Abstract
Deficiency in the deubiquitinating enzyme A20 causes severe inflammation in mice, and impaired A20 function is associated with human inflammatory diseases. A20 has been implicated in negatively regulating NF-κB signalling, cell death and inflammasome activation; however, the mechanisms by which A20 inhibits inflammation in vivo remain poorly understood. Genetic studies in mice revealed that its deubiquitinase activity is not essential for A20 anti-inflammatory function. Here we show that A20 prevents inflammasome-dependent arthritis by inhibiting macrophage necroptosis and that this function depends on its zinc finger 7 (ZnF7). We provide genetic evidence that RIPK1 kinase-dependent, RIPK3–MLKL-mediated necroptosis drives inflammasome activation in A20-deficient macrophages and causes inflammatory arthritis in mice. Single-cell imaging revealed that RIPK3-dependent death caused inflammasome-dependent IL-1β release from lipopolysaccharide-stimulated A20-deficient macrophages. Importantly, mutation of the A20 ZnF7 ubiquitin binding domain caused arthritis in mice, arguing that ZnF7-dependent inhibition of necroptosis is critical for A20 anti-inflammatory function in vivo.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout








Similar content being viewed by others
Data availability
Source data for Figs. 1–8 and Supplementary Figs. 1–4, 6 and 7 have been provided in Supplementary Table 3. Uncropped images of the immunoblots presented in the figures are included in Supplementary Fig. 9. All other data supporting the findings of this study are available from the corresponding author on reasonable request55,56,58,61.
References
Lork, M., Verhelst, K. & Beyaert, R. CYLD, A20 and OTULIN deubiquitinases in NF-κB signaling and cell death: so similar, yet so different. Cell Death Differ. 24, 1172–1183 (2017).
Ma, A. & Malynn, B. A. A20: linking a complex regulator of ubiquitylation to immunity and human disease. Nat. Rev. Immunol. 12, 774–785 (2012).
Das, T., Chen, Z., Hendriks, R. W. & Kool, M. A20/tumor necrosis factor α-induced protein 3 in immune cells controls development of autoinflammation and autoimmunity: lessons from mouse models. Front. Immunol. 9, 104 (2018).
Vereecke, L., Beyaert, R. & van Loo, G. Genetic relationships between A20/TNFAIP3, chronic inflammation and autoimmune disease. Biochem. Soc. Trans. 39, 1086–1091 (2011).
Novak, U. et al. The NF-κB negative regulator TNFAIP3 (A20) is inactivated by somatic mutations and genomic deletions in marginal zone lymphomas. Blood 113, 4918–4921 (2009).
Kato, M. et al. Frequent inactivation of A20 in B-cell lymphomas. Nature 459, 712–716 (2009).
Schmitz, R. et al. TNFAIP3 (A20) is a tumor suppressor gene in Hodgkin lymphoma and primary mediastinal B cell lymphoma. J. Exp. Med. 206, 981–989 (2009).
Compagno, M. et al. Mutations of multiple genes cause deregulation of NF-κB in diffuse large B-cell lymphoma. Nature 459, 717–721 (2009).
Aeschlimann, F. A. et al. A20 haploinsufficiency (HA20): clinical phenotypes and disease course of patients with a newly recognised NF-kB-mediated autoinflammatory disease. Ann. Rheum. Dis. 77, 728–735 (2018).
Duncan, C. J. A. et al. Early-onset autoimmune disease due to a heterozygous loss-of-function mutation in TNFAIP3 (A20). Ann. Rheum. Dis. 77, 783–786 (2018).
Kadowaki, T. et al. Haploinsufficiency of A20 causes autoinflammatory and autoimmune disorders. J. Allergy Clin. Immunol. 141, 1485–1488 (2018).
Takagi, M. et al. Haploinsufficiency of TNFAIP3 (A20) by germline mutation is involved in autoimmune lymphoproliferative syndrome. J. Allergy Clin. Immunol. 139, 1914–1922 (2017).
Zhou, Q. et al. Loss-of-function mutations in TNFAIP3 leading to A20 haploinsufficiency cause an early-onset autoinflammatory disease. Nat. Genet. 48, 67–73 (2016).
Lee, E. G. et al. Failure to regulate TNF-induced NF-κB and cell death responses in A20-deficient mice. Science 289, 2350–2354 (2000).
Onizawa, M. et al. The ubiquitin-modifying enzyme A20 restricts ubiquitination of the kinase RIPK3 and protects cells from necroptosis. Nat. Immunol. 16, 618–627 (2015).
Newton, K. et al. RIPK3 deficiency or catalytically inactive RIPK1 provides greater benefit than MLKL deficiency in mouse models of inflammation and tissue injury. Cell Death Differ. 23, 1565–1576 (2016).
Tavares, R. M. et al. The ubiquitin modifying enzyme A20 restricts B cell survival and prevents autoimmunity. Immunity 33, 181–191 (2010).
Vereecke, L. et al. Enterocyte-specific A20 deficiency sensitizes to tumor necrosis factor-induced toxicity and experimental colitis. J. Exp. Med. 207, 1513–1523 (2010).
Chu, Y. et al. B cells lacking the tumor suppressor TNFAIP3/A20 display impaired differentiation and hyperactivation and cause inflammation and autoimmunity in aged mice. Blood 117, 2227–2236 (2011).
Hammer, G. E. et al. Expression of A20 by dendritic cells preserves immune homeostasis and prevents colitis and spondyloarthritis. Nat. Immunol. 12, 1184–1193 (2011).
Kool, M. et al. The ubiquitin-editing protein A20 prevents dendritic cell activation, recognition of apoptotic cells, and systemic autoimmunity. Immunity 35, 82–96 (2011).
Lippens, S. et al. Keratinocyte-specific ablation of the NF-κB regulatory protein A20 (TNFAIP3) reveals a role in the control of epidermal homeostasis. Cell Death Differ. 18, 1845–1853 (2011).
Matmati, M. et al. A20 (TNFAIP3) deficiency in myeloid cells triggers erosive polyarthritis resembling rheumatoid arthritis. Nat. Genet. 43, 908–912 (2011).
Heger, K. et al. A20-deficient mast cells exacerbate inflammatory responses in vivo. PLoS Biol. 12, e1001762 (2014).
Catrysse, L. et al. A20 prevents chronic liver inflammation and cancer by protecting hepatocytes from death. Cell Death Dis. 7, e2250 (2016).
Voet, S. et al. A20 critically controls microglia activation and inhibits inflammasome-dependent neuroinflammation. Nat. Commun. 9, 2036 (2018).
Catrysse, L., Vereecke, L., Beyaert, R. & van Loo, G. A20 in inflammation and autoimmunity. Trends Immunol. 35, 22–31 (2014).
Wertz, I. E. et al. Phosphorylation and linear ubiquitin direct A20 inhibition of inflammation. Nature 528, 370–375 (2015).
Wertz, I. E. et al. De-ubiquitination and ubiquitin ligase domains of A20 downregulate NF-κB signalling. Nature 430, 694–699 (2004).
Lu, T. T. et al. Dimerization and ubiquitin mediated recruitment of A20, a complex deubiquitinating enzyme. Immunity 38, 896–905 (2013).
De, A., Dainichi, T., Rathinam, C. V. & Ghosh, S. The deubiquitinase activity of A20 is dispensable for NF-κB signaling. EMBO Rep. 15, 775–783 (2014).
Skaug, B. et al. Direct, noncatalytic mechanism of IKK inhibition by A20. Mol. Cell 44, 559–571 (2011).
Tokunaga, F. et al. Specific recognition of linear polyubiquitin by A20 zinc finger 7 is involved in NF-κB regulation. EMBO J. 31, 3856–3870 (2012).
Verhelst, K. et al. A20 inhibits LUBAC-mediated NF-κB activation by binding linear polyubiquitin chains via its zinc finger 7. EMBO J. 31, 3845–3855 (2012).
Yamaguchi, N. & Yamaguchi, N. The seventh zinc finger motif of A20 is required for the suppression of TNF-α-induced apoptosis. FEBS Lett. 589, 1369–1375 (2015).
Bosanac, I. et al. Ubiquitin binding to A20 ZnF4 is required for modulation of NF-κB signaling. Mol. Cell 40, 548–557 (2010).
Draber, P. et al. LUBAC-recruited CYLD and A20 regulate gene activation and cell death by exerting opposing effects on linear ubiquitin in signaling complexes. Cell Rep. 13, 2258–2272 (2015).
Vande Walle, L. et al. Negative regulation of the NLRP3 inflammasome by A20 protects against arthritis. Nature 512, 69–73 (2014).
Duong, B. H. et al. A20 restricts ubiquitination of pro-interleukin-1β protein complexes and suppresses NLRP3 inflammasome activity. Immunity 42, 55–67 (2015).
Lamkanfi, M. & Dixit, V. M. Mechanisms and functions of inflammasomes. Cell 157, 1013–1022 (2014).
Gais, P. et al. Cutting edge: divergent cell-specific functions of MyD88 for inflammatory responses and organ injury in septic peritonitis. J. Immunol. 188, 5833–5837 (2012).
McInnes, I. B. & Schett, G. The pathogenesis of rheumatoid arthritis. N. Engl. J. Med. 365, 2205–2219 (2011).
Smolen, J. S., Aletaha, D. & McInnes, I. B. Rheumatoid arthritis. Lancet 388, 2023–2038 (2016).
Armaka, M. et al. Mesenchymal cell targeting by TNF as a common pathogenic principle in chronic inflammatory joint and intestinal diseases. J. Exp. Med. 205, 331–337 (2008).
Moriwaki, K. & Chan, F. K. The inflammatory signal adaptor RIPK3: functions beyond necroptosis. Int. Rev. Cell Mol. Biol. 328, 253–275 (2017).
Pasparakis, M. & Vandenabeele, P. Necroptosis and its role in inflammation. Nature 517, 311–320 (2015).
Polykratis, A. et al. Cutting edge: RIPK1 kinase inactive mice are viable and protected from TNF-induced necroptosis in vivo. J. Immunol. 193, 1539–1543 (2014).
Garlanda, C., Dinarello, C. A. & Mantovani, A. The interleukin-1 family: back to the future. Immunity 39, 1003–1018 (2013).
Liu, T. et al. Single-cell imaging of caspase-1 dynamics reveals an all-or-none inflammasome signaling response. Cell Rep. 8, 974–982 (2014).
Shirasaki, Y. et al. Real-time single-cell imaging of protein secretion. Sci. Rep. 4, 4736 (2014).
Wright, H. L., Moots, R. J. & Edwards, S. W. The multifactorial role of neutrophils in rheumatoid arthritis. Nat. Rev. Rheumatol. 10, 593–601 (2014).
Conos, S. A. et al. Active MLKL triggers the NLRP3 inflammasome in a cell-intrinsic manner. Proc. Natl Acad. Sci. USA 114, E961–E969 (2017).
Kang, T. B., Yang, S. H., Toth, B., Kovalenko, A. & Wallach, D. Caspase-8 blocks kinase RIPK3-mediated activation of the NLRP3 inflammasome. Immunity 38, 27–40 (2013).
Vince, J. E. et al. Inhibitor of apoptosis proteins limit RIP3 kinase-dependent interleukin-1 activation. Immunity 36, 215–227 (2012).
Pasparakis, M. et al. TNF-mediated inflammatory skin disease in mice with epidermis-specific deletion of IKK2. Nature 417, 861–866 (2002).
Vlantis, K. et al. TLR-independent anti-inflammatory function of intestinal epithelial TRAF6 signalling prevents DSS-induced colitis in mice. Gut 65, 935–943 (2016).
Drexler, S. K. et al. Tissue-specific opposing functions of the inflammasome adaptor ASC in the regulation of epithelial skin carcinogenesis. Proc. Natl Acad. Sci. USA 109, 18384–18389 (2012).
Sodhi, C. P. et al. Intestinal epithelial Toll-like receptor 4 regulates goblet cell development and is required for necrotizing enterocolitis in mice. Gastroenterology 143, 708–718 (2012).
Clausen, B. E., Burkhardt, C., Reith, W., Renkawitz, R. & Forster, I. Conditional gene targeting in macrophages and granulocytes using LysMcre mice. Transgenic Res. 8, 265–277 (1999).
Newton, K., Sun, X. & Dixit, V. M. Kinase RIP3 is dispensable for normal NF-κBs, signaling by the B-cell and T-cell receptors, tumor necrosis factor receptor 1, and Toll-like receptors 2 and 4. Mol. Cell. Biol. 24, 1464–1469 (2004).
Lin, J. et al. RIPK1 counteracts ZBP1-mediated necroptosis to inhibit inflammation. Nature 540, 124–128 (2016).
Adachi, O. et al. Targeted disruption of the MyD88 gene results in loss of IL-1- and IL-18-mediated function. Immunity 9, 143–150 (1998).
Armaka, M., Ospelt, C., Pasparakis, M. & Kollias, G. The p55TNFR–IKK2–Ripk3 axis orchestrates arthritis by regulating death and inflammatory pathways in synovial fibroblasts. Nat. Commun. 9, 618 (2018).
Armaka, M., Gkretsi, V., Kontoyiannis, D. L. & Kollias, G. A standardized protocol for the isolation and culture of normal and arthritogenic murine synovial fibroblasts. Protoc. Exch. https://doi.org/10.1038/nprot.2009.102 (2009).
Acknowledgements
We are grateful to J. Buchholz, C. Uthoff-Hachenberg, E. Mahlberg, B. Kühnel, E. Stade, T. Liu, B. Yao, K. Igarashi, M. Sze, L. Bellen, S. Lalos, P. Athanasakis and A. Kateveni for their excellent technical assistance, and T. Hochepied for help with the generation of A20mZnF7/mZnF7 mice. We also thank D. Hackam for providing Tlr4FL/FL mice, V. Dixit and Genentech for providing Ripk3−/− mice, G. Vassilopoulos for providing access to microscope facilities and the InfrafrontierGR infrastructure (NSRF 2007–2013 and NSRF 2014–2020) for providing the mouse hosting and micro-computed tomography facilities. This work was supported by funding from the European Research Council (ERC grant nos AdG 323040 and 787826 to M.P.; grant no. AdG 340217 to G.K.), the Greek GSRT project INNATE FIBLROBLAST to G.K. (ERC06, co-financed by the ESF and NSRF 2007–2013), the European Commission (FP7 grant ‘Masterswitch’ 223404 to M.P. and G.K.), JSPS KAKENHI (grant no. JP16H06385 to M.M., JP26110005 to Y.Y., and JP15H01366 and JP17H05496 to Y.S.), JST PRESTO (grant no. JP17940748 to Y.S.), and the Japan Agency for Medical Research and Development (grant nos JP17gm0610004 and JP18gm5010001 to M.M.). The research in the G.v.L. lab is supported by research grants from the FWO, ‘Geneeskundige Stichting Koningin Elisabeth’ (GSKE), CBC Banque Prize, the Charcot Foundation, the ‘Belgian Foundation against Cancer’ and ‘Kom op tegen Kanker’. A.M. is supported by a grant from the ‘Concerted Research Actions’ (GOA) of the Ghent University. Research in the M.A. lab is supported by a startup grant from the Stavros Niarchos Foundation donation to BSRC ‘Alexander Fleming’. M.A. and G.K. also acknowledge support of this work by project MIS 5002562 funded by NSRF 2014–2020, co-financed by Greece and the European Union (ERDF). R.O.E. was supported by a postdoctoral fellowship from the Alexander von Humboldt foundation.
Author information
Authors and Affiliations
Contributions
A.P., G.v.L., M.A. and M.P. conceived the study and designed the experiments. A.P., A.M., R.O.E., Y.S., M.Y., Y.Y. and M.A. performed and analysed the experiments. B.H. provided the mice. Y.Y., S.U., M.M., G.K., M.A., G.v.L. and M.P. supervised the experiments. A.P., M.A., G.v.L. and M.P. interpreted the data and wrote the paper.
Corresponding authors
Ethics declarations
Competing interests
M.P. received consulting and speaker fees from Genentech, GSK and Boehringer.
Additional information
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Integrated supplementary information
Supplementary Figure 1 Leucocyte and cytokine analysis in the blood of wild type, A20MYC-KO, A20MYC-KO ASCMYC-KO, A20MYC-KO MyD88MYC-KO, and A20MYC-KO MyD88MYC mice.
(a) Graphs indicating the number of white blood cells (WBC), lymphocytes (LY), monocytes (MO), and neutrophils (NE) in peripheral blood from mice with the indicated genotypes (wt, n=54; A20MYC-KO, n=33; A20MYC-KO AscMYC-KO, n=23 mice). (b) Levels of the indicated cytokines and chemokines in the serum of mice with the indicated genotypes (n=10 mice per genotype). (c) Graphs indicating the number of white blood cells (WBC), lymphocytes (LY), monocytes (MO), and neutrophils (NE) in peripheral blood from mice with the indicated genotypes. Wild type and A20MYC-KO mice are the same shown in Supplementary Fig. 1 and are included for comparison (wt, n=54; A20MYC-KO, n=33; A20MYC-KO MyD88MYC-KO, n=22; A20MYC-KO MyD88MYC, n=12 mice). (d) Levels of the indicated cytokines and chemokines in the serum of mice with the indicated genotypes (n=10 mice per genotype). Dots in the graphs indicate individual mice. In all graphs average ± SEM is also shown for each group of mice. *, ** and *** represent p<0.05, p<0.01 and p<0.001 respectively (one-way ANOVA with Bonferroni correction between indicated genotypes). All statistical tests are two-tailed. Raw data are provided in Supplementary Table 3.
Supplementary Figure 2 MyD88 regulates LPS- and IL-1β-induced inflammatory signalling in synovial fibroblasts.
a) Synovial fibroblasts with the indicated genotypes were stimulated with 1 µg/ml of LPS (left panel) or 10 ng/ml of IL-1β (right panel) and analysis of NF-κB and MAPK signalling was performed by immunoblotting with the indicated antibodies. Results of one representative out of two independent experiments are shown. (b-c) Synovial fibroblasts with the indicated genotypes were incubated with 1 µg/ml of LPS (b) or 10 ng/ml of IL-1β (c) and mRNA expression of the indicated genes was evaluated at the indicated time points after stimulation. Graphs depict pooled results from two independent experiments in which five independent isolations of synovial fibroblasts for each genotype (n=5 independent isolations of synovial fibroblasts) were used. (d) Graphs depicting spleen weight as well as the numbers of white blood cells (WBC), lymphocytes (LY), monocytes (MO), and neutrophils (NE) in peripheral blood from mice with the indicated genotypes. Dots indicate individual mice (wt to wt, n=7; wt to MyD88SF-KO n=8; A20MYC-KO to wt, n=11, A20MYC-KO to MyD88SF-KO, n=10 mice). In all graphs average ± SEM is also shown for each group of mice. *, ** and *** represent p<0.05, p<0.01 and p<0.001 respectively (non-parametric Mann-Whitney test between indicated genotypes for d; two way ANOVA with Bonferroni correction for b, c). All statistical tests are two-tailed. Raw data are provided in Supplementary Table 3 and unprocessed immunoblots are provided in Supplementary Figure 9.
Supplementary Figure 3 Synovial fibroblast-specific TLR4 knockout does not inhibit arthritis caused by myeloid cell specific A20 deficiency.
a) Representative macroscopic and histological images of the ankle joints of mice with the indicated genotypes analysed 32 weeks after adoptive transfer of bone marrow from wt or A20MYC-KO animals (scale bar: 500μm). (b) Graphs depicting clinical scores, average thickness of rear paws at the ankle area, as well as histological scores for inflammation, bone erosion, and cartilage destruction in mice with the indicated genotypes transferred with bone marrow from wt or A20MYC-KO animals. Dots in the graphs indicate individual mice (wt to wt, n=9; wt to TLR4SF-KO n=12; A20MYC-KO to wt, n=24, A20MYC-KO to TLR4SF-KO, n=27 mice for a-b). (c) Graphs depicting spleen weight, the number of white blood cells (WBC), lymphocytes (LY), monocytes (MO), and neutrophils (NE) in peripheral blood from mice with the indicated genotypes. (wt to wt, n=9; wt to TLR4SF-KO n=10; A20MYC-KO to wt, n=18, A20MYC-KO to TLR4SF-KO, n=22 mice). In all graphs average ± SEM is shown for each group of mice (non-parametric Mann-Whitney test between indicated genotypes). All statistical tests are two-tailed. Raw data are provided in Supplementary Table 3.
Supplementary Figure 4 RIPK3 or MLKL deficiency or lack of RIPK1 kinase activity, does not affect TNF- and LPS-induced NF-κB and MAPK activation as well as inflammatory gene expression in A20-/- macrophages.
(a-b) BMDMs with the indicated genotypes were stimulated with 20 ng/ml of TNF for the indicated time points. (a) Analysis of NF-κB and MAPK signalling was performed by immunoblotting with the indicated antibodies. Results of one representative out of two independent experiments are shown. (b) mRNA expression of the indicated genes was analysed by qRT-PCR Results shown were obtained from one experiment in which three independent isolations of BMDMs for each genotype (n=3 independent BMDM isolations) were included. (c-d) BMDMs with the indicated genotypes were stimulated with 20 ng/ml of LPS for the indicated time points. (c) Analysis of NF-κB and MAPK signalling was performed by immunoblotting with the indicated antibodies. Results of one representative out of two independent experiments are shown. (d) mRNA expression of the indicated genes was analysed by qRT-PCR. Results shown were obtained from one experiment in which three independent isolations of BMDMs for each genotype (n=3 independent BMDM isolations) were included. In all graphs average ± SEM is shown for each group of mice. *, ** and *** represent p<0.05, p<0.01 and p<0.001 respectively (two way ANOVA with Bonferroni correction). All statistical tests are two-tailed. Raw data are provided in Supplementary Table 3 and unprocessed immunoblots are provided in Supplementary Figure 9.
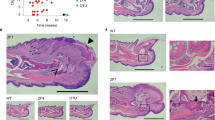
Supplementary Figure 5 A20mZnF7/mZnF7 knock-in mice generated using CRISPR/Cas9-mediated gene targeting develop dactylitis.
(a) Schematic representation of the A20 gene indicating the location of the point mutations introduced in exon 9 to change cysteine (C) residues at positions 764 and 767 to alanines (A). Two silent mutations were also introduced to avoid re-editing of the DNA by Cas9 after homology-directed repair. Sanger sequencing of wt and A20mZnF7/mZnF7 mouse tail DNA to confirm correct introduction of the designed mutations into the genome. (b) mCT analysis of forepaws of mice with the indicated genotypes depicts the extensive bone erosions in the digits of A20mZnF7/mZnF7 and the milder erosions in carpal bones compared to A20MYC-KO mice (c) Representative histological images from forepaws (right panel; transversal sections) and hindpaws (left panel; sagittal sections) of mice with the indicated genotypes. Note the development of dactylitis in A20mZnF7/mZnF7 mice with a characteristic severe tenosynovitis leading to disruption of muscle/tendon fibres and the pannus orchestrating destruction of bones (arrow), all being more evident in the distal and intermediate (arrowhead) than in proximal phalanxes (scale bar: 1mm) (A20wt/mZnF7, n=8 and A20mZnF7/mZnF7, n=10 mice).
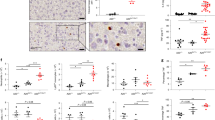
Supplementary Figure 6 Comparison of immune cell infiltration and inflammatory cytokine and chemokine expression in joints from A20MYC-KO and A20mZnF7/mZnF7 mice.
(a) Representative images of histological serial sections from the ankle joints of mice with the indicated genotypes, which were immunostained with the indicated antibodies (A20MYC-KO, n=6 and A20ZnF7 n=5 mice). Immune cell numbers were evaluated within the affected areas (dotted line). Data represents mean ± SEM (non-parametric Mann-Whitney test between indicated genotypes). (b) The mRNA expression of the indicated cytokines and chemokines was analyzed by qRT-PCR in RNA from hind paws of mice with the indicated genotypes. Each dot represents an individual mouse (A20wt/wt, n=9-10; A20wt/mZnF7, n=11-12 and A20MYC-KO, n=9 mice). Average ± SEM is also shown for each group of mice. *, ** and *** represent p<0.05, p<0.01 and p<0.001 respectively (Kruskal-Wallis one-way ANOVA test between indicated genotypes). All statistical tests are two-tailed. Raw data are provided in Supplementary Table 3.
Supplementary Figure 7 MyD88 deficiency prevents the development of arthritis and dactylitis in A20mZnF7/mZnF7 mice.
a) Graphs depicting the body weight (BW) of mice with the indicated genotypes at the age of 20-30 weeks. (b) Graph depicting spleen weight of mice with the indicated genotypes. (c) Representative pictures of forepaws and hindpaws of mice with the indicated genotypes at the age of 20 weeks. (d) Representative histological images of ankle joints from 20-30-week-old littermate mice with the indicated genotypes. Scale bar: 500 μm. Graph depicts histological scores for inflammation, bone erosion, and cartilage destruction in mice with the indicated genotypes. Dots in the graphs indicate individual mice (A20mZnF7/+ MyD88-/+ n=4; A20mZnF7/mZnF7 MyD88-/+ n=5; A20mZnF7/mZnF7 MyD88-/- n=3 mice for a,b; A20mZnF7/mZnF7 MyD88-/+ n=5; A20mZnF7/mZnF7 MyD88-/- n=5 mice for d). In all graphs average ± SEM is also shown for each group of mice. *, **, and *** represent p<0.05, p<0.01, and p<0.001 respectively (One-way ANOVA with Bonferroni correction for a; two-way ANOVA with Bonferroni correction for b). All statistical tests are two-tailed. Raw data are provided in Supplementary Table 3.
Supplementary Figure 8 Schematic model depicting the mechanisms regulating the pathogenesis of inflammatory arthritis in A20MYC-KO mice.
A20 prevents inflammasome activation and the release of mature IL-1β and IL-18 but also IL-1α in macrophages by inhibiting RIPK1-RIPK3-MLKL-dependent necroptosis. In A20-deficient macrophages, RIPK1-RIPK3-MLKL-dependent signalling causes necroptosis that results in the release of IL-1α and other DAMPs. In addition, MLKL-dependent plasma membrane permeabilization triggers K+ efflux, which activates the NLRP3 inflammasome and the caspase-1-dependent processing and subsequent release of IL-1β and IL-18. IL-1α and IL-1β, likely together with IL-18 and other DAMPs released by necroptotic A20-deficient macrophages activate MyD88-dependent proinflammatory signalling in synovial fibroblasts causing joint tissue inflammation, as well as cartilage and bone destruction resulting in the development of arthritis.
Supplementary Figure 9
Unprocessed immunoblots.
Supplementary information
Supplementary information
Supplementary Figures 1–9 and Supplementary Table titles/legends.
Supplementary Table 1
List of antibodies used.
Supplementary Table 2
List of primers using for qRT-PCR gene expression analysis.
Supplementary Table 3
Statistical source data.
Rights and permissions
About this article
Cite this article
Polykratis, A., Martens, A., Eren, R.O. et al. A20 prevents inflammasome-dependent arthritis by inhibiting macrophage necroptosis through its ZnF7 ubiquitin-binding domain. Nat Cell Biol 21, 731–742 (2019). https://doi.org/10.1038/s41556-019-0324-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41556-019-0324-3
This article is cited by
-
Cellular heterogeneity in TNF/TNFR1 signalling: live cell imaging of cell fate decisions in single cells
Cell Death & Disease (2024)
-
Ripks and Neuroinflammation
Molecular Neurobiology (2024)
-
Sinomenine ameliorates adjuvant-induced arthritis by inhibiting the autophagy/NETosis/inflammation axis
Scientific Reports (2023)
-
Roles of RIPK1 as a stress sentinel coordinating cell survival and immunogenic cell death
Nature Reviews Molecular Cell Biology (2023)
-
Necroptosis of macrophage is a key pathological feature in biliary atresia via GDCA/S1PR2/ZBP1/p-MLKL axis
Cell Death & Disease (2023)