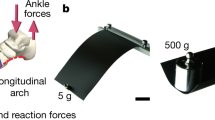
Abstract
The longitudinal arch of the human foot is viewed as a pivotal adaptation for bipedal walking and running. Fossil footprints from Laetoli, Tanzania, and Ileret, Kenya, are believed to provide direct evidence of longitudinally arched feet in hominins from the Pliocene and Pleistocene, respectively. We studied the dynamics of track formation using biplanar X-ray, three-dimensional animation and discrete element particle simulation. Here, we demonstrate that longitudinally arched footprints are false indicators of foot anatomy; instead they are generated through a specific pattern of foot kinematics that is characteristic of human walking. Analyses of fossil hominin tracks from Laetoli show only partial evidence of this walking style, with a similar heel strike but a different pattern of propulsion. The earliest known evidence for fully modern human-like bipedal kinematics comes from the early Pleistocene Ileret tracks, which were presumably made by members of the genus Homo. This result signals important differences in the foot kinematics recorded at Laetoli and Ileret and underscores an emerging picture of locomotor diversity within the hominin clade.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 digital issues and online access to articles
$119.00 per year
only $9.92 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
Data availability
Raw data from biplanar X-ray experiments are publicly available through the XMAPortal at the following link: https://xmaportal.org/webportal/larequest.php?request=CollectionView&StudyID=43&instit=BROWN&collectionID=20.
Code availability
Source data and code used to generate the figures in this manuscript are publicly available at the following address: https://doi.org/10.6084/m9.figshare.20736697.
References
Darwin, C. The Descent of Man, and Selection in Relation to Sex (J. Murray, 1871).
Morton, D. J. Evolution of the longitudinal arch of the human foot. J. Bone Jt Surg. 6, 56–90 (1924).
Holowka, N. B. & Lieberman, D. E. Rethinking the evolution of the human foot: insights from experimental research. J. Exp. Biol. 221, jeb174425 (2018).
Bramble, D. M. & Lieberman, D. E. Endurance running and the evolution of Homo. Nature 432, 345–352 (2004).
Leakey, M. D. & Hay, R. L. Pliocene footprints in the Laetolil Beds at Laetoli, northern Tanzania. Nature 278, 317–323 (1979).
Day, M. H. & Wickens, E. H. Laetoli Pliocene hominid footprints and bipedalism. Nature 286, 385–387 (1980).
White, T. D. & Suwa, G. Hominid footprints at Laetoli: facts and interpretations. Am. J. Phys. Anthropol. 72, 485–514 (1987).
Raichlen, D. A., Gordon, A. D., Harcourt-Smith, W. E. H., Foster, A. D. & Haas, W. R. Laetoli footprints preserve earliest direct evidence of human-like bipedal biomechanics. PLoS ONE 5, e9769 (2010).
Crompton, R. H. et al. Human-like external function of the foot, and fully upright gait, confirmed in the 3.66 million year old Laetoli hominin footprints by topographic statistics, experimental footprint-formation and computer simulation. J. R. Soc. Interface 9, 707–719 (2012).
Hatala, K. G., Demes, B. & Richmond, B. G. Laetoli footprints reveal bipedal gait biomechanics different from those of modern humans and chimpanzees. Proc. R. Soc. B 283, 20160235 (2016).
Bennett, M. R. et al. Early hominin foot morphology based on 1.5-million-year-old footprints from Ileret, Kenya. Science 323, 1197–1201 (2009).
Ward, C. V., Kimbel, W. H. & Johanson, D. C. Complete fourth metatarsal and arches in the foot of Australopithecus afarensis. Science 331, 750–753 (2011).
Pontzer, H. et al. Locomotor anatomy and biomechanics of the Dmanisi hominins. J. Hum. Evol. 58, 492–504 (2010).
Falkingham, P. L. & Gatesy, S. M. The birth of a dinosaur footprint: subsurface 3D motion reconstruction and discrete element simulation reveal track ontogeny. Proc. Natl Acad. Sci. USA 111, 18279–18284 (2014).
Falkingham, P. L., Turner, M. L. & Gatesy, S. M. Constructing and testing hypotheses of dinosaur foot motions from fossil tracks using digitization and simulation. Palaeontology 63, 865–880 (2020).
Hatala, K. G., Gatesy, S. M. & Falkingham, P. L. Integration of biplanar X-ray, three-dimensional animation and particle simulation reveals details of human ‘track ontogeny’. Interface Focus 11, 20200075 (2021).
Hatala, K. G. et al. Footprints reveal direct evidence of group behavior and locomotion in Homo erectus. Sci. Rep. 6, 28766 (2016).
Usherwood, J. R., Channon, A. J., Myatt, J. P., Rankin, J. W. & Hubel, T. Y. The human foot and heel–sole–toe walking strategy: a mechanism enabling an inverted pendular gait with low isometric muscle force? J. R. Soc. Interface 9, 2396–2402 (2012).
Webber, J. T. & Raichlen, D. A. The role of plantigrady and heel-strike in the mechanics and energetics of human walking with implications for the evolution of the human foot. J. Exp. Biol. 219, 3729–3737 (2016).
Masao, F. T. et al. New footprints from Laetoli (Tanzania) provide evidence for marked body size variation in early hominins. eLife 5, e19568 (2016).
Hatala, K. G. et al. Hominin track assemblages from Okote Member deposits near Ileret, Kenya, and their implications for understanding fossil hominin paleobiology at 1.5 Ma. J. Hum. Evol. 112, 93–104 (2017).
Morse, S. A. et al. Holocene footprints in Namibia: the influence of substrate on footprint variability. Am. J. Phys. Anthropol. 151, 265–279 (2013).
McNutt, E. J. et al. Footprint evidence of early hominin locomotor diversity at Laetoli, Tanzania. Nature 600, 468–471 (2021).
Zeininger, A., Schmitt, D. & Wunderlich, R. E. Mechanics of heel-strike plantigrady in African apes. J. Hum. Evol. 145, 102840 (2020).
Elftman, H. & Manter, J. Chimpanzee and human feet in bipedal walking. Am. J. Phys. Anthropol. 20, 69–79 (1935).
Latimer, B. & Lovejoy, C. O. The calcaneus of Australopithecus afarensis and its implications for the evolution of bipedality. Am. J. Phys. Anthropol. 78, 369–386 (1989).
Prang, T. C. Calcaneal robusticity in Plio-Pleistocene hominins: implications for locomotor diversity and phylogeny. J. Hum. Evol. 80, 135–146 (2015).
Fernández, P. J. et al. Evolution and function of the hominin forefoot. Proc. Natl Acad. Sci. USA 115, 8746–8751 (2018).
Venkadesan, M. et al. Stiffness of the human foot and evolution of the transverse arch. Nature 579, 97–100 (2020).
Latimer, B. & Lovejoy, C. O. Hallucal tarsometatarsal joint in Australopithecus afarensis. Am. J. Phys. Anthropol. 82, 125–133 (1990).
DeSilva, J. M., Gill, C. M., Prang, T. C., Bredella, M. A. & Alemseged, Z. A nearly complete foot from Dikika, Ethiopia and its implications for the ontogeny and function of Australopithecus afarensis. Sci. Adv. 4, eaar7723 (2018).
DeSilva, J. M. et al. Midtarsal break variation in modern humans: functional causes, skeletal correlates, and paleontological implications. Am. J. Phys. Anthropol. 156, 543–552 (2015).
Kelly, L. A., Cresswell, A. G., Racinais, S., Whiteley, R. & Lichtwark, G. Intrinsic foot muscles have the capacity to control deformation of the longitudinal arch. J. R. Soc. Interface 11, 20131188 (2014).
Kelly, L. A., Lichtwark, G. & Cresswell, A. G. Active regulation of longitudinal arch compression and recoil during walking and running. J. R. Soc. Interface 12, 20141076 (2015).
Holowka, N. B., Richards, A., Sibson, B. E. & Lieberman, D. E. The human foot functions like a spring of adjustable stiffness during running. J. Exp. Biol. 224, jeb219667 (2021).
Wood, B. & Collard, M. The human genus. Science 284, 65–71 (1999).
Brainerd, E. L. et al. X-ray reconstruction of moving morphology (XROMM): precision, accuracy and applications in comparative biomechanics research. J. Exp. Zool. 313A, 262–279 (2010).
Knörlein, B. J., Baier, D. B., Gatesy, S. M., Laurence-Chasen, J. D. & Brainerd, E. L. Validation of XMALab software for marker-based XROMM. J. Exp. Biol. 219, 3701–3711 (2016).
Kloss, C. & Goniva, C. in Supplemental Proceedings: Materials Fabrication, Properties, Characterization, and Modeling Vol. 2 (ed. TMS) 781–788 (John Wiley & Sons, 2011).
Stukowski, A. Visualization and analysis of atomistic simulation data with OVITO—the Open Visualization Tool. Model. Simul. Mater. Sci. Eng. 18, 015012 (2010).
R Core Team. R: A Language and Environment for Statistical Computing (R Foundation for Statistical Computing, 2019).
Wickham, H., François, R., Henry, L. & Müller, K. dplyr: A grammar of data manipulation. R package version 1.0.7 (2019).
Wickham, H. ggplot2: Elegant Graphics for Data Analysis (Springer-Verlag, 2016).
Acknowledgements
We thank D. Baier, B. Brainerd, S. Cheleden, F. Drury, K. Fiske, K. Huffman, B. Knörlein, D. Laidlaw, K. Tani Little, S. Megherhi, J. Novotny, D. North, M. Turner and the students of CS137 for assistance directly related to the design and implementation of this project. We thank the anonymous volunteers who participated in biplanar X-ray experiments. We are grateful to A. Manafzadeh for feedback at many stages of analysis. Discrete element simulations were made possible through a PRACE allocation of supercomputer resources (project 2021250007, Irene-Rome). This study received funding support from the National Science Foundation (BCS-1825403 to K.G.H. and P.L.F.; BCS-1824821 to S.M.G.) and from the Chatham University Research & Sabbatical Committee (to K.G.H.).
Author information
Authors and Affiliations
Contributions
All authors participated in the conceptualization, planning and administration of this project. K.G.H. and S.M.G. carried out biplanar X-ray experiments with input from P.L.F. P.L.F. carried out discrete element simulations with input from K.G.H. and S.M.G. All authors participated in analysing the data and in writing and editing the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Ecology & Evolution thanks the anonymous reviewers for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Extended data
Extended Data Fig. 2 Origins of kinematic hypotheses.
(a) Maya visualization of the foot at midstance directly above the track that this foot produced during a walking trial. (b) An ‘animation snapshot’ positioned at the same height as the foot in A, directly above the 3-D model of the track that this foot and its motion produced. The foot at midstance is relatively flat compared with the longitudinally arched track. However, the animation snapshot and the track are similarly arched. A sequence of similar observations led us to hypothesize that track arch morphology was a product of foot kinematics and not foot anatomy.
Extended Data Fig. 3 RAVs and morphologies of human and chimpanzee tracks.
(a) As an example we focus on one Laetoli track, one Walvis Bay track and one experimental chimpanzee track, with similar RAV and relative depth measurements (red circle). (b) The morphologies of the two hominin tracks and their respective arch models are similar to each other and readily distinguished from those of the bipedal chimpanzee (all models from right feet). The arch volume of hominin tracks is concentrated beneath the medial midfoot, while that of the bipedal chimpanzee track is concentrated distally, in between the first and second rays. Thus, the hominin tracks are arched longitudinally, while the bipedal chimpanzee track is not. Two different colour scales are applied to map relative heights, one for tracks and one for arch models, to optimize visualization of each set. Scale bar at right is 10 cm.
Extended Data Fig. 4 Photograph of trackway setup used for biplanar X-ray experiments.
Two X-ray emitters (foreground) project overlapping collimated X-rays that are received by two circular image intensifiers (background) equipped with video cameras. At the intersection of the biplanar X-ray beams is a container that is filled with mud (‘wet 5’ variety pictured here). Atop the remainder of the trackway is a deformable foam whose thickness matches the depth of mud within the container, which therefore allows subjects to sink to a similar extent with each step.
Extended Data Fig. 5 Interobserver variation in RAV measurements from track and foot 3-D models.
Paired observations and line of identity are plotted. Average interobserver difference was 0.42 (95% confidence interval of −1.00 to 0.15). RAV measurements were more consistent between observers for deeper tracks.
Supplementary information
Supplementary Information
Supplementary Notes 1–3.
Supplementary Video 1
Oblique isometric view of an animated foot (animated using biplanar X-ray experimental data) moving through a DEM-simulated mud. The foot is semitransparent, allowing for observation of foot–substrate interactions. Playback of simulation allows for visualization of continuous track arch formation throughout stance phase.
Supplementary Video 2
Cross-sectional view of simulated track formation. Same animation and simulation as presented in Supplementary Video 1 but with an opaque foot and with the simulated mud sectioned from heel-to-hallux, as in Fig. 2. This provides a more direct perspective for visualizing track arch formation over time. The track’s arch begins to form soon after heel strike and is continually shaped through push-off.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Hatala, K.G., Gatesy, S.M. & Falkingham, P.L. Arched footprints preserve the motions of fossil hominin feet. Nat Ecol Evol 7, 32–41 (2023). https://doi.org/10.1038/s41559-022-01929-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41559-022-01929-2