Abstract
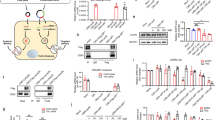
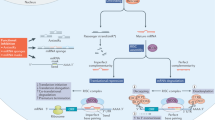
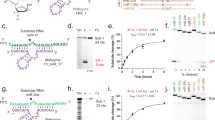
A small percentage of the short interfering RNA (siRNA) delivered via passive lipid nanoparticles and other delivery vehicles reaches the cytoplasm of cells. The high doses of siRNA and delivery vehicle that are thus required to achieve therapeutic outcomes can lead to toxicity. Here, we show that the integration of siRNA sequences into a Dicer-independent RNA stem–loop based on pre-miR-451 microRNA—which is highly enriched in small extracellular vesicles secreted by many cell types—reduces the expression of the genes targeted by the siRNA in the liver, intestine and kidney glomeruli of mice at siRNA doses that are at least tenfold lower than the siRNA doses typically delivered via lipid nanoparticles. Small extracellular vesicles that efficiently package siRNA can significantly reduce its therapeutic dose.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 digital issues and online access to articles
$99.00 per year
only $8.25 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout








Similar content being viewed by others
Data availability
The main data supporting the results of this study are available within the paper and its Supplementary Information. The raw and analysed datasets are too numerous to be readily shared publicly, but can be obtained for research purposes from the corresponding author on reasonable request.
References
Ha, M. & Kim, V. N. Regulation of microRNA biogenesis. Nat. Rev. Mol. Cell Biol. 15, 509–524 (2014).
Fitzgerald, K. et al. A highly durable RNAi therapeutic inhibitor of PCSK9. N. Engl. J. Med. 376, 41–51 (2017).
Coelho, T. et al. Safety and efficacy of RNAi therapy for transthyretin amyloidosis. N. Engl. J. Med. 369, 819–829 (2013).
Whitehead, Ka, Langer, R. & Anderson, D. G. Knocking down barriers: advances in siRNA delivery. Nat. Rev. Drug Discov. 8, 129–138 (2009).
Kanasty, R., Dorkin, J. R., Vegas, A. & Anderson, D. Delivery materials for siRNA therapeutics. Nat. Mater. 12, 967–977 (2013).
Wittrup, A. et al. Visualizing lipid-formulated siRNA release from endosomes and target gene knockdown. Nat. Biotechnol. 33, 1–9 (2015).
Pei, Y. et al. Quantitative evaluation of siRNA delivery in vivo. RNA 16, 2553–2563 (2010).
Veldhoen, S., Laufer, S. D., Trampe, A. & Restle, T. Cellular delivery of small interfering RNA by a non-covalently attached cell-penetrating peptide: quantitative analysis of uptake and biological effect. Nucleic Acids Res. 34, 6561–6573 (2006).
Gilleron, J. et al. Image-based analysis of lipid nanoparticle-mediated siRNA delivery, intracellular trafficking and endosomal escape. Nat. Biotechnol. 31, 638–646 (2013).
Garber, K. Alnylam terminates revusiran program, stock plunges. Nat. Biotechnol. 34, 1213–1214 (2016).
Fitzgerald, K. et al. A highly durable RNAi therapeutic inhibitor of PCSK9. N. Engl. J. Med. 376, 41–51 (2017).
Colombo, M., Raposo, G. & Théry, C. Biogenesis, secretion, and intercellular interactions of exosomes and other extracellular vesicles. Annu. Rev. Cell Dev. Biol. 30, 255–289 (2014).
Masciopinto, F. et al. Association of hepatitis C virus envelope proteins with exosomes. Eur. J. Immunol. 34, 2834–2842 (2004).
Baj-Krzyworzeka, M. et al. Tumour-derived microvesicles carry several surface determinants and mRNA of tumour cells and transfer some of these determinants to monocytes. Cancer Immunol. Immunother. 55, 808–818 (2006).
Ratajczak, J. et al. Embryonic stem cell-derived microvesicles reprogram hematopoietic progenitors: evidence for horizontal transfer of mRNA and protein delivery. Leukemia 20, 847–856 (2006).
Valadi, H. et al. Exosome-mediated transfer of mRNAs and microRNAs is a novel mechanism of genetic exchange between cells. Nat. Cell Biol. 9, 654–659 (2007).
Skog, J. et al. Glioblastoma microvesicles transport RNA and proteins that promote tumour growth and provide diagnostic biomarkers. Nat. Cell Biol. 10, 1470–1476 (2008).
Pegtel, D. M. et al. Functional delivery of viral miRNAs via exosomes. Proc. Natl Acad. Sci. USA 107, 6328–6333 (2010).
Alvarez-Erviti, L. et al. Delivery of siRNA to the mouse brain by systemic injection of targeted exosomes. Nat. Biotechnol. 29, 3–4 (2011).
Kamerkar, S. et al. Exosomes facilitate therapeutic targeting of oncogenic KRAS in pancreatic cancer. Nature 546, 498–503 (2017).
Didiot, M.-C. et al. Exosome-mediated delivery of hydrophobically modified siRNA for Huntingtin mRNA silencing. Mol. Ther. 24, 1836–1847 (2016).
Kooijmans, S. A. A. et al. Electroporation-induced siRNA precipitation obscures the efficiency of siRNA loading into extracellular vesicles. J. Control. Release 172, 229–238 (2013).
Chevillet, J. R. et al. Quantitative and stoichiometric analysis of the microRNA content of exosomes. Proc. Natl Acad. Sci. USA 111, 14888–14893 (2014).
Shurtleff, M., Karfilis, K. V., Temoche-Diaz, M., Ri, S. & Schekman, R. Y-box protein 1 is required to sort microRNAs into exosomes in cells and in a cell-free reaction. eLife 5, e19276 (2016).
Mukherjee, K. et al. Reversible HuR‐microRNA binding controls extracellular export of miR‐122 and augments stress response. EMBO Rep. 17, 1184–1203 (2016).
Villarroya-Beltri, C. et al. Sumoylated hnRNPA2B1 controls the sorting of miRNAs into exosomes through binding to specific motifs. Nat. Commun. 4, 2980 (2013).
Yang, J. S. & Lai, E. C. Alternative miRNA biogenesis pathways and the interpretation of core miRNA pathway mutants. Mol. Cell 43, 892–903 (2011).
Cifuentes, D. et al. A novel miRNA processing pathway independent of Dicer requires Argonaute2 catalytic activity. Science 328, 1694–1698 (2010).
Cheloufi, S., Dos Santos, C. O., Chong, M. M. & Hannon, G. J. A dicer-independent miRNA biogenesis pathway that requires Ago catalysis. Nature 465, 584–589 (2010).
Yang, J.-S. et al. Conserved vertebrate mir-451 provides a platform for Dicer-independent, Ago2-mediated microRNA biogenesis. Proc. Natl Acad. Sci. USA 107, 15163–15168 (2010).
Stevanato, L., Thanabalasundaram, L., Vysokov, N. & Sinden, J. D. Investigation of content, stoichiometry and transfer of miRNA from human neural stem cell line derived exosomes. PLos ONE 11, e0146353 (2016).
Shelke, G. V., Lässer, C., Gho, Y. S. & Lötvall, J. Importance of exosome depletion protocols to eliminate functional and RNA-containing extracellular vesicles from fetal bovine serum. J. Extracell. Vesicles 3, 24783 (2014).
Wei, Z. & Batagov, A. O. & Carter, D. R. F. & Krichevsky, A. M. Fetal bovine serum RNA interferes with the cell culture derived extracellular RNA. Sci. Rep. 6, 31175 (2016).
McKenzie, A. J. et al. KRAS–MEK signaling controls Ago2 sorting into exosomes. Cell Rep. 15, 978–987 (2016).
Melo, S. A. et al. Cancer exosomes perform cell-independent microRNA biogenesis and promote tumorigenesis. Cancer Cell 26, 707–721 (2014).
Eldh, M., Lötvall, J., Malmhäll, C. & Ekström, K. Importance of RNA isolation methods for analysis of exosomal RNA: evaluation of different methods. Mol. Immunol. 50, 278–286 (2012).
Meerson, A. & Ploug, T. Assessment of six commercial plasma small RNA isolation kits using qRT-PCR and electrophoretic separation: higher recovery of microRNA following ultracentrifugation. Biol. Methods Protoc. 1, bpw003 (2016).
Gantier, M. P. et al. Analysis of microRNA turnover in mammalian cells following Dicer1 ablation. Nucleic Acids Res. 39, 5692–5703 (2011).
Bartlett, D. W. & Davis, M. E. Insights into the kinetics of siRNA-mediated gene silencing from live-cell and live-animal bioluminescent imaging. Nucleic Acids Res. 34, 322–333 (2006).
Furuyama, K. et al. Continuous cell supply from a Sox9-expressing progenitor zone in adult liver, exocrine pancreas and intestine. Nat. Genet. 43, 34–41 (2011).
Love, K. T. et al. Lipid-like materials for low-dose, in vivo gene silencing. Proc. Natl Acad. Sci. USA 107, 1864–1869 (2010).
Bobrie, A., Colombo, M., Krumeich, S. & Raposo, G. Diverse subpopulations of vesicles secreted by different intracellular mechanisms are present in exosome preparations obtained by differential ultracentrifugation. J. Extracell. Vesicles 1, 18397 (2012).
Kowal, J. et al. Proteomic comparison defines novel markers to characterize heterogeneous populations of extracellular vesicle subtypes. Proc. Natl Acad.Sci. USA 113, 968–977 (2016).
Zhang, H. et al. Identification of distinct nanoparticles and subsets of extracellular vesicles by asymmetric flow field-flow fractionation. Nat. Cell Biol. 20, 332–343 (2018).
Théry, C., Amigorena, S., Raposo, G. & Clayton, A. Isolation and characterization of exosomes from cell culture supernatants. Curr. Protoc. Cell Biol. Ch. 3, 1–29 (2006).
Okabe, M., Ikawa, M., Kominami, K., Nakanishi, T. & Nishimune, Y. ‘Green mice’ as a source of ubiquitous green cells. FEBS Lett. 407, 313–319 (1997).
Gurney, M. E. et al. Motor neuron degeneration in mice that express a human Cu,Zn superoxide dismutase mutation. Science 264, 1772–1775 (1994).
Miller, V. M., Gouvion, C. M., Davidson, B. L. & Paulson, H. L. Targeting Alzheimer’s disease genes with RNA interference: an efficient strategy for silencing mutant alleles. Nucleic Acids Res. 32, 661–668 (2004).
Foust, K. D. et al. Therapeutic AAV9-mediated suppression of mutant SOD1 slows disease progression and extends survival in models of inherited ALS. Mol. Ther. 21, 2148–2159 (2013).
Lin, X. et al. A robust in vivo positive-readout system for monitoring siRNA delivery to xenograft tumors. RNA 17, 603–612 (2011).
Taylor, S. C., Carbonneau, J., Shelton, D. N. & Bolvin, G. Optimization of droplet digital PCR from RNA and DNA extracts with direct comparison to RT–qPCR: clinical implications for quantification of oseltamivir-resistant subpopulations. J. Virol. Methods 224, 58–66 (2015).
Acknowledgements
The authors acknowledge E. Lai (Memorial Sloan-Kettering Cancer Center) for providing mouse embryonic fibroblast cell lines (WT, Ago2−/− and Ago2−/− rescued with Ago2). R.R. was funded in part by a scholarship in translational research from the Centre for Neuromuscular Disease and the University of Ottawa Brain and Mind Research Institute. This research was funded by grants from the Canadian Institutes of Health Research (proof of principle grant, PPP-141720), the National Research and Engineering Council of Canada (discovery grant no. 436104), The Quebec Consortium for Drug Discovery (CQDM Explore grant) and the ALS Association Treat Program (grant no. 15-LGCA-290) awarded to D.G.
Author information
Authors and Affiliations
Contributions
J.A.T. performed cloning, lentivirus production, northern blots, analysis of RNA enrichment in sEVs and absolute quantification of RNA in sEVs. A.S. maintained mouse colonies, performed injections and tissue collections, helped analyse sEVs distribution, performed western blots of sEVs, generated cultures of primary mixed motor neurons, performed some RT–qPCR and performed and analysed microscopy. R.R. produced sEVs, performed nanoparticle tracking analysis, performed tissue collections, labelled sEVs and analysed their distribution, and analysed RNA enrichment in sEVs and mRNA target knockdown by RT–qPCR. M.T.T. maintained mouse colonies, generated mouse protocols, genotyped mice and performed injections and tissue collections. C.C. helped establish protocol for primary mixed motor neuron culture and performed some analyses of miRNA levels in sEVs. H.G. performed western blots of sEVs and density gradient analyses of sEVs. L.H.R. and P.S.K. helped design lipid nanoparticle experiments and produced C12–200 lipid nanoparticles. D.G.A. helped design lipid nanoparticle experiments and supervised L.H.R. and P.S.K. W.L. helped design experiments. J.A.T., A.S. and R.R. analysed experiments and helped design experiments. D.G. conceived the project, designed experiments and wrote the manuscript.
Corresponding author
Ethics declarations
Competing interests
J.A.T. and D.G. are inventors on a filed patent that claims the use of the pre-miR-451 backbone for enrichment of small RNAs in sEVs. The remaining authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Supplementary Information
Supplementary Tables 1–3, Supplementary Figs. 1–5 and unprocessed western blots.
Rights and permissions
About this article
Cite this article
Reshke, R., Taylor, J.A., Savard, A. et al. Reduction of the therapeutic dose of silencing RNA by packaging it in extracellular vesicles via a pre-microRNA backbone. Nat Biomed Eng 4, 52–68 (2020). https://doi.org/10.1038/s41551-019-0502-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41551-019-0502-4
This article is cited by
-
Regulation of cargo selection in exosome biogenesis and its biomedical applications in cancer
Experimental & Molecular Medicine (2024)
-
Cell-free therapy based on extracellular vesicles: a promising therapeutic strategy for peripheral nerve injury
Stem Cell Research & Therapy (2023)
-
CRISPR/Cas9 therapeutics: progress and prospects
Signal Transduction and Targeted Therapy (2023)
-
Cardiac progenitor cell-derived extracellular vesicles promote angiogenesis through both associated- and co-isolated proteins
Communications Biology (2023)
-
Context-specific regulation of extracellular vesicle biogenesis and cargo selection
Nature Reviews Molecular Cell Biology (2023)