na) for Synechocystis sp. PCC 6803 wild type, K6A, or K6E cells (*p < 0.05, ns = not significant, two-way ANOVA of data from Table 1 with post hoc Tukey test, n = 6 independent replicates, mean ± 95% CI). Source data are provided as a Source Data file.
Together, the data support the hypothesis that carbamate formation at ApcA K6 breaks a salt bridge with D100 and enhances EET in whole cells.
Discussion
The Calvin–Benson–Bassham (CBB) cycle rate depends on multiple factors. However, PCO2 is particularly important as it determines the relative rate of RuBisCO carboxylation compared to the competing oxygenation reaction. Following ribulose-1,5-biphosphate carboxylation, the CBB cycle requires ATP and NADPH, whose availability is controlled by light-dependent reactions. Therefore, although the light-dependent reactions might be independent of substrate availability, reduced PCO2 could represent a bottleneck for the ensuing light-independent reactions. It follows that an organism should optimise the light-dependent reaction rate to fit the changing environmental conditions that, under specific adverse scenarios, could lead to damage of the photosystems. Therefore, it seems paramount that CO2 can have a regulatory effect at the level of light-harvesting and/or light-dependent reactions for ‘sink to source’ regulation. The finding of a CO2-mediated PTM in the PBS might represent such a mechanism.
Methods
CO2 trapping
All CO2 trapping experiments were conducted in phosphate buffer (4 mL, 50 mM, pH 7.4). The solution was transferred to a potentiometric titrator (902 Titrando; Metrohm) and incubated at 25 °C with stirring. Next, a freshly made solution of triethyloxonium tetrafluoroborate (Et3OBF4; (280 mg, 1.47 mmol) in dH2O (1 mL)) was added stepwise with a constant pH maintained (pH 7.4) through the slow addition of 1 M NaOH solution via the automatic burette. The reaction mixture was stirred, and the pH was maintained for 1 h after the final Et3OBF4 addition to ensure that all TEO was hydrolysed. The reaction mixture was then dialysed against dH2O (1 L) overnight.
Mass spectrometry
The post-dialysis reaction trapping sample supernatant was removed using vacuum centrifugation. The dried protein sample was resuspended in 8 M urea and reduced with dithiothreitol (DTT, 25 mM final concentration) at 37 °C for 1 h. The sample was then alkylated with iodoacetamide (40 mM final concentration) in the dark for 1 h. This sample was then centrifuged at 1000 g for 5 min and the soluble supernatant removed. The sample was diluted to 1 M urea and digested with trypsin gold (mass spectrometry grade, Promega) in a 1:25 (w/w) ratio overnight at 37 °C. The digested solution was then desalted on a C18 column and analysed by ESI-MS/MS on an LTQ Orbitrap XL mass spectrometer (Thermo) coupled to an Ultimate 3000 nano-HPLC instrument. Peptides eluted from the LC gradient were injected online to the mass spectrometer (lock mass enabled, mass range 400–1800 Da, resolution 60,000 at 400 Da, 10 MS/MS spectra per cycle, collision-induced dissociation (CID) at 35% normalised CE, rejection of singly charged ions). The post-run raw data files were converted into.mgf files using the freeware MSConvert provided by Proteowizard29. mgf files were analysed using PEAKS Studio 10.5 software30, including the variable modifications ethylation (28.03 at D or E), carboxyethylation (72.02 at K or protein N-terminal groups), oxidation (M), acetylation (N-terminal) and the fixed modification carbimidomethyl (C). These data were then refined using a false discovery rate of 1%, two unique peptides per protein and a PTM AScore of 50. Three carbamates PTMs were discovered (including on ApcA). The two additional carbamate PTMs will be reported elsewhere.
NMR
Recombinant allophycocyanin alpha subunit wild type and K6A mutant protein were prepared in phosphate saline buffer, pH 7.4, 50 mM NaH13CO3 with 20–50% D2O. 13C-NMR experiments spectra were acquired with a Varian 600 MHz spectrometer equipped with an Agilent OneNMR Probe to deliver a maximum pulsed-field gradient strength of 62 G cm−1. A 1H spectrum was acquired to examine for small molecule impurities. Thirteen 1H experiments were recorded in 12 h, collecting 131072 complex points. The repetition time was 6.7 s, of which 1.7 s comprised the acquisition time. The excitation pulse angle was set to 45 degrees. The strong interfering H2O signal was eliminated using the Robust-5 pulse sequence31. Thirty-two 13C scans were collected, comprising 65 536 complex data points and a spectral width of 10 kHz. The repetition time was 6.3 s, of which 3.3 s comprised the acquisition time. The W5 inter-pulse delay was set to 240 µs. Rectangular 1 ms pulsed-field gradients were used in all cases with a strength of G1 = 28.3 G cm−1 (first pair) and G2 = 4.9 G cm−1 (second pair). The gradient stabilisation delay was 0.5 ms. The first pair of lock pre-focusing field gradients were separated from the first radio-frequency pulse by a 1.5 ms delay.
Cyanobacterial genetic manipulation
Synechocystis sp. PCC 6803 was cultivated in a BG-11 liquid medium with a pH of 8.0 at 28 °C. Phycobilisome isolation was performed according to the method of ref. 32. The plasmid pSL2680 containing cpf1, the native Francisella novicida CRISPR array and LacZ, amplified from the pCrispomyces-2, served as the base plasmid for construction of slr2067 K6 editing plasmids33. pSL2680 expressing full-length pre-crRNAs was constructed by cloning annealed oligonucleotides (5′- AGA TAT TTC GTG ACG ATA CTC ATG G-3′ and 5′- AGA CCC ATG AGT ATC GTC ACG AAA T-3′) into the AarI sites to yield pSL2680apcAgRNA. PCR was used to synthesise the homology regions which were cloned into the KpnI site on the plasmids containing the matching crRNA. The point mutation homology region was constructed in two pieces using oligonucleotides 5′-CAT TTT TTT GTC TAG CTT TAA TGC GGT AGT TGG TAC CCT TTA ATA AGC TTG GGT ACA CAG AC-3′/ 5′-GCT TCT GCA TCA GCA TTC ACG ATC GAT GCC GTG ACG ATA CTC ATG G-3′ and 5′- GCC CGG ATT ACA GAT CCT CTA GAG TCG ACG GTA CCG GCT CAC CTG TGA TGC CAT TG-3′/5′-CCA TGA GTA TCG TCA CGG CAT CGA TCG TGA ATG CTG ATG CAG AAG C-3′ for the K6A mutation and 5′-CAT TTT TTT GTC TAG CTT TAA TGC GGT AGT TGG TAC CCT TTA ATA AGC TTG GGT ACA CAG AC-3′/ 5′-GCT TCT GCA TCA GCA TTC ACG ATC GAT TCC GTG ACG ATA CTC ATG G-3′ and 5′- GCC CGG ATT ACA GAT CCT CTA GAG TCG ACG GTA CCG GCT CAC CTG TGA TGC CAT TG-3′/5′-CC ATG AGT ATC GTC ACG GAA TCG ATC GTG AAT GCT GAT GCA GAA GC-3′ for the K6E mutation. The homology templates were assembled, linearised with KpnI, and PCR was used to generate pSL2680-K6AapcA and pSL2680-K6EapcA. E. coli with cargo plasmid either pSL2680-K6AapcA or pSL2680-K6EapcA and E. coli with conjugal plasmid pRL443 were grown overnight at 37 °C. 10 mL of each culture was pelleted and resuspended in 10 mL LB media without antibiotics. Cargo and conjugal cultures were mixed and 200 µl combined with 100 µl of Synechocystis culture. The mixture was incubated for 5 h at 30 °C before plating on BG11 agar plates without antibiotics. Plates were incubated for 2 days at 30 °C. 15 μg mL−1 kanamycin was added, and plates were incubated at 30 °C with light for 10 days until colonies appeared. At least four rounds of streak purification were performed to ensure complete segregation. Colonies were cured of plasmids on BG11 agar without antibiotics. Two mutant strains were produced for each point mutation.
Cyanobacterial manipulation for spectroscopy
Synechocystis sp. PCC 6803 cells were grown at 30 °C with constant shaking in BG-11 pH 8 medium under continuous cool-white fluorescent light at 30 μmol photons m−2 s−1. Cells were harvested at the late exponential phase by centrifugation, washed in degassed BG11 media, and resuspended with fresh degassed BG11 to an optical density of ~1.0 at 730 nm. Cells were incubated in a Clark type O2 electrode chamber under light at 25 °C until no further O2 was evolved, indicating CO2 depletion.
Recombinant protein production
The ApcAB protein of Synechococcus sp. PCC 7002 is 86.3 % identical and 93.8 % similar to the ApcAB protein of Synechococcus sp. PCC 6803 (Supplementary Fig. 1b). The ApcAB protein from Synechococcus sp. PCC 7002 can be produced as a recombinant in Escherichia coli as a recombinant protein and was, therefore, used for in vitro biochemistry. Expression plasmid carrying Synechococcus sp. PCC 7002 ApcAB were cotransformed into E. coli BL21(DE3) cells with plasmids for chromophorylation. A 50-ml starter culture was added to 1 L of LB medium with the appropriate combination of antibiotics and shaken at 37 °C for 4 h until the optical density at 600 nm (OD600) was 0.6. Recombinant protein production was induced by addition of 0.5 mM isopropyl-γ-D-thiogalactoside (IPTG). Cells were incubated with shaking at 30 °C for another 4 h before they were harvested by centrifugation at 10,000 × g. Cells containing recombinant protein were thawed and resuspended in buffer O (50 mM Tris-HCl, 150 mM NaCl; pH 8.0) at 1 g (wet weight) cells/2.5 ml and then were lysed by French Press. The lysed cell suspension was centrifuged at 13,000 × g, and the recombinant protein purified using a nickel-nitrilotriacetic acid column34.
Plasmid construction for production of recombinant Synechococcus sp. PCC 7002 ApcAB mutant plasmid was performed using standard molecular techniques and mutagenic primers 5′-ATG AGT ATT GTC ACG GCA TCC ATC GTG AAT GCC GAC-3′ and 5′-GTC GGC ATT CAC GAT GGA TGC CGT GAC AAT ACT CAT-3′ for K6A and 5′-GTC ACG GAA TCC ATC GTG AAT GCC GAC GCT G-3′ and 5′-GAT GGA TTC CGT GAC AAT ACT CAT GGT GAA GGG ATG-3′ for K6E.
Molecular Dynamics simulations
Comparing the structures of (αβ)3 allophycocyanin trimers from Thermosynechococcus vulcanus (PDB: 3DBJ [https://www.rcsb.org/structure/3DBJ])25, Synechococcus sp. PCC 7002 (PDB: 7EXT [https://www.rcsb.org/structure/7EXT])35 and Synechocystis PCC 6803 (PDB: 4PO5 [https://www.rcsb.org/structure/4PO5])36, shows that they are very similar (Supplementary Fig. 13). Accordingly, structural superpositions provided low RMSD values between the (αβ)3 trimers; 0.78 Å RMSD between trimers from 3DBJ and 4PO5, 1.56 Å RMSD between trimers from 3DBJ and 7EXT, and 1.82 Å RMSD between trimers from 4PO5 and 7EXT. All-atom molecular dynamics simulations of Thermosynechococcus vulcanus (PDB: 3DBJ [https://www.rcsb.org/structure/3DBJ]) ApcAB (αβ)3 trimer in its apo and CO2-bound form were carried out using the AMBER18 molecular modelling software37. The topology and the initial coordinate files were generated using the LEaP module of AmberTools18. The systems simulated features no histidine, all other titratable groups were assigned to their default protonation state at 7.4 pH. The ff14SB38 version of the all-atom Amber force field was used to represent the protein systems. The parameters for chromophore and non-standard amino acid residues (N-methyl asparagine and CO2-bound lysine) were taken from the GAFF2 forcefield39, and the missing ones were generated with Antechamber40 (see Supplementary Data file). Each system was solvated with an explicit TIP3P water model and neutralised with the required number of randomly placed K+/Cl− ions. The ion count was adjusted to account for an osmolarity of 0.15 M.
The systems were independently subjected to 2-step energy minimisation with a combination of steepest descent and conjugate gradient methods using the pmemd programme implemented in AMBER to relax internal constraints by gradually releasing positional restraints. The first minimisation step had positional restraints on protein backbone atoms (Cα, C, N) and the chromophore with a restraint force constant of 10.0 kcal/mol/Å2, which were then removed in the second minimisation step. Following this, the systems were gradually heated to 300 K under constant volume (NVT) for 500 ps with positional restraints (restraint force constant of 5.0 kcal/mol/Å2) on the protein Cα atoms and the entire ligand. They were then density equilibrated for 5 ns under constant pressure (NPT) to bring the atmospheric pressure of the system to 1 bar. Microsecond long production run was performed for each system under NPT conditions. Langevin thermostat (collision frequency of 1 ps−1) was used to maintain a constant temperature and Berendsen barostat for pressure scaling. An integration time step of 2 fs was used, with all bonds to hydrogen atoms constrained with the SHAKE algorithm. The particle-mesh Ewald (PME) algorithm was used to evaluate long-range electrostatic forces with a non-bonded cut-off of 9 Å. Coordinates were saved every 10 ps. MD trajectories were analysed using the cpptraj module of AmberTools18 and VMD1.9.241.
Steady-state absorption and fluorescence spectroscopy
Absorption spectra and fluorescence spectra were obtained using a Synergy H4 Plate Reader and/or a Cary 5000 UV-Vis-NIR. Steady-state fluorescence was measured using a Jobin Yvon Horiba Fluorlog 3. Photoluminescence quantum yield measurements on solutions were carried out according to the absolute method, using an integrating sphere. The optical density of recombinant proteins was adjusted to 0.1 at their fluorescence maximum. For in vitro PLQY measurements using recombinant protein, measurements were made before and after the addition of 20 mM CO2/HCO3− or NaCl to calculate the impact of the addition. Data is presented at the ratio of PLQYs with CO2/HCO3− or NaCl.
Circular dichroism
Spectra were collected in high transparency quartz cuvettes with a 0.1 cm path length using a Jasco J-1500 spectropolarimeter equipped with a mini-circulation bath and Peltier stage. Spectra for secondary structure determination were measured from 190–260 nm in PBS, pH 7.4, at a protein concentration of 12.6 μM.
Time-correlated single photon counting
Time-correlated single-photon counting (TCSPC) was carried out using a DeltaFlex. The data were subsequently fitted to a sum of exponentials;
by deconvolution with the instrument response function (IRF). The IRF was obtained through light scattering from Ludox particles dispersed in solution. The FWHM was ~200 ps, which afforded a temporal time-resolution of ≥100 ps.
Ultrafast transient absorption spectroscopy
Ultrafast transient absorption spectroscopy was performed using a custom-built transient absorption (TA) set-up42. All laser pulses used were derived from a Yb:KGW laser system (Carbide, Light Conversion), providing pulses with 1028 nm, 54 µJ, 250 fs at 60 kHz repetition rate. Approximately 4 µJ pulse−1 was used to generate white light (WL) generation in a 5 mm-thick sapphire window, which served as the probe (range ~550–850 nm). The remaining ~50 µJ pulse−1 was used to generate 514 nm light by second-harmonic generation in a BBO crystal, which served as the pump. The polarization of the probe was at the magic angle (54.7°) relative to the pump. The pump pulse train was chopped at 30 kHz, attenuated to deliver ~0.8 µJ pulse−1 at the sample, and delayed relative to the pump using a motorised translation stage. Both pump and probe pulses were independently focussed onto the sample using curved mirrors. The transmitted WL was recollimated using a lens. A polariser was used to minimise the scattering of the pump by the sample. The WL was passed through a slit to select the central portion of the beam and dispersed via a prism onto a line-scan camera (LightWise Allegro, Imaging Solutions Group). The sample was contained in a standard cuvette of 1 mm path length, which was translated to reduce sample degradation. TA experiments on a given sample typically took 10 min to complete, which avoided overall sample degradation. The concentration of samples was 5 mg ml−1.
Statistical analysis
All error bars represent a 95% confidence interval. All statistics and graphical analyses were performed using GraphPad Prism 8 (GraphPad Software, inc.). All data points represent independent experiments.
Reporting summary
Further information on research design is available in the Nature Research Reporting Summary linked to this article.
Data availability
The authors declare that the data supporting the findings of this study are available within the article, the supplementary information file, and Source Data file. The structural data used in this study are available in the Protein Databank database under accession codes 3DBJ, 7EXT and 4PO5. Source data are provided with this paper.
References
Holland, H. D. The oxygenation of the atmosphere and oceans. Philos. Trans. R. Soc. Lond. Ser. B, Biol. Sci. 361, 903–915 (2006).
Watanabe, M. & Ikeuchi, M. Phycobilisome: architecture of a light-harvesting supercomplex. Photosynth Res 116, 265–276 (2013).
Sidler, W. A. In The Molecular Biology of Cyanobacteria 139–216 (Kluwer, 1994).
Sharkov, A. V. et al. Femtosecond energy-transfer between chromophores in allophycocyanin trimers. Chem. Phys. Lett. 191, 633–638 (1992).
Zhang, J. et al. Structure of phycobilisome from the red alga Griffithsia pacifica. Nature 551, 57–63 (2017).
Bryant, D. A., Guglielmi, G., Tandeaudemarsac, N., Castets, A. M. & Cohenbazire, G. Structure of cyanobacterial phycobilisomes—model. Arch. Microbiol. 123, 113–127 (1979).
Chang, L. et al. Structural organization of an intact phycobilisome and its association with photosystem II. Cell Res. 25, 726–737 (2015).
Palmqvist, K., Sundblad, L. G., Wingsle, G. & Samuelsson, G. Acclimation of photosynthetic light reactions during induction of inorganic carbon accumulation in the green alga chlamydomonas reinhardtii. Plant Physiol. 94, 357–366 (1990).
Sultemeyer, D. F., Miller, A. G., Espie, G. S., Fock, H. P. & Canvin, D. T. Active CO(2) transport by the green alga chlamydomonas reinhardtii. Plant Physiol. 89, 1213–1219 (1989).
Ihnken, S., Kromkamp, J. C., Beardall, J. & Silsbe, G. M. State-transitions facilitate robust quantum yields and cause an over-estimation of electron transport in Dunaliella tertiolecta cells held at the CO(2) compensation point and re-supplied with DIC. Photosynth Res. 119, 257–272 (2014).
Iwai, M., Kato, N. & Minagawa, J. Distinct physiological responses to a high light and low CO2 environment revealed by fluorescence quenching in photoautotrophically grown Chlamydomonas reinhardtii. Photosynth Res. 94, 307–314 (2007).
Ueno, Y., Shimakawa, G., Miyake, C. & Akimoto, S. Light-harvesting strategy during CO2-Dependent photosynthesis in the green alga chlamydomonas reinhardtii. J. Phys. Chem. Lett. 9, 1028–1033 (2018).
Wobbe, L., Bassi, R. & Kruse, O. Multi-level light capture control in plants and green algae. Trends Plant Sci. 21, 55–68 (2016).
Shimakawa, G. et al. Diversity in photosynthetic electron transport under [CO2]-limitation: the cyanobacterium Synechococcus sp. PCC 7002 and green alga Chlamydomonas reinhardtii drive an O2-dependent alternative electron flow and non-photochemical quenching of chlorophyll fluorescence during CO2-limited photosynthesis. Photosynth Res. 130, 293–305 (2016).
Brinkert, K., De Causmaecker, S., Krieger-Liszkay, A., Fantuzzi, A. & Rutherford, A. W. Bicarbonate-induced redox tuning in Photosystem II for regulation and protection. Proc. Natl Acad. Sci. USA 113, 12144–12149 (2016).
Koroidov, S., Shevela, D., Shutova, T., Samuelsson, G. & Messinger, J. Mobile hydrogen carbonate acts as proton acceptor in photosynthetic water oxidation. Proc. Natl Acad. Sci. USA 111, 6299–6304 (2014).
Lorimer, G. H. & Miziorko, H. M. Carbamate formation on the epsilon-amino group of a lysyl residue as the basis for the activation of ribulosebisphosphate carboxylase by CO2 and Mg2. Biochemistry 19, 5321–5328 (1980).
Matthew, J. B., Morrow, J. S., Wittebort, R. J. & Gurd, F. R. Quantitative determination of carbamino adducts of alpha and beta chains in human adult hemoglobin in presence and absence of carbon monoxide and 2,3-diphosphoglycerate. J. Biol. Chem. 252, 2234–2244 (1977).
Linthwaite, V. L. et al. Ubiquitin is a carbon dioxide-binding protein. Sci. Adv. 7, eabi5507 (2021).
Linthwaite, V. L. et al. The identification of carbon dioxide mediated protein post-translational modifications. Nat. Commun. 9, 3092 (2018).
Morrow, J. S., Keim, P. & Gurd, F. R. CO2 adducts of certain amino acids, peptides, and sperm whale myoglobin studied by carbon 13 and proton nuclear magnetic resonance. J. Biol. Chem. 249, 7484–7494 (1974).
Liran, O., Shemesh, E. & Tchernov, D. Investigation into the CO2 concentrating step rates within the carbon concentrating mechanism of Synechocystis sp PCC6803 at various pH and light intensities reveal novel mechanistic properties. Algal Res. 33, 419–429 (2018).
Porres, L. et al. Absolute measurements of photoluminescence quantum yields of solutions using an integrating sphere. J. Fluoresc. 16, 267–272 (2006).
Loos, D., Cotlet, M., De Schryver, F., Habuchi, S. & Hofkens, J. Single-molecule spectroscopy selectively probes donor and acceptor chromophores in the phycobiliprotein allophycocyanin. Biophys. J. 87, 2598–2608 (2004).
McGregor, A., Klartag, M., David, L. & Adir, N. Allophycocyanin trimer stability and functionality are primarily due to polar enhanced hydrophobicity of the phycocyanobilin binding pocket. J. Mol. Biol. 384, 406–421 (2008).
MacColl, R. Allophycocyanin and energy transfer. Biochim Biophys. Acta 1657, 73–81 (2004).
Mirkovic, T. et al. Light absorption and energy transfer in the antenna complexes of photosynthetic organisms. Chem. Rev. 117, 249–293 (2017).
Edington, M. D., Riter, R. E. & Beck, W. F. Interexciton-state relaxation and exciton localization in allophycocyanin trimers. J. Phys. Chem.-Us 100, 14206–14217 (1996).
Chambers, M. C. et al. A cross-platform toolkit for mass spectrometry and proteomics. Nat. Biotechnol. 30, 918–920 (2012).
Ma, B. et al. PEAKS: powerful software for peptide de novo sequencing by tandem mass spectrometry. Rapid Commun. Mass Spectrom. 17, 2337–2342 (2003).
Aguilar, J. A. & Kenwright, S. J. Robust NMR water signal suppression for demanding analytical applications. Analyst 141, 236–242 (2016).
Ducret, A., Sidler, W., Wehrli, E., Frank, G. & Zuber, H. Isolation, characterization and electron microscopy analysis of a hemidiscoidal phycobilisome type from the cyanobacterium Anabaena sp. PCC 7120. Eur. J. Biochem. 236, 1010–1024 (1996).
Ungerer, J. & Pakrasi, H. B. Cpf1 is a versatile tool for CRISPR genome editing across diverse species of cyanobacteria. Sci. Rep. 6, 39681 (2016).
Biswas, A. et al. Biosynthesis of cyanobacterial phycobiliproteins in Escherichia coli: chromophorylation efficiency and specificity of all bilin lyases from Synechococcus sp. strain PCC 7002. Appl. Environ. Microbiol. 76, 2729–2739 (2010).
Zheng, L. et al. Structural insight into the mechanism of energy transfer in cyanobacterial phycobilisomes. Nat. Commun. 12, 5497 (2021).
Peng, P. P. et al. The structure of allophycocyanin B from Synechocystis PCC 6803 reveals the structural basis for the extreme redshift of the terminal emitter in phycobilisomes. Acta Crystallogr D. Biol. Crystallogr 70, 2558–2569 (2014).
Case, D. A. et al. AMBER 18. University of California, San Francisco (2018).
Maier, J. A. et al. ff14SB: improving the accuracy of protein side chain and backbone parameters from ff99SB. J. Chem. Theory Comput 11, 3696–3713 (2015).
Wang, J., Wolf, R. M., Caldwell, J. W., Kollman, P. A. & Case, D. A. Development and testing of a general amber force field. J. Comput Chem. 25, 1157–1174 (2004).
Wang, J., Wang, W., Kollman, P. A. & Case, D. A. Automatic atom type and bond type perception in molecular mechanical calculations. J. Mol. Graph Model 25, 247–260 (2006).
Humphrey, W., Dalke, A. & Schulten, K. VMD: visual molecular dynamics. J. Mol. Graph 14, 33–38 (1996). 27-38.
Tyson, A. L. & Verlet, J. R. R. On the mechanism of phenolate photo-oxidation in aqueous solution. J. Phys. Chem. B 123, 2373–2379 (2019).
Acknowledgements
We thank Andrew Foster, Nigel Robinson, and Justin Ungerer for helpful discussion and Durham University for computer time on its HPC facility, Hamilton. We thank Wendy Schlucter and Himradi Pakrasi for the gift of recombinant plasmids for this work. This work was funded by the Leverhulme Trust grant F/00/128/AU (MJC), the Biotechnology and Biological Sciences Research Council grant BB/S015132/1 (MJC) and the Engineering and Physical Sciences Research Council grant EP/P016499/1 (MTD).
Author information
Authors and Affiliations
Contributions
A.G.G., S.G., C.J., V.R., V.L., E.B. and A.B. performed experiments and analysed data. D.H., T.B., J.V., M.D., L.O.P. and M.C. designed the experiments. L.O.P. and M.C. conceived and managed the project. M.C. wrote the manuscript. All authors discussed the results and implications and commented on the manuscript at all stages.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests
Peer review
Peer review information
Nature Communications thanks Oded Liran, Frank Müh and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Source data
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Guillén-García, A., Gibson, S.E.R., Jordan, C.J.C. et al. Allophycocyanin A is a carbon dioxide receptor in the cyanobacterial phycobilisome. Nat Commun 13, 5289 (2022). https://doi.org/10.1038/s41467-022-32925-6
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41467-022-32925-6
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.