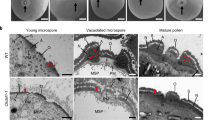
Abstract
Pollen apertures are an interesting model for the formation of specialized plasma-membrane domains. The plant-specific protein INP1 serves as a key aperture factor in such distantly related species as Arabidopsis, rice and maize. Although INP1 orthologues probably play similar roles throughout flowering plants, they show substantial sequence divergence and often cannot substitute for each other, suggesting that INP1 might require species-specific partners. Here, we present a new aperture factor, INP2, which satisfies the criteria for being a species-specific partner for INP1. Both INP proteins display similar structural features, including the plant-specific DOG1 domain, similar patterns of expression and mutant phenotypes, as well as signs of co-evolution. These proteins interact with each other in a species-specific manner and can restore apertures in a heterologous system when both are expressed but not when expressed individually. Our findings suggest that the INP proteins form a species-specific functional module that underlies formation of pollen apertures.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 digital issues and online access to articles
$119.00 per year
only $9.92 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout








Similar content being viewed by others
Data availability
All data supporting the findings of this study are available within the article, Supplementary Information files or from the corresponding author on reasonable request. Source data are provided with this paper.
References
Furness, C. A. & Rudall, P. J. Pollen aperture evolution—a crucial factor for eudicot success? Trends Plant Sci. 9, 154–158 (2004).
Zhou, Y. & Dobritsa, A. A. Formation of aperture sites on the pollen surface as a model for development of distinct cellular domains. Plant Sci. 288, 110222 (2019).
Heslop-Harrison, J. An interpretation of the hydrodynamics of pollen. Am. J. Bot. 66, 737–743 (1979).
Katifori, E., Alben, S., Cerda, E., Nelson, D. R. & Dumais, J. Foldable structures and the natural design of pollen grains. Proc. Natl Acad. Sci. USA 107, 7635–7639 (2010).
Vieira, A. M. & Feijó, J. A. Hydrogel control of water uptake by pectins during in vitro pollen hydration of Eucalyptus globulus. Am. J. Bot. 103, 437–451 (2016).
Zhang, X. et al. Rice pollen aperture formation is regulated by the interplay between OsINP1 and OsDAF1. Nat. Plants 6, 394–403 (2020).
Wodehouse, R. P. Pollen Grains: Their Structure, Identification and Significance in Science and Medicine (McGraw-Hill, 1935).
PalDat—A Palynological Database (PalDat, accessed 30 April 2018); www.paldat.org
Dobritsa, A. A. & Coerper, D. The novel plant protein INAPERTURATE POLLEN1 marks distinct cellular domains and controls formation of apertures in the Arabidopsis pollen exine. Plant Cell 24, 4452–4464 (2012).
Dobritsa, A. A., Kirkpatrick, A. B., Reeder, S. H., Li, P. & Owen, H. A. Pollen aperture factor INP1 acts late in aperture formation by excluding specific membrane domains from exine deposition. Plant Physiol. 176, 326–339 (2018).
Lee, B. H. et al. Arabidopsis protein kinase D6PKL3 is involved in formation of distinct plasma-membrane aperture domains on the pollen surface. Plant Cell 30, 2038–2056 (2018).
Li, P. et al. INP1 involvement in pollen aperture formation is evolutionarily conserved and may require species-specific partners. J. Exp. Bot. 69, 983–996 (2018).
Dobritsa, A. A. et al. A large-scale genetic screen in Arabidopsis to identify genes involved in pollen exine production. Plant Physiol. 157, 947–970 (2011).
Reeder, S. H., Lee, B. H., Fox, R. & Dobritsa, A. A. A ploidy-sensitive mechanism regulates aperture formation on the Arabidopsis pollen surface and guides localization of the aperture factor INP1. PLoS Genet. 12, e1006060 (2016).
Klepikova, A. V., Kasianov, A. S., Gerasimov, E. S., Logacheva, M. D. & Penin, A. A. A high resolution map of the Arabidopsis thaliana developmental transcriptome based on RNA-seq profiling. Plant J. 88, 1058–1070 (2016).
Sall, K. et al. DELAY OF GERMINATION 1-LIKE 4 acts as an inducer of seed reserve accumulation. Plant J. 100, 7–19 (2019).
Kelley, L. A., Mezulis, S., Yates, C. M., Wass, M. N. & Sternberg, M. J. E. The Phyre2 web portal for protein modeling, prediction and analysis. Nat. Protoc. 10, 845–858 (2015).
Magnani, E. et al. A comprehensive analysis of microProteins reveals their potentially widespread mechanism of transcriptional regulation. Plant Physiol. 165, 149–159 (2014).
Amborella Genome Project. The Amborella genome and the evolution of flowering plants. Science 342, 1241089 (2013).
Kellogg, E. A. Evolutionary history of the grasses. Plant Physiol. 125, 1198–1205 (2001).
Plourde, S. M., Amom, P., Tan, M., Dawes, A. T. & Dobritsa, A. A. Changes in morphogen kinetics and pollen grain size are potential mechanisms of aberrant pollen aperture patterning in previously observed and novel mutants of Arabidopsis thaliana. PLoS Comput. Biol. 15, e1006800 (2019).
Lukowitz, W., Gillmor, C. S. & Scheible, W.-R. Positional cloning in Arabidopsis. Why it feels good to have a genome initiative working for you. Plant Physiol. 123, 795–806 (2000).
1001 Genomes Consortium. 1,135 genomes reveal the global pattern of polymorphism in Arabidopsis thaliana. Cell 166, 481–491 (2016).
Hou, X. et al. A platform of high-density INDEL/CAPS markers for map-based cloning in Arabidopsis. Plant J. 63, 880–888 (2010).
Wickett, N. J. et al. Phylotranscriptomic analysis of the origin and early diversification of land plants. Proc. Natl Acad. Sci. USA 111, E4859–E4868 (2014).
Van Bel, M. et al. PLAZA 4.0: an integrative resource for functional, evolutionary and comparative plant genomics. Nucleic Acids Res. 46, D1190–D1196 (2018).
Capella-Gutiérrez, S., Silla-Martínez, J. M. & Gabaldón, T. trimAl: a tool for automated alignment trimming in large-scale phylogenetic analyses. Bioinformatics 25, 1972–1973 (2009).
Kalyaanamoorthy, S., Minh, B. Q., Wong, T. K. F., von Haeseler, A. & Jermiin, L. S. ModelFinder: fast model selection for accurate phylogenetic estimates. Nat. Methods 14, 587–589 (2017).
Trifinopoulos, J., Nguyen, L.-T., von Haeseler, A. & Minh, B. Q. W-IQ-TREE: a fast online phylogenetic tool for maximum likelihood analysis. Nucleic Acids Res. 44, W232–W235 (2016).
Minh, B. Q. et al. IQ-TREE 2: new models and efficient methods for phylogenetic inference in the genomic era. Mol. Biol. Evol. 37, 1530–1534 (2020).
Letunic, I. & Bork, P. Interactive tree of life (iTOL) v4: recent updates and new developments. Nucleic Acids Res. 47, W256–W259 (2019).
Xie, K., Zhang, J. & Yang, Y. Genome-wide prediction of highly specific guide RNA spacers for CRISPR-Cas9-mediated genome editing in model plants and major crops. Mol. Plant 7, 923–926 (2014).
Wang, Z.-P. et al. Egg cell-specific promoter-controlled CRISPR/Cas9 efficiently generates homozygous mutants for multiple target genes in Arabidopsis in a single generation. Genome Biol. 16, 144 (2015).
Xing, H.-L. et al. A CRISPR/Cas9 toolkit for multiplex genome editing in plants. BMC Plant Biol. 14, 327 (2014).
Clough, S. J. & Bent, A. F. Floral dip: a simplified method for Agrobacterium-mediated transformation of Arabidopsis thaliana. Plant J. 16, 735–743 (1998).
Dobritsa, A. A. et al. LAP5 and LAP6 encode anther-specific proteins with similarity to chalcone synthase essential for pollen exine development in Arabidopsis thaliana. Plant Physiol. 153, 937–955 (2010).
García-Nafría, J., Watson, J. F. & Greger, I. H. IVA cloning: a single-tube universal cloning system exploiting bacterial In Vivo Assembly. Sci. Rep. 6, 27459 (2016).
Wang, L., Kim, J. & Somers, D. E. Transcriptional corepressor TOPLESS complexes with pseudoresponse regulator proteins and histone deacetylases to regulate circadian transcription. Proc. Natl Acad. Sci. USA 110, 761–766 (2013).
Voinnet, O., Pinto, Y. M. & Baulcombe, D. C. Suppression of gene silencing: a general strategy used by diverse DNA and RNA viruses of plants. Proc. Natl Acad. Sci. USA 96, 14147–14152 (1999).
Kim, J., Geng, R., Gallenstein, R. A. & Somers, D. E. The F-box protein ZEITLUPE controls stability and nucleocytoplasmic partitioning of GIGANTEA. Development 140, 4060–4069 (2013).
Shen, M. et al. HOS15 is a transcriptional corepressor of NPR1-mediated gene activation of plant immunity. Proc. Natl Acad. Sci. USA 117, 30805–30815 (2020).
Acknowledgements
Funding for this project was provided to A.A.D. by the US National Science Foundation (MCB-1817835). We acknowledge the support from the US National Institutes of Health grant no. R35GM131760 (to I.B.Z.), the TRONDBUSS program (OSU and Norwegian Science and Technology University) (to I. M.M.), Herta Camerer Gross Postdoctoral Research Fellowship (to R.W.), University graduate fellowships (to P.C. and K.A.), OSU Mayers Undergraduate Summer Research Scholarship (to P.A.), NSF-REU supplement mechanism (to P.A. and A.H.), iCAPS internship from the Center for Applied Science (to A.H.), Undergraduate Research Scholarship from the OSU Arts and Sciences Honors Committee (to A.H.) and a Dr. Elizabeth Wagner Scholarship from the Department of Molecular Genetics at OSU (to A.H.). We thank members of the Dobritsa laboratory for discussions, Y. Zhou for help with phylogenetic analysis, V. Edwards, N. Weyrick and S. Knapp for technical help, D. Somers and D. Mackey for vectors, Arabidopsis Biological Resource Center for DNA stocks and the NCI-subsidized Genomics Facility at the OSU Comprehensive Cancer Center (CCSG:P30CA016058) for sequencing.
Author information
Authors and Affiliations
Contributions
B.H.L., R.W. and A.A.D. conceived and designed the experiments. B.H.L., R.W., I.M.M., S.H.R., P.A., M.H.T., K.A., P.C., A.H. and A.A.D. performed the experiments. E.P.A., A.A.D. and I.B.Z. performed phylogenetic analysis. B.H.L., R.W., I.M.M., K.A., P.C., E.P.A. and A.A.D. analysed the data. A.A.D. wrote the article and all authors revised and approved the final manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Peer review information Nature Plants thanks Sheila McCormick, Dabing Zhang and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Extended data
Extended Data Fig. 1 INP2 and INP1 display similar expression patterns, with both genes showing highest expression in young developing buds.
The RNA-seq data for INP2 (a) and INP1 (b) are from the dataset of Klepikova et al.15 and visualized with the BAR eFP Browser.
Extended Data Fig. 2 INP1 and INP2 proteins both contain the DOG1 domain and have similar structural organization predicted for their C-terminal parts.
a, Protein alignment between INP1 and INP2 proteins. Identical and similar (V/I/L, D/E, K/R, N/Q and S/T) residues are shaded, respectively, in blue and green. The positions of the DOG1 domains predicted by Pfam are indicated by purple lines. b-c, Protein structures predicted by Phyre2 for C-terminal parts of INP1 (b) and INP2 (c) (confidence: >97% for both proteins). In both cases, the modelled regions cover 114 amino acids, which constitute, respectively, 42% of INP1 and 37% of INP2. The same template (c4clvB, nickel-cobalt-cadmium resistance protein NccX from Cupriavidus metallidurans 31a) was selected by the program in both cases.
Extended Data Fig. 3 Alignment of INP2 proteins from representatives of different angiosperm taxa.
The following species were used (from top to bottom): Papaver somniferum (basal eudicots, Papaveraceae), Arabidopsis thaliana (rosids, Brassicaceae) Capsella rubella (rosids, Brassicaceae), Olea europea (asterids, Oleaceae), Mimulus guttatus (asterids, Phrymacae), Manihot esculenta (rosids, Euphorbiaceae), Solanum lycopersicum (asterids, Solanaceae), Nymphaea colorata (basal angiosperms, ANA, Nympheaceae), Oryza sativa (monocots, Poaceae), Zea mays (monocots, Poaceae), Elaeis guineensis (monocots, Arecaceae), Ananas comosus (monocots, Bromeliaceae). The seven regions selected for creating AtINP2/SlINP2 chimeras are indicated by differently coloured rectangles. Aspartate (D) and glutamate (E) residues in the acidic region are shaded in blue. Black shading indicates identical amino acids and grey shading indicates similar amino acids present at the same position in at least half of the aligned proteins.
Extended Data Fig. 4 Arabidopsis INP2 likely contains a transmembrane domain at its N terminus.
Multiple TM discovery algorithms predict existence of the transmembrane domain at the N terminus of INP2 from Arabidopsis thaliana (AtINP2), with the consensus score of 0.85 generated by the plant membrane protein database Aramemnon (AramTMCon).
Extended Data Fig. 5 None of the seven AtINP2 regions is sufficient on its own to convert SlINP2 into a protein able to function in Arabidopsis.
Confocal images of pollen grains produced by the transgenic inp2 plants expressing seven versions of chimeric SlINP2 constructs in which one region at a time was replaced with the corresponding regions from AtINP2. At least 10 independent T1 lines were tested for each construct (≥ 50 pollen grains per line), with similar results. Scale bars = 10 µm.
Supplementary information
Supplementary Information
Supplementary Tables 1 and 2.
Source data
Source Data Fig. 4
Unprocessed western blots.
Rights and permissions
About this article
Cite this article
Lee, B.H., Wang, R., Moberg, I.M. et al. A species-specific functional module controls formation of pollen apertures. Nat. Plants 7, 966–978 (2021). https://doi.org/10.1038/s41477-021-00951-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41477-021-00951-9