Abstract
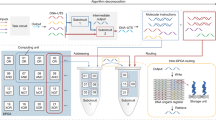
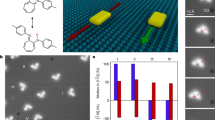
DNA has become the biomolecule of choice for molecular computation that may one day complement conventional silicon-based processors. In general, DNA computation is conducted in individual tubes, is slow in generating chemical outputs in response to chemical inputs and requires fluorescence readout. Here, we introduce a new paradigm for DNA computation where the chemical input is processed and transduced into a mechanical output using dynamic DNA-based motors operating far from equilibrium. We show that DNA-based motors with onboard logic (DMOLs) can perform Boolean functions (NOT, YES, AND and OR) with 15 min readout times. Because DMOLs are micrometre-sized, massive arrays of DMOLs that are identical or uniquely encoded by size and refractive index can be multiplexed and perform motor-to-motor communication on the same chip. Finally, DMOL computational outputs can be detected using a conventional smartphone camera, thus transducing chemical information into the electronic domain in a facile manner, suggesting potential applications.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
Data availability
Raw data acquisitions for Figs. 2–6, S3, S5, S6, and S10 can be found at https://doi.org/10.15139/S3/ZKRS8Z. Additional datasets generated in this study are available from the corresponding author upon reasonable request. Source data are provided with this paper.
Code availability
Python script from bright-field acquisition data regarding net displacements and particle ensemble trajectories can be found at https://github.com/spiranej/particle_tracking_.
References
Wadhams, G. H. & Armitage, J. P. Making sense of it all: bacterial chemotaxis. Nat. Rev. Mol. Cell Biol. 5, 1024–1037 (2004).
Srinivas, N. et al. On the biophysics and kinetics of toehold-mediated DNA strand displacement. Nucleic Acids Res. 41, 10641–10658 (2013).
Genot, A. J., Zhang, D. Y., Bath, J. & Turberfield, A. J. Remote toehold: a mechanism for flexible control of DNA hybridization kinetics. J. Am. Chem. Soc. 133, 2177–2182 (2011).
Yurke, B., Turberfield, A. J., Mills, A. P., Simmel, F. C. & Neumann, J. L. A DNA-fuelled molecular machine made of DNA. Nature 406, 605–608 (2000).
Zhang, D. Y. & Seelig, G. Dynamic DNA nanotechnology using strand-displacement reactions. Nat. Chem. 3, 103–113 (2011).
Dirks, R. M. & Pierce, N. A. Triggered amplification by hybridization chain reaction. Proc. Natl Acad. Sci. USA 101, 15275–15278 (2004).
Augspurger, E. E., Rana, M. & Yigit, M. V. Chemical and biological sensing using hybridization chain reaction. ACS Sens. 3, 878–902 (2018).
Ge, Z. et al. Hybridization chain reaction amplification of microRNA detection with a tetrahedral DNA nanostructure-based electrochemical biosensor. Anal. Chem. 86, 2124–2130 (2014).
Bi, S., Chen, M., Jia, X., Dong, Y. & Wang, Z. Hyperbranched hybridization chain reaction for triggered signal amplification and concatenated logic circuits. Angew. Chem. Int. Ed. 54, 8144–8148 (2015).
Qian, L. & Winfree, E. A simple DNA gate motif for synthesizing large-scale circuits. J. R. Soc. Interface 8, 1281–1297 (2011).
Qian, L. & Winfree, E. Scaling up digital circuit computation with DNA strand displacement cascades. Science 332, 1196–1201 (2011).
Song, X., Eshra, A., Dwyer, C. & Reif, J. Renewable DNA seesaw logic circuits enabled by photoregulation of toehold-mediated strand displacement. RSC Adv. 7, 28130–28144 (2017).
Benenson, Y. et al. Programmable and autonomous computing machine made of biomolecules. Nature 414, 430–434 (2001).
Seelig, G., Soloveichik, D., Zhang, D. Y. & Winfree, E. Enzyme-free nucleic acid logic circuits. Science 314, 1585–1588 (2006).
Thubagere, A. J. et al. A cargo-sorting DNA robot. Science 357, eaan6558 (2017).
Cherry, K. M. & Qian, L. Scaling up molecular pattern recognition with DNA-based winner-take-all neural networks. Nature 559, 370–376 (2018).
Zhou, C., Geng, H., Wang, P. & Guo, C. Programmable DNA nanoindicator‐based platform for large‐scale square root logic biocomputing. Small 15, 1903489 (2019).
Benenson, Y. Biomolecular computing systems: principles, progress and potential. Nat. Rev. Genet. 13, 455–468 (2012).
Wang, F. et al. Implementing digital computing with DNA-based switching circuits. Nat. Commun. 11, 121 (2020).
Wang, K. et al. Autonomous DNA nanomachine based on cascade amplification of strand displacement and DNA walker for detection of multiple DNAs. Biosens. Bioelectron. 105, 159–165 (2018).
You, M., Zhu, G., Chen, T., Donovan, M. J. & Tan, W. Programmable and multiparameter DNA-based logic platform for cancer recognition and targeted therapy. J. Am. Chem. Soc. 137, 667–674 (2015).
Zhu, J., Zhang, L., Zhou, Z., Dong, S. & Wang, E. Aptamer-based sensing platform using three-way DNA junction-driven strand displacement and its application in DNA logic circuit. Anal. Chem. 86, 312–316 (2014).
Chen, Y. et al. A DNA logic gate based on strand displacement reaction and rolling circle amplification, responding to multiple low-abundance DNA fragment input signals, and its application in detecting miRNAs. Chem. Commun. 51, 6980–6983 (2015).
Song, T. et al. Fast and compact DNA logic circuits based on single-stranded gates using strand-displacing polymerase. Nat. Nanotechnol. 14, 1075–1081 (2019).
Shah, S. et al. Using strand displacing polymerase to program chemical reaction networks. J. Am. Chem. Soc. 21, 9587–9593 (2020).
Douglas, S. M., Bachelet, I. & Church, G. M. A logic-gated nanorobot for targeted transport of molecular payloads. Science 335, 831–834 (2012).
Kang, H. et al. DNA dynamics and computation based on toehold-free strand displacement. Nat. Commun. 12, 4994 (2021).
Wang, D. et al. Molecular logic gates on DNA origami nanostructures for microRNA diagnostics. Anal. Chem. 86, 1932–1936 (2014).
Yehl, K. et al. High-speed DNA-based rolling motors powered by RNAseH. Nat. Nanotechnol. 11, 184–190 (2016).
Bazrafshan, A. et al. Tunable DNA origami motors translocate ballistically over μm distances at nm/s speeds. Angew. Chem. Int. Ed. 59, 9514–9521 (2020).
Credi, A., Balzani, V., Langford, S. J. & Stoddart, J. F. Logic operations at the molecular level. An XOR gate based on a molecular machine. J. Am. Chem. Soc. 119, 2679–2681 (1997).
Hu, L., Lu, C.-H. & Willner, I. Switchable catalytic DNA catenanes. Nano Lett. 15, 2099–2103 (2015).
Blanchard, A. T. et al. Highly polyvalent DNA motors generate 100+ pN of force via autochemophoresis. Nano Lett. 19, 6977–6986 (2019).
McKinnon, K. M. Flow cytometry: an overview. Curr. Protoc. Immunol. 120, 5.1.1–5.1.11 (2018).
Chatterjee, G., Dalchau, N., Muscat, R. A., Phillips, A. & Seelig, G. A spatially localized architecture for fast and modular DNA computing. Nat. Nanotechnol. 12, 920–927 (2017).
Vashist, S. K., Mudanyali, O., Schneider, E. M., Zengerle, R. & Ozcan, A. Cellphone-based devices for bioanalytical sciences. Anal. Bioanal. Chem. 406, 3263–3277 (2014).
Ghonge, T. et al. Smartphone-imaged microfluidic biochip for measuring CD64 expression from whole blood. Analyst 144, 3925–3935 (2019).
Acknowledgements
We acknowledge support through the following grants: NIH U01AA029345-01, NSF DMR 1905947 and NSF MSN 2004126. We thank S. Urazhdin for access to the thermal evaporator and W. Lam for cellscope.
Author information
Authors and Affiliations
Contributions
S.P. conceptualized the project, designed all experiments, analysed the data and compiled the figures. A.B. helped in the data analysis and discussion of the data. K.S. conceptualized and supervised the project. S.P. and K.S. wrote the manuscript with contributions from A.B.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Nanotechnology thanks Eyal Nir, Zhisong Wang and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Supplementary Information
Supplementary Figs. 1–10, Table 1 and captions for Videos 1–7.
Supplementary Video 1
Time-lapse videos of Cy3 (red) and Cy5 (blue) fluorescence channels overlaid, acquired at 5 s intervals for a duration of 15 min. The video was acquired ~30 min after RNaseH addition using a ×100 1.49 NA objective. YES-gated DMOLs modified with 10% staple-lock CED are shown translocating on a 1% surface-lock D* chip after the addition of 1 μM anti-lock DNA. Note that the Cy5 signal gradually bleaches over time. Scale bar, 10 μm.
Supplementary Video 2
Time-lapse videos of Cy3 (red), Cy5 (blue) and FAM (green) fluorescence channels overlaid, acquired at 5 s intervals for a duration of 15 min. The video was acquired ~30 min after RNaseH addition using a ×100 1.49 NA objective. AND-gated DMOLs modified with 50% staple-lock DNA (25% CED and 25% MND) are shown translocating on a 5% surface-lock D* chip after the addition of inputs A + B (1 μm each). Note that the Cy5 and FAM signals gradually bleach over time. Scale bar, 10 μm.
Supplementary Video 3
Time-lapse videos of Cy3 (red), Cy5 (blue) and FAM (green) fluorescence channels overlaid, acquired at 5 s intervals for a duration of 15 min. The video was acquired ~30 min after RNaseH addition using a ×100 1.49 NA objective. DMOL1 (located at the top of the frame) modified with 50% staple-lock CED is shown translocating on a 5% surface-lock DNA chip after the addition of input A. DMOL2 (located at the bottom of the frame) modified with 50% staple-lock (25% CED and 25% MND) is shown stalled on a 5% surface-lock D* chip after the addition of input A as it requires input A + B to unlock and translocate. Note that the Cy5 and FAM signals gradually bleach over time. Scale bar, 10 μm.
Supplementary Video 4
Representative time-lapse bright-field video acquired at 5 s intervals for a duration of 15 min using cellscope. DMOL2 (6 μm polystyrene), DMOL3 (3 μm polystyrene) and DMOL6 (5 μm silica) are shown. DMOLs were added to a 5% surface-lock D* chip and introduced to input A, which rescued the motion of DMOL3. Scale bar, 10 μm.
Supplementary Video 5
Representative time-lapse bright-field video acquired at 5 s intervals for a duration of 15 min using cellscope. DMOL2 (6 μm polystyrene), DMOL3 (3 μm polystyrene) and DMOL6 (5 μm silica) are shown. DMOLs were added to a 5% surface-lock D* chip and introduced to input A + B, which rescued the motion of DMOL2 and DMOL3. Scale bar, 10 μm.
Supplementary Video 6
Representative time-lapse bright-field video acquired at 5 s intervals for a duration of 15 min using cellscope. DMOL2 (6 μm polystyrene), DMOL3 (3 μm polystyrene) and DMOL6 (5 μm silica) are shown. DMOLs were added to a 5% surface-lock D* chip and introduced to input A + B + C, which rescued the motion of DMOL2 and DMOL3. Scale bar, 10 μm.
Supplementary Video 7
Representative time-lapse bright-field video acquired at 5 s intervals for a duration of 15 min using cellscope. DMOL2 (6 μm polystyrene), DMOL3 (3 μm polystyrene) and DMOL6 (5 μm silica) are shown. DMOLs were added to a 5% surface-lock D* chip and introduced to input A + B + C + F, which rescued the motion of DMOL2, DMOL3 and DMOL6. Scale bar, 10 μm.
Source data
Source Data Fig. 2
Statistical source data.
Source Data Fig. 3
Statistical source data.
Source Data Fig. 4
Statistical source data.
Source Data Fig. 5
Statistical source data.
Source Data Fig. 6
Statistical source data.
Rights and permissions
About this article
Cite this article
Piranej, S., Bazrafshan, A. & Salaita, K. Chemical-to-mechanical molecular computation using DNA-based motors with onboard logic. Nat. Nanotechnol. 17, 514–523 (2022). https://doi.org/10.1038/s41565-022-01080-w
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41565-022-01080-w
This article is cited by
-
DNA as a universal chemical substrate for computing and data storage
Nature Reviews Chemistry (2024)
-
A temporally resolved DNA framework state machine in living cells
Nature Machine Intelligence (2023)
-
High-throughput microbead assay system with a portable, cost-effective Wi-Fi imaging module, and disposable multi-layered microfluidic cartridges for virus and microparticle detection, and tracking
Biomedical Microdevices (2023)
-
General-purpose DNA computation
Science China Chemistry (2023)