Abstract
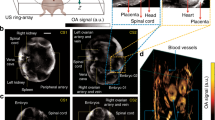
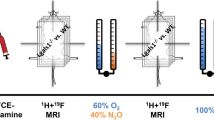
Direct assessment of blood oxygenation in the human placenta can provide information about placental function. However, the monitoring of placental oxygenation involves invasive sampling or imaging techniques that are poorly suited for bedside use. Here we show that placental oxygen haemodynamics can be non-invasively probed in real time and up to 4.2 cm below the body surface via concurrent frequency-domain diffuse optical spectroscopy and ultrasound imaging. We developed a multimodal instrument to facilitate the assessment of the properties of the anterior placenta by leveraging image-reconstruction algorithms that integrate ultrasound information about the morphology of tissue layers with optical information on haemodynamics. In a pilot investigation involving placentas with normal function (15 women) or abnormal function (9 women) from pregnancies in the third trimester, we found no significant differences in baseline haemoglobin properties, but statistically significant differences in the haemodynamic responses to maternal hyperoxia. Our findings suggest that the non-invasive monitoring of placental oxygenation may aid the early detection of placenta-related adverse pregnancy outcomes and maternal vascular malperfusion.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 digital issues and online access to articles
$99.00 per year
only $8.25 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
Data availability
The main data supporting the results in this study are available within the paper and its supplementary information. All optical data generated in this study, including source data and the data used to make the figures, are available from figshare with identifiers at https://doi.org/10.6084/m9.figshare.19451882, https://doi.org/10.6084/m9.figshare.19451879 and https://doi.org/10.6084/m9.figshare.19451876. The raw clinical and ultrasound data are available from the corresponding author, subject to approval from the Institutional Review Board of the University of Pennsylvania.
Code availability
The custom code employed for processing the optical data and for performing the statistical analysis are available from figshare with identifiers at https://doi.org/10.6084/m9.figshare.19451882, https://doi.org/10.6084/m9.figshare.19451879 and https://doi.org/10.6084/m9.figshare.19451876. The LabVIEW code and simulation code are also available from the corresponding author on request.
References
Burton, G. J., Fowden, A. L. & Thornburg, K. L. Placental origins of chronic disease. Physiol. Rev. 96, 1509–1565 (2016).
Thornburg, K. L., O’Tierney, P. F. & Louey, S. Review: the placenta is a programming agent for cardiovascular disease. Placenta 31, S54–S59 (2010).
Hodyl, N. A. et al. Child neurodevelopmental outcomes following preterm and term birth: what can the placenta tell us? Placenta 57, 79–86 (2017).
AIUM-ACR-ACOG-SMFM-SRU practice parameter for the performance of standard diagnostic obstetric ultrasound examinations. J. Ultrasound Med. 37, E13–E24 (2018).
Turco, M. Y. et al. Trophoblast organoids as a model for maternal–fetal interactions during human placentation. Nature 564, 263–281 (2018).
Hemberger, M., Hanna, C. W. & Dean, W. Mechanisms of early placental development in mouse and humans. Nat. Rev. Genet. 21, 27–43 (2020).
Carter, A. M. Animal models of human placentation – a review. Placenta 28, S41–S47 (2007).
Schmidt, A., Morales-Prieto, D. M., Pastuschek, J., Fröhlich, K. & Markert, U. R. Only humans have human placentas: molecular differences between mice and humans. J. Reprod. Immunol. 108, 65–71 (2015).
Nye, G. A. et al. Human placental oxygenation in late gestation: experimental and theoretical approaches. J. Physiol. 596, 5523–5534 (2018).
Horsman, M. R., Mortensen, L. S., Petersen, J. B., Busk, M. & Overgaard, J. Imaging hypoxia to improve radiotherapy outcome. Nat. Rev. Clin. Oncol. 9, 674–687 (2012).
You, W. et al. Hemodynamic responses of the placenta and brain to maternal hyperoxia in fetuses with congenital heart disease by using blood oxygen-level dependent MRI. Radiology 294, 141–148 (2020).
Logothetis, N. K. What we can do and what we cannot do with fMRI. Nature 453, 869–878 (2008).
Durduran, T., Choe, R., Yodh, A. G. & Baker, W. B. Diffuse optics for tissue monitoring and tomography. Rep. Prog. Phys. 73, 076701 (2010).
Yodh, A. G. & Boas, D. A. in Biomedical Photonics Handbook: Biomedical Diagnostics (ed Vo-Dinh, Tuan) Ch. 21 (2014).
Boas, D. A., O’Leary, M. A., Chance, B. & Yodh, A. G. Scattering of diffuse photon density waves by spherical inhomogeneities within turbid media: analytic solution and applications. Proc. Natl Acad. Sci. USA 91, 4887–4891 (1994).
Waterhouse, D. J., Fitzpatrick, C. R. M., Pogue, B. W., O’Connor, J. P. B. & Bohndiek, S. E. A roadmap for the clinical implementation of optical-imaging biomarkers. Nat. Biomed. Eng. 3, 339–353 (2019).
Baker, W. B. et al. Continuous non-invasive optical monitoring of cerebral blood flow and oxidative metabolism after acute brain injury. J. Cereb. Blood Flow Metab. 39, 1469–1485 (2019).
Choe, R. et al. Transabdominal near infrared oximetry of hypoxic stress in fetal sheep brain in utero. Proc. Natl Acad. Sci. USA 100, 12950–12954 (2003).
Tromberg, B. J. et al. Predicting responses to neoadjuvant chemotherapy in breast cancer: ACRIN 6691 trial of diffuse optical spectroscopic imaging. Cancer Res. 76, 5933–5944 (2016).
Boas, D. A., Elwell, C. E., Ferrari, M. & Taga, G. Twenty years of functional near-infrared spectroscopy: introduction for the special issue. Neuroimage 85, 1–5 (2014).
Yun, S. H. & Kwok, S. J. J. Light in diagnosis, therapy and surgery. Nat. Biomed. Eng. 1, 8 (2017).
Zhu, Q. et al. Breast cancer: assessing response to neoadjuvant chemotherapy by using US-guided near-infrared tomography. Radiology 266, 433–442 (2013).
Eggebrecht, A. T. et al. Mapping distributed brain function and networks with diffuse optical tomography. Nat. Photonics 8, 448–454 (2014).
Konecky, S. D. et al. Imaging complex structures with diffuse light. Opt. Express 16, 5048–5060 (2008).
Ntziachristos, V. Going deeper than microscopy: the optical imaging frontier in biology. Nat. Methods 7, 603–614 (2010).
Kakogawa, J., Sumimoto, K., Kawamura, T., Minoura, S. & Kanayama, N. Noninvasive monitoring of placental oxygenation by near-infrared spectroscopy. Am. J. Perinatol. 27, 463–468 (2010).
Hasegawa, J. et al. Evaluation of placental function using near infrared spectroscopy during fetal growth restriction. J. Perinat. Med. 38, 29–32 (2010).
Kawamura, T. et al. Measurement of placental oxygenation by transabdominal near-infrared spectroscopy. Am. J. Perinatol. 24, 161–166 (2007).
Scholkmann, F. et al. A review on continuous wave functional near-infrared spectroscopy and imaging instrumentation and methodology. NeuroImage 85, 6–27 (2014).
Boas, D. A., Pitris, C. & Ramanujam, N. (eds) Handbook of biomedical optics (CRC Press, 2016). https://doi.org/10.1201/b10951
Fantini, S. & Sassaroli, A. in Handbook of Optical Biomedical Diagnostics 2nd edn (ed Valery V. Tuchin) Ch. 7. (SPIE Press, 2016). https://doi.org/10.1117/3.2219603.ch7
Choi, J. et al. Noninvasive determination of the optical properties of adult brain: near-infrared spectroscopy approach. J. Biomed. Opt. 9, 221–229 (2004).
Liebert, A. et al. Assessment of inflow and washout of indocyanine green in the adult human brain by monitoring of diffuse reflectance at large source-detector separation. J. Biomed. Opt. 16, 046011 (2011).
Pifferi, A. et al. New frontiers in time-domain diffuse optics, a review. J. Biomed. Opt. 21, 091310 (2016).
Wright, E. et al. Maternal vascular malperfusion and adverse perinatal outcomes in low-risk nulliparous women. Obstet. Gynecol. 130, 1112–1120 (2017).
Ernst, L. M. Maternal vascular malperfusion of the placental bed. APMIS 126, 551–560 (2018).
Hallacoglu, B., Sassaroli, A. & Fantini, S. Optical characterization of two-layered turbid media for non-invasive, absolute oximetry in cerebral and extracerebral tissue. PLoS ONE 8, E64095 (2013).
Liemert, A. & Kienle, A. Light diffusion in N-layered turbid media: frequency and time domains. J. Biomed. Opt. 15, 025002 (2010).
Ripoll, J. et al. Recovery of optical parameters in multiple-layered diffusive media: theory and experiments. J. Opt. Soc. Am. A 18, 821–830 (2001).
Corlu, A. et al. Diffuse optical tomography with spectral constraints and wavelength optimization. Appl. Opt. 44, 2082–2093 (2005).
Pogue, B. W. & Patterson, M. S. Review of tissue simulating phantoms for optical spectroscopy, imaging and dosimetry. J. Biomed. Opt. 11, 041102 (2006).
Schweiger, M. & Arridge, S. The Toast++ software suite for forward and inverse modeling in optical tomography. J. Biomed. Opt. 19, 040801 (2014).
Lee, S. W. Y., Khaw, K. S., Kee, W. D. N., Leung, T. Y. & Critchley, L. A. H. Haemodynamic effects from aortocaval compression at different angles of lateral tilt in non-labouring term pregnant women. Br. J. Anaesth. 109, 950–956 (2012).
O’Gorman, N., Tampakoudis, G., Wright, A., Wright, D. & Nicolaides, K. H. Uterine artery pulsatility index at 12, 22, 32 and 36 weeks’ gestation in screening for pre-eclampsia. Ultrasound Obstet. Gynecol. 47, 565–572 (2016).
Nguyen, T. et al. Non-invasive transabdominal measurement of placental oxygenation: a step toward continuous monitoring. Biomed. Opt. Express 12, 4119–4130 (2021).
American College of Obstetricians and Gynecologists. Management of intrapartum fetal heart rate tracings. Obstet. Gynecol. 116, 1232–1240 (2010).
Luo, J. et al. In vivo quantification of placental insufficiency by BOLD MRI: a human study. Sci. Rep. 7, 3713 (2017).
Catov, J. M. et al. Neonatal outcomes following preterm birth classified according to placental features. Am. J. Obstet. Gynecol. 216, 411.e1–411.e14 (2017).
Liemert, A. Light diffusion in N-layered turbid media: steady-state domain. J. Biomed. Opt. 15, 025003 (2010).
Haskell, R. C. et al. Boundary conditions for the diffusion equation in radiative transfer. J. Opt. Soc. Am. A 11, 2727–2741 (1994).
Jacques, S. L. Optical properties of biological tissues: a review. Phys. Med. Biol. 58, R37–R61 (2013).
Hansen, P. C. in Computational Inverse Problems in Electrocardiology Vol. 4 (ed P. Johnston, Advances in Computational Bioengineering) 119–142 (WIT Press, Southampton, 2000).
Ugray, Z. et al. Scatter search and local NLP solvers: a multistart framework for global optimization. INFORMS J. Comput. 19, 328–340 (2007).
Gestational hypertension and preeclampsia: ACOG practice bulletin, number 222. Obstet. Gynecol. 135, e237–e260 (2020).
Fenton, T. R. & Kim, J. H. A systematic review and meta-analysis to revise the Fenton growth chart for preterm infants. BMC Pediatr. 13, 59 (2013).
Khong, T. Y. et al. Sampling and definitions of placental lesions: Amsterdam Placental Workshop Group Consensus Statement. Arch. Pathol. Lab. Med. 140, 698–713 (2016).
Taroni, P. et al. Breast tissue composition and its dependence on demographic risk factors for breast cancer: non-invasive assessment by time domain diffuse optical spectroscopy. PLoS ONE 10, e0128941 (2015).
Henry, B. et al. Hybrid diffuse optical techniques for continuous hemodynamic measurement in gastrocnemius during plantar flexion exercise. J. Biomed. Opt. 20, 125006 (2015).
Khare, S. M. et al. Evaluation of the human placenta optical scattering properties using continuous wave and frequency-domain diffuse reflectance spectroscopy. J. Biomed. Opt. 25, 116001 (2020).
Acknowledgements
The work was supported by NIH U01HD087180. J.M.C. was partially supported by NIH P41EB015893. T.K. was partially supported by NIH F31HD085731 and NIH T32HL007915. W.B. was partially supported by R01NS113945. A.G.Y. acknowledges partial support from NIH R01NS060653 and NIH P41EB015893. We thank D. Licht, B. White, J. Strauss and Y. H. Ong for useful discussions, advice and support, as well as the clinic research coordinators of the Maternal and Child Health Research Center at Perelman School of Medicine, University of Pennsylvania.
Author information
Authors and Affiliations
Contributions
L.W., A.G.Y. and N.S. designed the study. L.W. and T.K. developed the instrument with assistance from W.B.B., K.A., L.H., D.R.B. and V.K. L.W. and J.M.C. developed the three-layer reconstruction algorithm and conducted the computer simulations. L.W. and T.K. performed phantom experiments with help from W.B.B. and L.H. K.A. designed the optical probe with input from L.W. and W.B.B. L.W. collected and analysed the optical data. S.P. and N.S. advised on human participant data interpretation. N.S. collected and analysed the ultrasound data. R.L.L. performed placental histopathologic analysis. L.W., A.G.Y. and N.S. wrote the paper with input from all authors.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Biomedical Engineering thanks Carolyn Bayer, Christopher Contag and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Extended data
Extended Data Fig. 1
Detailed schematic of the custom heterodyne FD-DOS instrument.
Supplementary information
Supplementary dataset 1
Optically measured placental data for the 24 participants.
Supplementary dataset 2
Clinically monitored variables for the 24 participants.
Supplementary tables
Supplementary Tables 1–6.
Rights and permissions
About this article
Cite this article
Wang, L., Cochran, J.M., Ko, T. et al. Non-invasive monitoring of blood oxygenation in human placentas via concurrent diffuse optical spectroscopy and ultrasound imaging. Nat. Biomed. Eng 6, 1017–1030 (2022). https://doi.org/10.1038/s41551-022-00913-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41551-022-00913-2