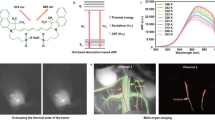
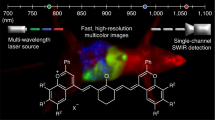
Abstract
Fluorescent nanosensors hold the potential to revolutionize life sciences and medicine. However, their adaptation and translation into the in vivo environment is fundamentally hampered by unfavourable tissue scattering and intrinsic autofluorescence. Here we develop wavelength-induced frequency filtering (WIFF) whereby the fluorescence excitation wavelength is modulated across the absorption peak of a nanosensor, allowing the emission signal to be separated from the autofluorescence background, increasing the desired signal relative to noise, and internally referencing it to protect against artefacts. Using highly scattering phantom tissues, an SKH1-E mouse model and other complex tissue types, we show that WIFF improves the nanosensor signal-to-noise ratio across the visible and near-infrared spectra up to 52-fold. This improvement enables the ability to track fluorescent carbon nanotube sensor responses to riboflavin, ascorbic acid, hydrogen peroxide and a chemotherapeutic drug metabolite for depths up to 5.5 ± 0.1 cm when excited at 730 nm and emitting between 1,100 and 1,300 nm, even allowing the monitoring of riboflavin diffusion in thick tissue. As an application, nanosensors aided by WIFF detect the chemotherapeutic activity of temozolomide transcranially at 2.4 ± 0.1 cm through the porcine brain without the use of fibre optic or cranial window insertion. The ability of nanosensors to monitor previously inaccessible in vivo environments will be important for life-sciences research, therapeutics and medical diagnostics.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
Data availability
Source data are available via Zenodo at https://doi.org/10.5281/zenodo.6049452. The data that support the findings of this study are available in the paper and Supplementary Information. All other data are available from the corresponding author upon reasonable request.
References
Aron, A. T., Ramos-Torres, K. M., Cotruvo, J. A. & Chang, C. J. Recognition- and reactivity-based fluorescent probes for studying transition metal signaling in living systems. Acc. Chem. Res. 48, 2434–2442 (2015).
Lin, V. S., Chen, W., Xian, M. & Chang, C. J. Chemical probes for molecular imaging and detection of hydrogen sulfide and reactive sulfur species in biological systems. Chem. Soc. Rev. 44, 4596–4618 (2015).
Cotruvo, J. J. A., Aron, A. T., Ramos-Torres, K. M. & Chang, C. J. Synthetic fluorescent probes for studying copper in biological systems. Chem. Soc. Rev. 44, 4400–4414 (2015).
Giuliano, K. A. & Taylor, D. L. Fluorescent-protein biosensors: new tools for drug discovery. Trends Biotechnol. 16, 135–140 (1998).
Wolff, M., Wiedenmann, J., Nienhaus, G. U., Valler, M. & Heilker, R. Novel fluorescent proteins for high-content screening. Drug Discov. Today 11, 1054–1060 (2006).
Bera, K. et al. Biosensors show the pharmacokinetics of S-ketamine in the endoplasmic reticulum. Front. Cell. Neurosci. 13, 499 (2019).
Chen, W.-T., Mahmood, U., Weissleder, R. & Tung, C.-H. Arthritis imaging using a near-infrared fluorescence folate-targeted probe. Arthritis Res. Ther. 7, R310 (2005).
Xie, D. Fluorescent dye labeled influenza virus mainly infects innate immune cells and activated lymphocytes and can be used in cell-mediated immune response assay. J. Immunol. Methods 343, 42–48 (2009).
Cruz Hernández, J. C. et al. Neutrophil adhesion in brain capillaries reduces cortical blood flow and impairs memory function in Alzheimer’s disease mouse models. Nat. Neurosci. 22, 413–420 (2019).
Lee, M. H. et al. Toward a chemical marker for inflammatory disease: a fluorescent probe for membrane-localized thioredoxin. J. Am. Chem. Soc. 136, 8430–8437 (2014).
Midde, K. et al. Single-cell imaging of metastatic potential of cancer cells. iScience 10, 53–65 (2018).
Mehrotra, P. Biosensors and their applications—a review. J. Oral Biol. Craniofac. Res. 6, 153–159 (2016).
Sieroń, A. et al. The role of fluorescence diagnosis in clinical practice. Onco Targets Ther. 6, 977–982 (2013).
Emanuel, G., Moffitt, J. R. & Zhuang, X. High-throughput, image-based screening of pooled genetic-variant libraries. Nat. Methods 14, 1159–1162 (2017).
Koch, M., Symvoulidis, P. & Ntziachristos, V. Tackling standardization in fluorescence molecular imaging. Nat. Photon. 12, 505–515 (2018).
Wang, S. et al. Anti-quenching NIR-II molecular fluorophores for in vivo high-contrast imaging and pH sensing. Nat. Commun. 10, 1058 (2019).
Zheng, X. et al. Successively activatable ultrasensitive probe for imaging tumour acidity and hypoxia. Nat. Biomed. Eng. 1, 0057 (2017).
Unruh, R. M. et al. Preclinical evaluation of poly(HEMA-co-acrylamide) hydrogels encapsulating glucose oxidase and palladium benzoporphyrin as fully implantable glucose sensors. J. Diabetes Sci. Technol. 9, 985–992 (2015).
Cash, K. J. & Clark, H. A. In vivo histamine optical nanosensors. Sensors 12, 11922–11932 (2012).
Yi, H. et al. M13 phage-functionalized single-walled carbon nanotubes as nanoprobes for second near-infrared window fluorescence imaging of targeted tumors. Nano Lett. 12, 1176–1183 (2012).
Yang, J. et al. Ultra-bright near-infrared-emitting HgS/ZnS core/shell nanocrystals for in vitro and in vivo imaging. J. Mater. Chem. B 3, 6928–6938 (2015).
Chen, G. et al. (α-NaYbF4:Tm3+)/CaF2 core/shell nanoparticles with efficient near-infrared to near-infrared upconversion for high-contrast deep tissue bioimaging. ACS Nano 6, 8280–8287 (2012).
Iverson, N. M. et al. In vivo biosensing via tissue-localizable near-infrared-fluorescent single-walled carbon nanotubes. Nat. Nanotechnol. 8, 873–880 (2013).
Bec, J. et al. In vivo label-free structural and biochemical imaging of coronary arteries using an integrated ultrasound and multispectral fluorescence lifetime catheter system. Sci. Rep. 7, 8960 (2017).
Boghossian, A. A. et al. Near-infrared fluorescent sensors based on single-walled carbon nanotubes for life sciences applications. ChemSusChem 4, 848–863 (2011).
Zhang, J. et al. Molecular recognition using corona phase complexes made of synthetic polymers adsorbed on carbon nanotubes. Nat. Nanotechnol. 8, 959–968 (2013).
Salem, D. P. et al. Ionic strength-mediated phase transitions of surface-adsorbed DNA on single-walled carbon nanotubes. J. Am. Chem. Soc. 139, 16791–16802 (2017).
Rieker, G. B., Jeffries, J. B. & Hanson, R. K. Measurements of high-pressure CO2 absorption near 2.0 μm and implications on tunable diode laser sensor design. Appl. Phys. B 94, 51–63 (2009).
Shimada, T. et al. Pharmacokinetic advantage of intraperitoneal injection of docetaxel in the treatment for peritoneal dissemination of cancer in mice. J. Pharm. Pharmacol. 57, 177–181 (2005).
Lu, Z., Wang, J., Wientjes, M. G. & Au, J. L. S. Intraperitoneal therapy for peritoneal cancer. Future Oncol. 6, 1625–1641 (2010).
Zempleni, J., Galloway, J. R. & McCormick, D. B. Pharmacokinetics of orally and intravenously administered riboflavin in healthy humans. Am. J. Clin. Nutr. 63, 54–66 (1996).
Park, M. et al. Measuring the accessible surface area within the nanoparticle corona using molecular probe adsorption. Nano Lett. 19, 7712–7724 (2019).
Koman, V. B., von Moos, N. R., Santschi, C., Slaveykova, V. I. & Martin, O. J. F. New insights into ROS dynamics: a multi-layered microfluidic chip for ecotoxicological studies on aquatic microorganisms. Nanotoxicology 10, 1041–1050 (2016).
Loock, H.-P. & Wentzell, P. D. Detection limits of chemical sensors: applications and misapplications. Sens. Actuators B 173, 157–163 (2012).
Krasnovsky, A. Jr. & Kovalev, Y. Spectral and kinetic parameters of phosphorescence of triplet chlorophyll a in the photosynthetic apparatus of plants. Biochemistry 79, 349–361 (2014).
Inoue, Y., Izawa, K., Kiryu, S., Tojo, A. & Ohtomo, K. Diet and abdominal autofluorescence detected by in vivo fluorescence imaging of living mice. Mol. Imaging 7, 7290.2008.0003 (2008).
Kim, H., Park, H. & Lee, S. J. Effective method for drug injection into subcutaneous tissue. Sci. Rep. 7, 9613 (2017).
Mortellaro, M. & DeHennis, A. Performance characterization of an abiotic and fluorescent-based continuous glucose monitoring system in patients with type 1 diabetes. Biosens. Bioelectron. 61, 227–231 (2014).
Rosenblum, D., Joshi, N., Tao, W., Karp, J. M. & Peer, D. Progress and challenges towards targeted delivery of cancer therapeutics. Nat. Commun. 9, 1410 (2018).
Fukui, H. Increased intestinal permeability and decreased barrier function: does it really influence the risk of inflammation? Inflamm. Intest. Dis. 1, 135–145 (2016).
Penet, M.-F. et al. Applications of molecular MRI and optical imaging in cancer. Future Med. Chem. 2, 975–988 (2010).
Gambhir, S. S. Molecular imaging of cancer with positron emission tomography. Nat. Rev. Cancer 2, 683–693 (2002).
Ibla, J. C. & Khoury, J. in Cell–Cell Interactions: Methods and Protocols Vol. 1066 (ed. Colgan, S. P.) 111–117 (Humana Press, 2006).
Jacques, S. L. Optical properties of biological tissues: a review. Phys. Med. Biol. 58, R37–R61 (2013).
Hambardzumyan, D. & Bergers, G. Glioblastoma: defining tumor niches. Trends Cancer 1, 252–265 (2015).
Stupp, R. et al. Radiotherapy plus concomitant and adjuvant temozolomide for glioblastoma. N. Engl. J. Med. 352, 987–996 (2005).
Friedman, H. S., Kerby, T. & Calvert, H. Temozolomide and treatment of malignant glioma. Clin. Cancer Res. 6, 2585–2597 (2000).
Holohan, C., Van Schaeybroeck, S., Longley, D. B. & Johnston, P. G. Cancer drug resistance: an evolving paradigm. Nat. Rev. Cancer 13, 714–726 (2013).
Portnow, J. et al. The neuropharmacokinetics of temozolomide in patients with resectable brain tumors: potential implications for the current approach to chemoradiation. Clin. Cancer Res. 15, 7092–7098 (2009).
Liu, H.-L. et al. Pharmacodynamic and therapeutic investigation of focused ultrasound-induced blood-brain barrier opening for enhanced temozolomide delivery in glioma treatment. PLoS ONE 9, e114311 (2014).
Kato, Y., Holm, D. A., Okollie, B. & Artemov, D. Noninvasive detection of temozolomide in brain tumor xenografts by magnetic resonance spectroscopy. Neuro. Oncol. 12, 71–79 (2010).
Arı, K. et al. 99mTc(I) carbonyl-radiolabeled lipid based drug carriers for temozolomide delivery and bioevaluation by in vitro and in vivo. Radiochim. Acta 107, 1185–1193 (2019).
Torricelli, A. et al. Time domain functional NIRS imaging for human brain mapping. NeuroImage 85, 28–50 (2014).
Strangman, G. E., Li, Z. & Zhang, Q. Depth sensitivity and source-detector separations for near infrared spectroscopy based on the Colin27 brain template. PLoS ONE 8, e66319 (2013).
Wellman, S. M. & Kozai, T. D. Y. Understanding the inflammatory tissue reaction to brain implants to improve neurochemical sensing performance. ACS Chem. Neurosci. 8, 2578–2582 (2017).
Dorand, R. D., Barkauskas, D. S., Evans, T. A., Petrosiute, A. & Huang, A. Y. Comparison of intravital thinned skull and cranial window approaches to study CNS immunobiology in the mouse cortex. IntraVital 3, e29728 (2014).
Wen, Q. et al. Association of diffusion and anatomic imaging parameters with survival for patients with newly diagnosed glioblastoma participating in two different clinical trials. Transl. Oncol. 8, 446–455 (2015).
Landry, M. P. et al. Experimental tools to study molecular recognition within the nanoparticle corona. Sensors 14, 16196–16211 (2014).
Hendler-Neumark, A. & Bisker, G. Fluorescent single-walled carbon nanotubes for protein detection. Sensors 19, 5403 (2019).
Landry, M. P. et al. Single-molecule detection of protein efflux from microorganisms using fluorescent single-walled carbon nanotube sensor arrays. Nat. Nanotechnol. 12, 368–377 (2017).
Jin, H. et al. Detection of single-molecule H2O2 signalling from epidermal growth factor receptor using fluorescent single-walled carbon nanotubes. Nat. Nanotechnol. 5, 302–309 (2010).
Bisker, G. et al. Insulin detection using a corona phase molecular recognition site on single-walled carbon nanotubes. ACS Sens. 3, 367–377 (2018).
Bisker, G. et al. Protein-targeted corona phase molecular recognition. Nat. Commun. 7, 10241 (2016).
Flock, S. T., Jacques, S. L., Wilson, B. C., Star, W. M. & van Gemert, M. J. C. Optical properties of intralipid: a phantom medium for light propagation studies. Lasers Surg. Med. 12, 510–519 (1992).
Koman, V. B., Santschi, C. & Martin, O. J. F. Maximal absorption regime in random media. Opt. Express 24, A1306–A1320 (2016).
Acknowledgements
The research is supported by the Koch Institute for Integrative Cancer Research at MIT and the Bridge Project Program. V.B.K. is supported by The Swiss National Science Foundation (project nos. P2ELP3_162149 and P300P2_174469). D.K. is supported by the Grant-in-Aid for JSPS Fellows (JSPS KAKENHI grant no. 15J07423) and Encouragement of Young Scientists (B) (JSPS KAKENHI grant no. JP16K17485) from the Japan Society for the Promotion of Science. X.J. is supported by the King Abdullah University of Science & Technology (OSR-2015 Sensors 2707). G.B. acknowledges support from the Zuckerman STEM Leadership Program and the Israeli Science Foundation (grant no. 456/18). F.T.N. is supported by the Arnold O. Beckman Postdoctoral Fellowship from the Arnold and Mabel Beckman Foundation. V.B.K. acknowledges helpful discussions with J. Yang.
Author information
Authors and Affiliations
Contributions
V.B.K. and M.S.S. conceived the idea and planned the experiments with the assistance of N.A.B., X.J. and F.T.N. V.B.K. developed the experimental setup, performed the in vitro experiments and analysed the data with the assistance of D.K., F.T.N., M.A.L., G.B. and J.D. F.T.N. and V.B.K. performed the tissue autofluorescence studies. N.A.B. and X.J. performed the ex vivo and in vivo experiments with the assistance of V.B.K. V.B.K. and M.S.S. wrote the manuscript with inputs from all the authors. All the authors contributed to discussions regarding the research.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Nanotechnology thanks Yun-Sheng Chen and Fan Zhang for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Supplementary Information
Supplementary Notes 1–5, Tables 1–4 and Figs. 1–35.
Rights and permissions
About this article
Cite this article
Koman, V.B., Bakh, N.A., Jin, X. et al. A wavelength-induced frequency filtering method for fluorescent nanosensors in vivo. Nat. Nanotechnol. 17, 643–652 (2022). https://doi.org/10.1038/s41565-022-01136-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41565-022-01136-x
This article is cited by
-
In-Vivo fluorescent nanosensor implants based on hydrogel-encapsulation: investigating the inflammation and the foreign-body response
Journal of Nanobiotechnology (2023)
-
Study of the durability and sustainability of fluorescent nanosensors based on cellulose nanocomposites incorporated with various carbon dots
Cellulose (2023)