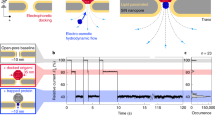
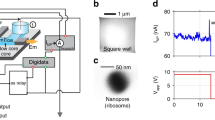
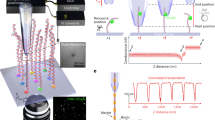
Abstract
Proteins, nucleic acids and ions secreted from single cells are the key signalling factors that determine the interaction of cells with their environment and the neighbouring cells. It is possible to study individual ion channels by pipette clamping, but it is difficult to dynamically monitor the activity of ion channels and transporters across the cellular membrane. Here we show that a solid-state nanopore integrated in an atomic force microscope can be used for the stochastic sensing of secreted molecules and the activity of ion channels in arbitrary locations both inside and outside a cell. The translocation of biomolecules and ions through the nanopore is observed in real time in live cells. The versatile nature of this approach allows us to detect specific biomolecules under controlled mechanical confinement and to monitor the ion-channel activities of single cells. Moreover, the nanopore microscope was used to image the surface of the nuclear membrane via high-resolution scanning ion conductance measurements.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
Data availability
All the data needed to evaluate the conclusions in the paper are present in the paper. Additional data and other findings of this study are available from the corresponding authors upon reasonable request.
References
Alberts, B., Johnson, A., Lewis, J., Raff, M., Roberts, K., Walter, P. Molecular Biology of the Cell (Garland Science, 2009).
Kollmannsberger, P., Bidan, C. M., Dunlop, J. W. C., Fratzl, P. & Vogel, V. Tensile forces drive a reversible fibroblast-to-myofibroblast transition during tissue growth in engineered clefts. Sci. Adv. 4, eaao4881 (2018).
Catterall, W. A. From ionic currents to molecular mechanisms: the structure and function of voltage-gated sodium channels. Neuron 26, 13–25 (2000).
Robert, C., Hue, I., McGraw, S., Gagné, D. & Sirard, M.-A. Quantification of cyclin B1 and p34(cdc2) in bovine cumulus–oocyte complexes and expression mapping of genes involved in the cell cycle by complementary DNA macroarrays. Biol. Reprod. 67, 1456–1464 (2002).
McDonald, M. P. et al. Visualizing single-cell secretion dynamics with single-protein sensitivity. Nano Lett. 18, 513–519 (2018).
Actis, P. et al. Compartmental genomics in living cells revealed by single-cell nanobiopsy. ACS Nano 8, 546–553 (2014).
Gatterdam, V. et al. Focal molography is a new method for the in situ analysis of molecular interactions in biological samples. Nat. Nanotechnol. 12, 1089–1095 (2017).
Li, X. et al. Label-free optofluidic nanobiosensor enables real-time analysis of single-cell cytokine secretion. Small 14, 1800698 (2018).
Kennedy, E. et al. Method for dynamically detecting secretions from single cells using a nanopore. Nano Lett. 18, 4263–4272 (2018).
Meister, A. et al. FluidFM: combining atomic force microscopy and nanofluidics in a universal liquid delivery system for single cell applications and beyond. Nano Lett. 9, 2501–2507 (2009).
Dorwling-Carter, L., Aramesh, M., Han, H., Zambelli, T. & Momotenko, D. Combined ion conductance and atomic force microscope for fast simultaneous topographical and surface charge imaging. Anal. Chem. 90, 11453–11460 (2018).
Li, J. et al. Ion-beam sculpting at nanometre length scales. Nature 412, 166–169 (2001).
Aramesh, M. Ion-beam sculpting of nanowires. Phys. Status Solidi Rapid Res. Lett. 12, 1700333 (2018).
Aramesh, M., Mayamei, Y., Wolff, A. & Ostrikov, K. Superplastic nanoscale pore shaping by ion irradiation. Nat. Commun. 9, 835 (2018).
Ossola, D. et al. Simultaneous scanning ion conductance microscopy and atomic force microscopy with microchanneled cantilevers. Phys. Rev. Lett. 115, 238103 (2015).
Lan, W. J., Kubeil, C., Xiong, J. W., Bund, A. & White, H. S. Effect of surface charge on the resistive pulse waveshape during particle translocation through glass nanopores. J. Phys. Chem. C 118, 2726–2734 (2014).
Ivanov, A. P. et al. On-demand delivery of single DNA molecules using nanopipets. ACS Nano 9, 3587–3594 (2015).
Klausen, L. H., Fuhs, T. & Dong, M. Mapping surface charge density of lipid bilayers by quantitative surface conductivity microscopy. Nat. Commun. 7, 12447 (2016).
Perry, D., Al Botros, R., Momotenko, D., Kinnear, S. L. & Unwin, P. R. Simultaneous nanoscale surface charge and topographical mapping. ACS Nano 9, 7266–7276 (2015).
Freedman, K. J. et al. Nanopore sensing at ultra-low concentrations using single-molecule dielectrophoretic trapping. Nat. Commun. 7, 10217 (2016).
Steinbock, L. J., Steinbock, J. F. & Radenovic, A. Controllable shrinking and shaping of glass nanocapillaries under electron irradiation. Nano Lett. 13, 1717–1723 (2013).
Dorwling-Carter, L. et al. Simultaneous scanning ion conductance and atomic force microscopy with a nanopore: effect of the aperture edge on the ion current images. J. Appl. Phys. 124, 174902 (2018).
Nadappuram, B. P. et al. Nanoscale tweezers for single-cell biopsies. Nat. Nanotechnol. 14, 80 (2018).
Pud, S. et al. Mechanical trapping of DNA in a double-nanopore system. Nano Lett. 16, 8021–8028 (2016).
Wanunu, M., Morrison, W., Rabin, Y., Grosberg, A. Y. & Meller, A. Electrostatic focusing of unlabelled DNA into nanoscale pores using a salt gradient. Nat. Nanotechnol. 5, 160–165 (2010).
Feng, J. et al. Identification of single nucleotides in MoS2 nanopores. Nat. Nanotechnol. 10, 1070–1076 (2015).
Briggs, K. et al. DNA translocations through nanopores under nanoscale preconfinement. Nano Lett. 18, 660–668 (2018).
Maglia, G., Restrepo, M. R., Mikhailova, E. & Bayley, H. Enhanced translocation of single DNA molecules through ɑ-hemolysin nanopores by manipulation of internal charge. Proc. Natl Acad. Sci. USA 105, 19720–19725 (2008).
Kasianowicz, J. J., Brandin, E., Branton, D. & Deamer, D. W. Characterization of individual polynucleotide molecules using a membrane channel. Proc. Natl Acad. Sci. USA 93, 13770–13773 (1996).
Meller, A., Nivon, L. & Branton, D. Voltage-driven DNA translocations through a nanopore. Phys. Rev. Lett. 86, 3435–3438 (2001).
Balme, S. et al. Influence of adsorption on proteins and amyloid detection by silicon nitride nanopore. Langmuir 32, 8916–8925 (2016).
Toyoda, Y. et al. Genome-scale single-cell mechanical phenotyping reveals disease-related genes involved in mitotic rounding. Nat. Commun. 8, 1266 (2017).
Klotzsch, E., Stiegler, J., Ben-Ishay, E. & Gaus, K. Do mechanical forces contribute to nanoscale membrane organisation in T cells? Biochim. Biophys. Acta Mol. Cell Res. 1853, 822–829 (2015).
Karner, A. et al. Tuning membrane protein mobility by confinement into nanodomains. Nat. Nanotechnol. 12, 260–266 (2017).
Aramesh, M., Shimoni, O., Ostrikov, K., Prawer, S. & Cervenka, J. Surface charge effects in protein adsorption on nanodiamonds. Nanoscale 7, 5726–5736 (2015).
Aramesh, M., Tran, P. A., Ostrikov, K. & Prawer, S. Conformal nanocarbon coating of alumina nanocrystals for biosensing and bioimaging. Carbon 122, 422–427 (2017).
Konradi, R., Pidhatika, B., Mühlebach, A. & Textor, M. Poly-2-methyl-2-oxazoline: a peptide-like polymer for protein-repellent surfaces. Langmuir 24, 613–616 (2008).
Weydert, S. et al. Easy to apply polyoxazoline-based coating for precise and long-term control of neural patterns. Langmuir 33, 8594–8605 (2017).
Yusko, E. C. et al. Real-time shape approximation and fingerprinting of single proteins using a nanopore. Nat. Nanotechnol. 12, 360–367 (2017).
Blondel, V. D., Guillaume, Jean-Loup, Lambiotte, R. & Lefebvre, E. Fast unfolding of communities in large networks. J. Stat. Mech. Theory Exp. 2008, P10008 (2008).
Sakai, T. et al. Plasma fibronectin supports neuronal survival and reduces brain injury following transient focal cerebral ischemia but is not essential for skin-wound healing and hemostasis. Nat. Med. 7, 324–330 (2001).
Ruoslahti, E. Fibronectin and its receptors. Annu. Rev. Biochem. 57, 375–413 (1988).
Gottlieb, P. A. in Current Topics in Membranes (ed. Gottlieb, P. A.) 1–36 (Current Topics in Membranes Vol. 79, Academic, 2017).
Guillaume-Gentil, O. et al. Force-controlled fluidic injection into single cell nuclei. Small 9, 1904–1907 (2013).
Larkin, J., Henley, R. Y., Muthukumar, M., Rosenstein, J. K. & Wanunu, M. High-bandwidth protein analysis using solid-state nanopores. Biophys. J. 106, 696–704 (2014).
Wei, R., Gatterdam, V., Wieneke, R., Tampé, R. & Rant, U. Stochastic sensing of proteins with receptor-modified solid-state nanopores. Nat. Nanotechnol. 7, 257–263 (2012).
Bell, N. A. W. & Keyser, U. F. Digitally encoded DNA nanostructures for multiplexed, single-molecule protein sensing with nanopores. Nat. Nanotechnol. 11, 645–651 (2016).
Ren, R. et al. Nanopore extended field-effect transistor for selective single-molecule biosensing. Nat. Commun. 8, 586 (2017).
Guillaume-Gentil, O. et al. Tunable single-cell extraction for molecular analyses. Cell 166, 506–517 (2016).
Misiunas, K., Ermann, N. & Keyser, U. F. QuipuNet: convolutional neural network for single-molecule nanopore sensing. Nano Lett. 18, 4040–4045 (2018).
Acknowledgements
This project received funding from the European Union’s Horizon 2020 research and innovation programme under the Marie Skłodowska-Curie Individual Fellowship (project reference 706930) and the Career Seed Grant from ETH Zürich (project reference 0-20440-18) to M.A. The work was partially funded by the EUROSTARS project grant E!11644. D.M. is supported by the Swiss National Science Foundation Ambizione grant PZ00P2_174217/1. I.S. is thankful to Empa for financial support and to the Swiss National Science Foundation for support in equipment procurement (R’Equip 206021_133823). This work was supported by the Human Frontiers Science Program RGY0065/2017 to E.K.
The authors acknowledge the valuable and insightful discussions with C. Frei, V. Gatterdam, O. Guillaume-Gentil and V. Vogel. We are grateful to members of our laboratories for assistance during the project. We appreciate the technical assistance from S. Wheeler. The PAcrAm-g-(PMOXA, NH2, Si) polymer was a kind donation of SuSoS AG, Switzerland. We are grateful to the Martinac lab and M. Vassalli for valuable discussions and the provided materials. The authors acknowledge support of the Scientific Center for Optical and Electron Microscopy ScopeM of the Swiss Federal Institute of Technology ETHZ.
Author information
Authors and Affiliations
Contributions
M.A. and J.V. designed the experiments. M.A., C.F., L.D.-C., I.L., T.S., S.J.I., I.S. and V.H. performed the experiments. M.A., C.F. and S.J.I. performed the statistical analysis and coding with support from J.V. All the authors discussed the results and commented on the manuscript. M.A. wrote the manuscript with support from J.V.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Peer review information: Nature Nanotechnology thanks Sebastien Balme and other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Supplementary Information
Experimental, supplementary figures, notes and references.
Rights and permissions
About this article
Cite this article
Aramesh, M., Forró, C., Dorwling-Carter, L. et al. Localized detection of ions and biomolecules with a force-controlled scanning nanopore microscope. Nat. Nanotechnol. 14, 791–798 (2019). https://doi.org/10.1038/s41565-019-0493-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41565-019-0493-z
This article is cited by
-
Spatially multiplexed single-molecule translocations through a nanopore at controlled speeds
Nature Nanotechnology (2023)
-
Population distributions of single-cell adhesion parameters during the cell cycle from high-throughput robotic fluidic force microscopy
Scientific Reports (2022)
-
FluidFM for single-cell biophysics
Nano Research (2022)
-
The emerging landscape of single-molecule protein sequencing technologies
Nature Methods (2021)
-
Single-entity electrochemistry at confined sensing interfaces
Science China Chemistry (2020)