Abstract
The biological and pathological importance of mutual interactions between the nervous system and cancer have become increasingly evident. The emerging field of cancer neuroscience aims to decipher key signalling factors of cancer–nervous system crosstalk and to exploit these modulators as targets for improved anticancer therapies. Here we discuss the key achievements in cancer neuroscience research, inspire further interactions on a variety of related research topics, and provide a roadmap for future studies.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout


Similar content being viewed by others
References
Hanahan, D. & Weinberg, R. A. Hallmarks of cancer: the next generation. Cell 144, 646–674 (2011).
Monje, M. et al. Roadmap for the emerging field of cancer neuroscience. Cell 181, 219–222 (2020).
Faulkner, S., Jobling, P., March, B., Jiang, C. C. & Hondermarck, H. Tumor neurobiology and the war of nerves in cancer. Cancer Discov. 9, 702–710 (2019).
Reiche, E. M., Nunes, S. O. & Morimoto, H. K. Stress, depression, the immune system and cancer. Lancet Oncol. 5, 617–625 (2004).
Li, L. & Hanahan, D. Hijacking the neuronal NMDAR signaling circuit to promote tumor growth and invasion. Cell 153, 86–100 (2013).
Li, L. et al. GKAP acts as a genetic modulator of NMDAR signaling to govern invasive tumor growth. Cancer Cell 33, 736–751 (2018).
Venkatesh, H. S. et al. Neuronal activity promotes glioma growth through neuroligin-3 secretion. Cell 161, 803–816 (2015).
Venkatesh, H. S. et al. Targeting neuronal activity-regulated neuroligin-3 dependency in high-grade glioma. Nature 549, 533–537 (2017).
Venkataramani, V. et al. Glutamatergic synaptic input to glioma cells drives brain tumour progression. Nature 573, 532–538 (2019).
Venkatesh, H. S. et al. Electrical and synaptic integration of glioma into neural circuits. Nature 573, 539–545 (2019).
Zeng, Q. et al. Synaptic proximity enables NMDAR signalling to promote brain metastasis. Nature 573, 526–531 (2019).
Latario, C. J. et al. Tumor microtubes connect pancreatic cancer cells in an Arp2/3 complex-dependent manner. Mol. Biol. Cell 31, 1259–1272 (2020).
Winkler, F. & Wick, W. Harmful networks in the brain and beyond. Science 359, 1100–1101 (2018).
Osswald, M. et al. Brain tumour cells interconnect to a functional and resistant network. Nature 528, 93–98 (2015).
Weil, S. et al. Tumor microtubes convey resistance to surgical lesions and chemotherapy in gliomas. Neuro Oncol. 19, 1316–1326 (2017).
Jung, E. et al. Tumor cell plasticity, heterogeneity and resistance in crucial microenvironmental niches in glioma. Nat. Commun. 12, 1014 (2021).
Jung, E. et al. Tweety-homolog 1 drives brain colonization of gliomas. J. Neurosci. 37, 6837–6850 (2017).
Magnon, C. et al. Autonomic nerve development contributes to prostate cancer progression. Science 341, 1236361 (2013).
Magnon, C. Role of the autonomic nervous system in tumorigenesis and metastasis. Mol. Cell Oncol. 2, e975643 (2015).
Renz, B. W. et al. β2 adrenergic-neurotrophin feedforward loop promotes pancreatic cancer. Cancer Cell 33, 75–90 e7 (2018).
Kamiya, A. et al. Genetic manipulation of autonomic nerve fiber innervation and activity and its effect on breast cancer progression. Nat. Neurosci. 22, 1289–1305 (2019).
Gillespie, S. & Monje, M. The neural regulation of cancer. Annu. Rev. Cancer Biol. 4, 371–390 (2020).
Zahalka, A. H. & Frenette, P. S. Nerves in cancer. Nat. Rev. Cancer 20, 143–157 (2020).
Pundavela, J. et al. Nerve fibers infiltrate the tumor microenvironment and are associated with nerve growth factor production and lymph node invasion in breast cancer. Mol. Oncol. 9, 1626–1635 (2015).
Yang, E. V. et al. Norepinephrine up-regulates the expression of vascular endothelial growth factor, matrix metalloproteinase (MMP)-2, and MMP-9 in nasopharyngeal carcinoma tumor cells. Cancer Res. 66, 10357–10364 (2006).
Azuma, H. et al. Gamma-aminobutyric acid as a promoting factor of cancer metastasis; induction of matrix metalloproteinase production is potentially its underlying mechanism. Cancer Res. 63, 8090–8096 (2003).
Bapat, A. A., Hostetter, G., Von Hoff, D. D. & Han, H. Perineural invasion and associated pain in pancreatic cancer. Nat. Rev. Cancer 11, 695–707 (2011).
Chen, S. H. et al. Perineural invasion of cancer: a complex crosstalk between cells and molecules in the perineural niche. Am. J. Cancer Res. 9, 1–21 (2019).
He, S. et al. GFRα1 released by nerves enhances cancer cell perineural invasion through GDNF-RET signaling. Proc. Natl Acad. Sci. USA 111, E2008–E2017 (2014).
Hirth, M. et al. CXCL10 and CCL21 promote migration of pancreatic cancer cells toward sensory neurons and neural remodeling in tumors in mice, associated with pain in patients. Gastroenterology 159, 665–681 (2020).
Deborde, S. et al. Schwann cells induce cancer cell dispersion and invasion. J. Clin. Invest. 126, 1538–1554 (2016).
Cavel, O. et al. Endoneurial macrophages induce perineural invasion of pancreatic cancer cells by secretion of GDNF and activation of RET tyrosine kinase receptor. Cancer Res. 72, 5733–5743 (2012).
Selvaraj, D. et al. A functional role for VEGFR1 expressed in peripheral sensory neurons in cancer pain. Cancer Cell 27, 780–796 (2015).
Zahalka, A. H. et al. Adrenergic nerves activate an angio-metabolic switch in prostate cancer. Science 358, 321–326 (2017).
Huang, S. et al. Lymph nodes are innervated by a unique population of sensory neurons with immunomodulatory potential. Cell 184, 441–459 (2021).
Mohammadpour, H. et al. β2 adrenergic receptor-mediated signaling regulates the immunosuppressive potential of myeloid-derived suppressor cells. J. Clin. Invest. 129, 5537–5552 (2019).
Dubeykovskaya, Z. et al. Neural innervation stimulates splenic TFF2 to arrest myeloid cell expansion and cancer. Nat. Commun. 7, 10517 (2016).
Gao, X. et al. Nociceptive nerves regulate haematopoietic stem cell mobilization. Nature 589, 591–596 (2021).
Shwartz, Y. et al. Cell types promoting goosebumps form a niche to regulate hair follicle stem cells. Cell 182, 578–593 (2020).
Qin, E. Y. et al. Neural precursor-derived pleiotrophin mediates subventricular zone invasion by glioma. Cell 170, 845–859 (2017).
Pan, Y. et al. NF1 mutation drives neuronal activity-dependent initiation of optic glioma. Nature 594, 277–282 (2021).
Chen, P. et al. Olfactory sensory experience regulates gliomagenesis via neuronal IGF1. Nature 606, 550–556 (2022).
Hayakawa, Y. et al. Nerve growth factor promotes gastric tumorigenesis through aberrant cholinergic signaling. Cancer Cell 31, 21–34 (2017).
Zhao, C. M. et al. Denervation suppresses gastric tumorigenesis. Sci. Transl. Med. 6, 250ra115 (2014).
Saloman, J. L. et al. Ablation of sensory neurons in a genetic model of pancreatic ductal adenocarcinoma slows initiation and progression of cancer. Proc. Natl Acad. Sci. USA 113, 3078–3083 (2016).
Peterson, S. C. et al. Basal cell carcinoma preferentially arises from stem cells within hair follicle and mechanosensory niches. Cell Stem Cell 16, 400–412 (2015).
Dobrenis, K., Gauthier, L. R., Barroca, V. & Magnon, C. Granulocyte colony-stimulating factor off-target effect on nerve outgrowth promotes prostate cancer development. Int. J. Cancer 136, 982–988 (2015).
Stopczynski, R. E. et al. Neuroplastic changes occur early in the development of pancreatic ductal adenocarcinoma. Cancer Res. 74, 1718–1727 (2014).
Pundavela, J. et al. ProNGF correlates with Gleason score and is a potential driver of nerve infiltration in prostate cancer. Am. J. Pathol. 184, 3156–3162 (2014).
Mehlen, P., Delloye-Bourgeois, C. & Chedotal, A. Novel roles for Slits and Netrins: axon guidance cues as anticancer targets? Nat. Rev. Cancer 11, 188–197 (2011).
Mauffrey, P. et al. Progenitors from the central nervous system drive neurogenesis in cancer. Nature 569, 672–678 (2019).
Amit, M. et al. Loss of p53 drives neuron reprogramming in head and neck cancer. Nature 578, 449–454 (2020).
Huberfeld, G. & Vecht, C. J. Seizures and gliomas—towards a single therapeutic approach. Nat. Rev. Neurol. 12, 204–216 (2016).
Yu, K. et al. PIK3CA variants selectively initiate brain hyperactivity during gliomagenesis. Nature 578, 166–171 (2020).
Svoboda, K. & Yasuda, R. Principles of two-photon excitation microscopy and its applications to neuroscience. Neuron 50, 823–839 (2006).
Jain, R. et al. Visualizing murine breast and melanoma tumor microenvironment using intravital multiphoton microscopy. STAR Protoc. 2, 100722 (2021).
Entenberg, D. et al. A permanent window for the murine lung enables high-resolution imaging of cancer metastasis. Nat. Methods 15, 73–80 (2018).
Rakhilin, N. et al. An intravital window to image the colon in real time. Nat. Commun. 10, 5647 (2019).
Richardson, D. S. et al. Tissue clearing. Nat. Rev. Methods Primers 1, 84 (2021).
Ueda, H. R. et al. Tissue clearing and its applications in neuroscience. Nat. Rev. Neurosci. 21, 61–79 (2020).
Almagro, J., Messal, H. A., Zaw Thin, M., van Rheenen, J. & Behrens, A. Tissue clearing to examine tumour complexity in three dimensions. Nat. Rev. Cancer 21, 718–730 (2021).
Kubota, S. I. et al. Whole-body profiling of cancer metastasis with single-cell resolution. Cell Rep. 20, 236–250 (2017).
Cai, R. et al. Panoptic imaging of transparent mice reveals whole-body neuronal projections and skull-meninges connections. Nat. Neurosci. 22, 317–327 (2019).
Pan, C. et al. Deep learning reveals cancer metastasis and therapeutic antibody targeting in the entire body. Cell 179, 1661–1676 e19 (2019).
Demir, I. E. et al. Clinically actionable strategies for studying neural influences in cancer. Cancer Cell 38, 11–14 (2020).
Demir, I. E. et al. Future directions in preclinical and translational cancer neuroscience research. Nat. Cancer 1, 1027–1031 (2021).
Coarfa, C. et al. Influence of the neural microenvironment on prostate cancer. Prostate 78, 128–139 (2018).
Peixoto, R., Pereira, M. L. & Oliveira, M. Beta-blockers and cancer: where are we?. Pharmaceuticals (Basel) 13, 105 (2020).
Lei, Y. et al. Gold nanoclusters-assisted delivery of NGF siRNA for effective treatment of pancreatic cancer. Nat. Commun. 8, 15130 (2017).
Schneider, M. et al. Meclofenamate causes loss of cellular tethering and decoupling of functional networks in glioblastoma. Neuro. Oncol 23, 1885–1897 (2021).
Jung, E. et al. Emerging intersections between neuroscience and glioma biology. Nat. Neurosci. 22, 1951–1960 (2019).
Acknowledgements
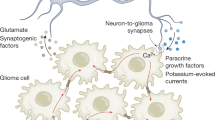
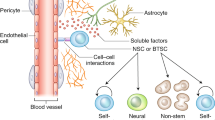
F.W. is supported by a grant from the Deutsche Forschungsgemeinschaft (SFB 1389) and by intramural funding from Heidelberg University (Cancer Neuroscience Initiative). C.P. is funded by the Deutsche Forschungsgemeinschaft (grant no. 451894423). Figures 1 and 2 are created with BioRender.com.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
F.W. is one of the inventors of patent WO 2017/020982A1 ‘Agents for use in the treatment of glioma’. This patent covers new treatment strategies that all target the formation and function of TMs in glioma. F.W. reports research collaboration with DC Europa Limited, Glaxo Smith Kline, Genentech and Boehringer, and is co-founder of Divide&Conquer. C.P. is one of the inventors of a patent on whole-body clearing and imaging-related technologies.
Peer review
Peer review information
Nature Cell Biology thanks Richard Wong and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Pan, C., Winkler, F. Insights and opportunities at the crossroads of cancer and neuroscience. Nat Cell Biol 24, 1454–1460 (2022). https://doi.org/10.1038/s41556-022-00978-w
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41556-022-00978-w
This article is cited by
-
Brain Tumor Networks in Diffuse Glioma
Neurotherapeutics (2022)