Abstract
High-throughput sequencing (HTS) is capable of broad virus detection encompassing both known and unknown adventitious viruses in a variety of sample matrices. We describe the development of a general-purpose HTS-based method for the detection of adventitious viruses. Performance was evaluated using 16 viruses equivalent to well-characterized National Institutes of Health (NIH) virus stocks and another six viruses of interest. A viral vaccine crude harvest and a cell substrate matrix were spiked with 22 viruses. Specificity was demonstrated for all 22 viruses at the species level. Our method was capable of detecting and identifying adventitious viruses spiked at 104 genome copies per milliliter in a viral vaccine crude harvest and 0.01 viral genome copies spiked per cell in a cell substrate matrix. Moreover, 9 of the 11 NIH model viruses with published in vivo data were detected by HTS with an equivalent or better sensitivity (in a viral vaccine crude harvest). Our general-purpose HTS method is unbiased and highly sensitive for the detection of adventitious viruses, and has a large breadth of detection, which may obviate the need to perform in vivo testing.
Similar content being viewed by others
Introduction
The potential contamination of biopharmaceuticals with adventitious (unintentionally introduced) viruses poses a serious safety risk and threatens public confidence in the use of biopharmaceuticals. This is particularly true in the case of vaccines administered to large numbers of healthy people, including children1,2. There have been a number of instances over previous decades where evidence for the presence of adventitious virus contamination in a marketed vaccine product has threatened public trust in immunization programs3. Examples include the detection of simian virus 40 (SV40) in the early polio vaccine in the 1960s, the finding of reverse transcriptase in measles and mumps vaccines in 1995 and of porcine circovirus (PCV) nucleic acid sequences and/or infectious circovirus in rotavirus vaccines in 20103,4.
These viruses could be unintentionally introduced at various manufacturing stages and may originate from multiple sources including raw materials, the cell substrate or the environment. Implementation of Good Manufacturing Practise and close monitoring of the manufacturing process can help reduce the likelihood of viral contamination. Therefore, adventitious virus testing at various stages of the manufacturing process is an integral part of the safety assessment for vaccines and other biological products, and there are well-defined regulatory requirements to ensure that these products are absent of adventitious viruses5,6.
Gaps exist in the current compendial adventitious virus testing package where some viral families are not detected or incompletely detected by the compendial methods7,8,9. Non-specific adventitious virus testing of biological materials has typically included in vivo tests and cell culture-based in vitro tests, the breadth and sensitivity of which are presumed from historical experience rather than from systematic assay validation9. In addition, routine testing for adventitious viruses following the compendial requirements generally requires testing the cell substrate and the viral crude harvest (at seed lot and/or bulk levels). Both of these manufacturing stages present complex matrices for in vivo and in vitro testing. Targeted PCR-based virus detection methods require prior knowledge of the adventitious virus with respect to primer design/selection to target specific nucleic acid sequences. As such, there is a need for a broad-range detection method for both anticipated viruses, as well as unanticipated viruses.
A study by the National Institutes of Health (NIH) in 2014 compared the sensitivity of in vivo assays and in vitro cell culture tests using a panel of 16 viruses and found the in vitro tests to be more sensitive for detecting most of the tested viruses9. The results from the NIH study support the use of tests for broader detection of adventitious viruses, particularly where no suitable animal models or appropriate culture methods for detection exist. The World Health Organization (WHO; WHO technical report series 978 [Annex 3])10 and the European Pharmacopoeia (legally binding standards and quality specifications; Ph. Eur. 2.6.16, Ph. Eur. 5.2.14, Ph. Eur. 5.2.3.)10,11,12,13 recommend or require that prior to the implementation of new alternative methods to detect adventitious agents, the specificity and sensitivity of the new and existing methods must be compared; and whether the new method has at least the same sensitivity as in vivo methods should be determined (Supplementary Table 1).
High-throughput sequencing (HTS) is a non-specific technique with the potential to detect both known and unknown adventitious agents including viruses7,14,15,16,17. High-throughput molecular biology methods (HTS combined with a pan-viral microarray) had succeeded in detecting the contamination of Rotarix vaccine by a porcine circovirus18. HTS may also be more sensitive than quantitative PCR (qPCR)19,20; however the method may be overly sensitive to the detection of background and cross-contaminating viral nucleic acids originating from the laboratory environment or from other sources21. Viral genome size can also influence the sensitivity of HTS22,23 as sensitivity is expected to be proportional to the mass ratio of nucleic acids in a given matrix. Although HTS results for adventitious virus testing may differ between laboratories24, the development of well-characterized model virus stocks would support standardization and validation of the different HTS platforms22. Currently there are no established viral reference standards with corresponding in vivo data, for assessing new techniques for the detection of adventitious viruses.
The panel of 16 virus stocks from the NIH is instrumental in our efforts to develop, qualify and validate the HTS adventitious virus test, by providing an important baseline against which new techniques for the detection of adventitious viruses can be compared9. Here, we describe the development of a general-purpose HTS-based test for the detection of adventitious viruses and its performance using virus stocks equivalent to the 16 NIH model viruses, plus another six viruses of interest. Detection of these viruses was assessed in a live Yellow Fever virus vaccine crude harvest matrix and a Vero cell substrate matrix to define the sensitivity (limit of detection [LOD]) and to demonstrate the specificity of the HTS test for adventitious virus testing.
Results
Identification of approximate LOD and viral spike detection in a viral vaccine crude harvest nucleic acid extraction
Nucleic acid recovery for both the Yellow Fever Virus vaccine crude harvest and the Vero cell substrate matrix are shown in Supplementary Note 1.
The number of sequence reads detected for each of the spiked viruses is listed in Table 1. All 22 model adventitious viruses were detected in at least one of the spike levels assessed. LOD was determined to be approximately 103 to 104 genome copies per mL of Yellow Fever Virus vaccine crude harvest. Specificity was assessed for all 22 viruses by determining whether the bioinformatics tool PhyloID was able to unequivocally detect each of the 22 spiked-in viruses and that none of these viruses was detected in the negative control (unspiked Yellow Fever virus vaccine crude harvest). Specificity was verified for all 22 viruses at the species level while specificity was verified for 20 of the 22 viruses at the strain level. For mammalian reovirus type 3 and coxsackievirus B3, the reads from the virus used for the spike were spread over two (for coxsackievirus B3) or three (for mammalian reovirus type 3) reference sequences of the correct viral species.
Assessment of LOD
Using a range of viral spikes from 105 to 103 genome copies per mL, most viruses were consistently detected at least at 104 genome copies per mL in viral vaccine crude harvest (Table 2).
Reproducibility of the number of reads detected by the HTS test across three replicate datasets in the viral vaccine crude harvest
Reproducibility of the test was evaluated using three independent replicates (over two spiking studies) at a spiking level of 104 genome copies per mL. The mean number of reads at 104 genome copies per mL is shown in Table 2; the coefficient of variation ranged from 11 to 107%.
Identification of approximate LOD and viral spike behavior in the cell substrate matrix
The number of sequence reads detected for each of the spiked viruses is listed in Table 3. For the Vero cell substrate with a cell count of 1 × 106 cells per mL, 19 of the 22 viruses were detected at least at 104 genome copies per mL (0.01 genome copies per cell) in the cell substrate matrix, with potentially better sensitivity for some viruses. Influenza A virus, minute virus of mice and reovirus 3 were not detected at this highest spike level of 0.01 genome copies per cell.
LOD overview
Preliminary evaluation indicates that the adventitious virus detection test using HTS can potentially achieve a limit of detection that is at or below 0.01 genome copies per cell in a Vero cell substrate matrix (Table 3). 19 of the 22 viruses were detectable at 0.01 genome copies per cell and 14 of the viruses were detected at 0.001 genome copies per cell in a Vero cell substrate matrix (Table 3).
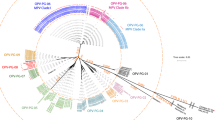
In the Yellow Fever Virus vaccine crude harvest, HTS can achieve a LOD that is at or below 104 genome copies per mL. Of the viruses assessed, 21 were detectable at 104 genome copies per mL, with 16 of these at 103 genome copies per mL in a matrix containing 109 viral vaccine genome copies per mL (Fig. 1).
Comparison to NIH in vivo testing results
In the previous NIH study, 11 of the 16 virus stocks were assessed for their detection in the in vivo test9. While the in vivo assays detect infectious virus, with levels reported as titers, the HTS method measures nucleic acids. We converted the sensitivity reported in the NIH study into genome copies using the infectious titer to genome copy ratio determined from the vial of original NIH viral stock. Nine of the 11 viruses previously assessed in vivo were detected with better sensitivity by our HTS method in the viral vaccine crude harvest matrix than reported in vivo in the NIH study; the remaining five viruses were not assessed in vivo within the NIH study (Table 4). Influenza A virus and VSV were detected with better sensitivity in vivo in the NIH study than using HTS in this study; these viruses were also detected at 104-fold and 105-fold lower genomic copy amounts in vivo compared to in vitro, respectively, as the animal model allows an efficient amplification of these replicating viral contaminants.
Discussion
Various reviews of HTS for adventitious agent detection have shown that there is a need for unbiased extraction methods, relevant controls, the use of spike recovery experiments, and quality control measures during library preparation7,14,15,16,17. Our HTS method is a general-purpose non-specific method that can be used to detect both known and unexpected viruses in multiple types of samples (viral seeds/crude harvest, cell banks, and cell supernatants). The LOD for a panel of 22 model adventitious viruses was assessed in a viral vaccine crude harvest matrix and a cell substrate matrix. Based on the results, the adventitious virus test using HTS technology is capable of detecting adventitious viruses at least at 104 genome copies per mL in the viral vaccine crude harvest matrix. Detection in the cell substrate matrix was at least at 0.01 viral genome copies per cell (104 genome copies per mL) for 19 of the 22 viruses. In a similar study evaluating the performance of HTS for the detection of adventitious viruses by spiking model viruses into a cellular matrix containing whole cells, all of the model viruses used (human respiratory syncytial virus [RSV], Epstein-Barr virus [EBV], feline leukemia virus and human reovirus) were detected when spiked at 3 genome copies per cell; only EBV and RSV were detected at 0.1 genome copies per cell22. This study showed that HTS can detect almost all viral contaminants evidenced by the large panel that was investigated, i.e., 22 different viruses that were enveloped/non-enveloped, single-stranded/double-stranded, RNA, DNA and of varying genome sizes. The use of the method for different sample types was also demonstrated, i.e., the nucleic acid recovery from the viral vaccine crude harvest matrix was higher than the cell substrate matrix, however a high proportion of contaminants was detected for both samples. The difference in detection levels observed for some of the viruses is likely attributed to a higher amount of background host nucleic acids from the cell substrate. Compared to the vaccine viral harvest, where even if the cells are lysed during manufacturing, the host cell nucleic acids are limited within the viral harvest, which is very different when testing the cell substrate matrix. These cells are lysed during sample preparation, releasing their nucleic acids, and will therefore make up a higher proportion of the sequence data leading to a poorer signal (viral spike) to noise ratio and a decrease in the number of reads detected for the viral spikes. Overall, the successful detection of the spiked-in viruses in these complex backgrounds demonstrates the applicability of HTS for adventitious virus detection.
Methods for the detection of adventitious agents continue to evolve and improve. The use of viral stocks equivalent to the well-characterized viral stocks from the NIH-supported studies allows us to compare our HTS performance against compendial in vivo testing, as proposed in the European Pharmacopoeia Chapter 5.2.1411. In addition, such well-characterized materials can be used as a reference to help ensure satisfactory test performance in many matrices and thus, enable more meaningful comparisons between laboratories24. Importantly, the use of HTS is in-line with the Directive 2010/63/EU of the European Parliament and of the Council of 22 September 2010 with the aim, and the need, to reduce the number of animals used for scientific purposes25.
The approach to this study is based on European Pharmacopeia chapter 5.2.14 (Supplementary Table 1) and uses 16 well-characterized model viruses that are representative of potential contaminants in vaccine manufacturing to demonstrate that HTS sensitivity is at least equivalent to the sensitivity of the in vivo test methods. In this study, we produced 16 viruses that were equivalent to the viruses assessed in the NIH study. In vivo test results were available for 11 of the viruses in the NIH study and 9 of our 11 viruses were detected with better sensitivity by HTS in the viral vaccine crude harvest compared with the NIH in vivo data. Influenza A virus and VSV were the two viruses detected with lower sensitivity by HTS (also detected with lower sensitivity in vitro) which most likely suggests that the animal models used provide a good environment for the replication of those viruses, while HTS does not involve any viral replication. Two viruses resolved to the species level, but not to the strain level (mammalian reovirus type 3 and coxsackievirus B3) when measured by the in-house Sanofi Pasteur tool PhyloID26. However, resolution at the strain level can be more difficult when counts are low and when strains are similar in their sequences, as the reads may get assigned to any of the reference genomes within that species. We are, however, working on improving the strain resolution of PhyloID.
A limitation of a HTS adventitious virus detection method is that the test only detects viral nucleic acid and further investigation is necessary to determine if the signal is from infectious particles. In some cases, for example gamma-irradiated raw materials, or inactivated samples, the presence of viral nucleic acids is not necessarily a concern. In addition, HTS may be prone to detection of background and cross-contaminating viral nucleic acids originating from the laboratory environment or from other sources due to its breadth of detection, as well as its sensitivity21. A thorough understanding of the testing environment, proper test controls and knowledge of the history of the test sample will help to determine if laboratory follow-up is necessary.
Possible laboratory follow-ups include confirmation of intact full-length viral genomes27,28, expression analysis29,30 and infectivity assays4,31. It is also essential to validate the HTS test under Good Manufacturing Practice in order to apply the method to new vaccine products as release specification tests, as a substitution of in vivo tests to detect adventitious virus and to potentially replace/substitute specific tests such as PCRs used in routine testing. HTS would need to be compared with qPCR if it is to become routinely used. The sensitivity of HTS is influenced by genomic size22,23, as well as genomic structure, and for RNA viruses, the relative efficiencies in reverse transcription and cDNA synthesis22. Nevertheless, HTS is virus-sequence independent and can detect all types of virus genomes, including single-stranded/ double-stranded RNA and DNA.
The HTS method for adventitious virus detection is a highly sensitive, unbiased non-specific method, which has a large breadth of detection compared to other tests, a favorable comparison to in vivo data and may obviate the need to perform in vivo testing for adventitious viruses.
Methods
A schematic overview of the HTS adventitious virus detection procedure is provided in Fig. 2.
Preparation of virus panel
Two vials of each of the 16 virus stocks were received from the NIH; the viruses are described in Supplementary Table 2. The genome copy (GC) of each stock was determined by qPCR by Clean Cells (https://clean-cells.com/), a contract organization for Sanofi Pasteur, using plasmids containing synthetic nucleic acids of the targeted sequence to establish a standard curve; the resultant GC was used in addition to the infectious titer to convert the in vivo and in vitro LOD values established in the previous NIH study9 (expressed in infectious units) into equivalent genome copy numbers (the unit used for HTS detection) (Supplementary Table 3). Using these initial vials, larger viral stocks of similar characteristics were produced using protocols provided by the NIH for virus propagation and titration assays; the same sourced cells, culture medium, multiplicity of infection, infection duration, and harvest conditions were used. The production of viral stocks was conducted by Clean Cells. Six additional virus stocks of interest to Sanofi Pasteur that were available internally or from Clean Cells with the necessary viral titer and equivalent genome copy were also analyzed (Human Cytomegalovirus, Reovirus Type 3, Minute Virus of Mice, Porcine Parvovirus, Bovine Coronavirus and Human Borna Disease Virus; Supplementary Table 4). A viral pool was created at 1 × 106 genome copies of each virus per mL in Tris EDTA. The volume of each virus necessary for the creation of the viral pool is listed in Supplementary Table 5; Tris-EDTA buffer solution was added to the viral pool to bring the final volume to 1 mL.
Spiking experiments
Spiking experiments were designed based upon previous internal and collaborative studies22,24. Samples were prepared in a serum-free live Yellow Fever virus vaccine crude harvest and serum-free Vero cell substrate matrix; the same starting materials and extraction method were used for all experiments for consistency. The live Yellow Fever virus vaccine crude harvest was selected to represent a viral matrix for which HTS testing will be used at Sanofi Pasteur. The vaccine virus was titered at approximately 109 viral vaccine genome copies per mL and the NIH-equivalent model adventitious viruses were spiked in at 105, 104, 103, and 102 genome copies per mL. Two replicate experiments were performed for spiking at both 103 and 102 genome copies per mL as it was hypothesized that the LOD for the assay may be close to this spiking level. We used a Vero cell-substrate matrix as a representative cell bank, which is used frequently at Sanofi Pasteur in the manufacture of several viral vaccines. Samples of the Vero cell substrate matrix (106 cells per mL) were spiked at levels of 104 and 103 genome copies per mL (0.01 and 0.001 virus genome copies per cell, respectively). Replicate spikes were made at each spike level.
Nucleic acid extraction, cDNA synthesis and assessment of the sample
A schematic overview of the viral nucleic acid extraction protocol is presented in Fig. 3. Optimization of nucleic acid extraction has been described previously32 and was undertaken with the Invitrogen PureLink Viral RNA/DNA Mini kit (Life Technologies cat #12280050) and the Wako® DNA Extractor Kit (WAKO cat #295-50201). cDNA synthesis was undertaken with the SuperScript® Double-Stranded cDNA Synthesis kit (Invitrogen cat #11917-010), followed by nucleic acid purification using the QIAquick PCR Purification Kit (Qiagen cat #28104). The nucleic acid extractions were assessed using the Agilent 2100 Bioanalyzer system.
Sequencing
Sequencing library preparation was performed using the Illumina Nextera XT DNA Library Prep kit. Sequencing was carried out on the Illumina HiSeq1500. Paired-end sequencing was carried out at 2 ×151 bp. Sequence data were converted from the Bcl to FASTQ formats using the Illumina Bcl2Fastq2 software.
Data analysis
Data analysis was undertaken using PhyloID (a Sanofi Pasteur in-house bioinformatics tool developed for adventitious virus detection) and has been described previously26. Sequence data were trimmed, assembled into contigs and identified using a phylogenomic approach based on the profile of matches against reference data. Sensitivity was determined as the lowest concentration that a virus was conclusively positive (here, defined as >2 reads in >1 contig or in 1 contig >200 nt from the PhyloID analysis).
Limit of detection (LOD)
The limit of detection of adventitious viruses using HTS was compared to the data obtained from the NIH study9. The LOD achieved by the in vivo test methods, as described in the NIH study, was converted to genome copies based on a determination of genome content of the NIH stocks by Clean Cells to facilitate the comparison with the HTS studies described here.
The genome copies at LOD for the in vivo test were calculated using the formula:
For example, for Herpes Simplex Virus Type 1:
*All obtained from the NIH stock9, †As determined by Clean Cells from the NIH stock.
(The limit of detection for viruses not detected in vivo is specified as greater than the genome copy number after conversion from the titer of the NIH stock virus [Supplementary Tables 2 and 3]).
Data availability
The data that support the findings of this study are available on request from the corresponding author (S.N.).
References
Folb, P. I. et al. A global perspective on vaccine safety and public health: the global advisory committee on vaccine safety. Am. J. Public Health 94, 1926–1931 (2004).
Barone, P. W. et al. Viral contamination in biologic manufacture and implications for emerging therapies. Nat. Biotechnol. 38, 563–572 (2020).
Petricciani, J., Sheets, R., Griffiths, E. & Knezevic, I. Adventitious agents in viral vaccines: lessons learned from 4 case studies. Biologicals 42, 223–236 (2014).
World Health Organization. Scientific Principles For Regulatory Risk Evaluation On Finding An Adventitious Agent In A Marketed Vaccine (Annex 2). WHO Expert Committee on Biological Standardization. https://www.who.int/biologicals/expert_committee/TRS_978_61st_report.pdf?ua=1 (2015). (Accessed May 2019).
EMEA, E. M. A. ICH Topic Q5A: Quality of Biotechnological Products: Viral Safety Evaluation of Biotechnology Products Derived from Cell Lines of Human or Animal Origin. ICH Harmonised Tripartite Guideline (2006).
U.S. Department of Health and Human Services, F. a. D. A., Center for Biologics Evaluation and Research. Guidance for Industry. Characterization and Qualification of Cell Substrates and Other Biological Materials Used in the Production of Viral Vaccines for Infectious Disease Indications [February 2010]. http://www.fda.gov/BiologicsBloodVaccines/GuidanceComplianceRegulatoryInformation/Guidances/default.htm (2010).
Mallet, L. & Gisonni-Lex, L. Need for new technologies for detection of adventitious agents in vaccines and other biological products. PDA J. Pharm. Sci. Technol. 68, 556–562 (2014).
Sheets, R. L. & Duncan, P. A. In Vaccine Analysis: Strategies, Principles, and Control (eds Brian K. Nunnally, Vincent E., Turula & Robert D. Sitrin) 397–443 (Springer Berlin Heidelberg, 2015).
Gombold, J. et al. Systematic evaluation of in vitro and in vivo adventitious virus assays for the detection of viral contamination of cell banks and biological products. Vaccine 32, 2916–2926 (2014).
World Health Organization. Expert Committee on Biological Standardization. https://www.who.int/biologicals/expert_committee/TRS_978_61st_report.pdf?ua=1 (Accessed May 2019).
European Pharmacopoeia General Chapter 5.2.14: “Substitution of in Vivo Method(s) by in Vitro Method(s) for the Quality Control of Vaccines”. version 9.3 published in July 2017, creation.
European Pharmacopoeia General Chapter 5.2.3: “Cell Substrates for the Production of Vaccines for Human Use”, version 9:0 and updated version 9.3 in July 2017, revision.
European Pharmacopoeia General Chapter 2.6.16: “Tests for Extraneous Agents in Viral Vaccines for Human Use”, version 9.3 published in July 2017, revision. Corrected version in Supplement 9.4.
Khan, A. S. et al. Report of the international conference on next generation sequencing for adventitious virus detection in biologicals. Biologicals 55, 1–16 (2018).
Khan, A. S. et al. Advanced Virus Detection Technologies Interest Group (AVDTIG): efforts on High Throughput Sequencing (HTS) for virus detection. PDA J. Pharm. Sci. Technol. 70, 591–595 (2016).
Lambert, C. et al. Considerations for optimization of high-throughput sequencing bioinformatics pipelines for virus detection. Viruses 10, https://doi.org/10.3390/v10100528 (2018).
Ng, S. H. et al. Current perspectives on high-throughput sequencing (hts) for adventitious virus detection: upstream sample processing and library preparation. Viruses 10, https://doi.org/10.3390/v10100566 (2018).
Victoria, J. G. et al. Viral nucleic acids in live-attenuated vaccines: detection of minority variants and an adventitious virus. J. Virol. 84, 6033–6040 (2010).
Ng, S. H. et al. Preliminary evaluation of next-generation sequencing performance relative to qPCR and in vitro cell culture tests for human cytomegalovirus. PDA J. Pharm. Sci. Technol. 68, 563–571 (2014).
Cabannes, E., Hebert, C. & Eloit, M. Whole genome: next-generation sequencing as a virus safety test for biotechnological products. PDA J. Pharm. Sci. Technol. 68, 631–638 (2014).
Petty, T. J. et al. Comprehensive human virus screening using high-throughput sequencing with a user-friendly representation of bioinformatics analysis: a pilot study. J. Clin. Microbiol. 52, 3351–3361 (2014).
Khan, A. S. et al. A multicenter study to evaluate the performance of high-throughput sequencing for virus detection. mSphere 2, https://doi.org/10.1128/mSphere.00307-17 (2017).
Paskey, A. C. et al. Enrichment post-library preparation enhances the sensitivity of high-throughput sequencing-based detection and characterization of viruses from complex samples. BMC Genomics 20, 155 (2019).
Mee, E. T., Preston, M. D., Minor, P. D. & Schepelmann, S. Development of a candidate reference material for adventitious virus detection in vaccine and biologicals manufacturing by deep sequencing. Vaccine 34, 2035–2043 (2016).
The European Parliament and the Council Of The European Union. Directive 2010/63/EU of the European Parliament and of the Council of 22 September 2010 on the protection of animals used for scientific purposes. Official Journal of the European Union, L 276/233-239 (2010).
Charlebois, R. L., Ng, S. H., Gisonni-Lex, L. & Mallet, L. Cataloguing the taxonomic origins of sequences from a heterogeneous sample using phylogenomics: applications in adventitious agent detection. PDA J. Pharm. Sci. Technol. 68, 602–618 (2014).
Machnik, G. et al. Sequence analysis of proviral DNA of porcine endogenous retroviruses. Transplant. Proc. 37, 4610–4614 (2005).
Tellier, R., Bukh, J., Emerson, S. U., Miller, R. H. & Purcell, R. H. Long PCR and its application to hepatitis viruses: amplification of hepatitis A, hepatitis B, and hepatitis C virus genomes. J. Clin. Microbiol. 34, 3085–3091 (1996).
Brussel, A. et al. Use of a new RNA next generation sequencing approach for the specific detection of virus infection in cells. Biologicals 59, 29–36 (2019).
Cheval, J. et al. Adventitious virus detection in cells by high-throughput sequencing of newly synthesized RNAs: unambiguous differentiation of cell infection from carryover of viral nucleic acids. mSphere 4, https://doi.org/10.1128/mSphere.00298-19 (2019).
Ma, H., Galvin, T. A., Glasner, D. R., Shaheduzzaman, S. & Khan, A. S. Identification of a novel rhabdovirus in Spodoptera frugiperda cell lines. J. Virol. 88, 6576–6585 (2014).
Sathiamoorthy, S., Malott, R. J., Gisonni-Lex, L. & Ng, S. H. S. Selection and evaluation of an efficient method for the recovery of viral nucleic acid extraction from complex biologicals. NPJ Vaccines 3, 31 (2018).
Acknowledgements
The authors would like to thank Dr. Rebecca Sheets from the NIH, the QBI and the Vaccine Research Program, Division of AIDS, NIAID who provided the viral strains through Quality Biological, Inc., the Vaccine Research Program (Contract No. HHSN272201100023C) and Jim Gombold for in-depth discussion regarding LOD expression. Editorial assistance with the preparation of the manuscript was provided by Ella Palmer, inScience Communications, Springer Healthcare Ltd., and was funded by Sanofi Pasteur. The authors also thank Jacek Remani and Lauren Rodrigues for support and oversight of the maintenance of the Illumina sequencer, and Sandrine Buisson, for editorial assistance and manuscript coordination on behalf of Sanofi Pasteur. This study was funded by Sanofi Pasteur.
Author information
Authors and Affiliations
Contributions
All authors participated in the conception or design of the study; C.L., S.N., and L.G.L. were the scientific leads for obtaining the NIH viral stocks and L.G.L. was head of the Microbiology/Virology platform in Toronto where all of the work was undertaken. R.C., S.S., and S.N. were involved in data acquisition or analysis; all authors contributed to the drafting and critical revision of the manuscript and approved the final version, and are accountable for all aspects of the work presented.
Corresponding author
Ethics declarations
Competing interests
R.C., C.L., L.G.L., and S.N. are employees of Sanofi Pasteur. L.M. and S.S. were employees of Sanofi Pasteur at the time of the study; L.M. is now employed by EDQM–Council of Europe, S.S. is now employed by Turnstone Biologics. R.C., S.N., L.G.L., L.M., and S.S. hold shares in Sanofi.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Charlebois, R.L., Sathiamoorthy, S., Logvinoff, C. et al. Sensitivity and breadth of detection of high-throughput sequencing for adventitious virus detection. npj Vaccines 5, 61 (2020). https://doi.org/10.1038/s41541-020-0207-4
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41541-020-0207-4