Abstract
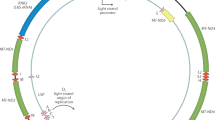
Variation in the mitochondrial DNA (mtDNA) sequence is common in certain tumours. Two classes of cancer mtDNA variants can be identified: de novo mutations that act as ‘inducers’ of carcinogenesis and functional variants that act as ‘adaptors’, permitting cancer cells to thrive in different environments. These mtDNA variants have three origins: inherited variants, which run in families, somatic mutations arising within each cell or individual, and variants that are also associated with ancient mtDNA lineages (haplogroups) and are thought to permit adaptation to changing tissue or geographic environments. In addition to mtDNA sequence variation, mtDNA copy number and perhaps transfer of mtDNA sequences into the nucleus can contribute to certain cancers. Strong functional relevance of mtDNA variation has been demonstrated in oncocytoma and prostate cancer, while mtDNA variation has been reported in multiple other cancer types. Alterations in nuclear DNA-encoded mitochondrial genes have confirmed the importance of mitochondrial metabolism in cancer, affecting mitochondrial reactive oxygen species production, redox state and mitochondrial intermediates that act as substrates for chromatin-modifying enzymes. Hence, subtle changes in the mitochondrial genotype can have profound effects on the nucleus, as well as carcinogenesis and cancer progression.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Warburg, O. The Metabolism of Tumors (ed. Smith, R. R.) (Springer, 1931).
Warburg, O. On the origin of cancer cells. Science 123, 309–314 (1956).
Pedersen, P. L. Tumor mitochondria and the bioenergetics of cancer cells. Prog. Exp. Tumor Res. 22, 190–274 (1978).
Anderson, S. et al. Sequence and organization of the human mitochondrial genome. Nature 290, 457–465 (1981). This study reports the human mtDNA sequence.
Andrews, R. M. et al. Reanalysis and revision of the Cambridge reference sequence for human mitochondrial DNA. Nat. Genet. 23, 147 (1999).
Bibb, M. J., Van Etten, R. A., Wright, C. T., Walberg, M. W. & Clayton, D. A. Sequence and gene organization of mouse mitochondrial DNA. Cell 26, 167–180 (1981).
Wallace, D. C. Mitochondrial genetic medicine. Nat. Genet. 50, 1642–1649 (2018).
Wallace, D. C. Mitochondrial DNA variation in human radiation and disease. Cell 163, 33–38 (2015).
Wallace, D. C. et al. Mitochondrial DNA mutation associated with Leber’s hereditary optic neuropathy. Science 242, 1427–1430 (1988). This study reports the first mtDNA nucleotide substitution disease.
Shoffner, J. M. et al. Myoclonic epilepsy and ragged-red fiber disease (MERRF) is associated with a mitochondrial DNA tRNALys mutation. Cell 61, 931–937 (1990).
Holt, I. J., Harding, A. E., Petty, R. K. & Morgan-Hughes, J. A. A new mitochondrial disease associated with mitochondrial DNA heteroplasmy. Am. J. Hum. Genet. 46, 428–433 (1990).
Cortopassi, G. A. & Arnheim, N. Detection of a specific mitochondrial DNA deletion in tissues of older humans. Nucleic Acids Res. 18, 6927–6933 (1990).
Corral-Debrinski, M. et al. Hypoxemia is associated with mitochondrial DNA damage and gene induction. Implications for cardiac disease. JAMA 266, 1812–1816 (1991).
Corral-Debrinski, M. et al. Mitochondrial DNA deletions in human brain: regional variability and increase with advanced age. Nat. Genet. 2, 324–329 (1992).
Mishmar, D. et al. Natural selection shaped regional mtDNA variation in humans. Proc. Natl Acad. Sci. USA 100, 171–176 (2003).
Ruiz-Pesini, E., Mishmar, D., Brandon, M., Procaccio, V. & Wallace, D. C. Effects of purifying and adaptive selection on regional variation in human mtDNA. Science 303, 223–226 (2004). This study reports that human mtDNA variants can be adaptive.
Brandon, M., Baldi, P. & Wallace, D. C. Mitochondrial mutations in cancer. Oncogene 25, 4647–4662 (2006). This article is the first proposal that cancer mtDNA mutations may be adaptive.
Petros, J. A. et al. mtDNA mutations increase tumorigenicity in prostate cancer. Proc. Natl Acad. Sci. USA 102, 719–724 (2005). This article shows that mtDNA mutations are important in prostate cancer.
Arnold, R. S. et al. An inherited heteroplasmic mutation in mitochondrial gene COI in a patient with prostate cancer alters reactive oxygen, reactive nitrogen and proliferation. Biomed. Res. Int. 2013, 239257 (2013).
Wallace, D. C., Fan, W. & Procaccio, V. Mitochondrial energetics and therapeutics. Annu. Rev. Path. 5, 297–348 (2010).
Dang, L. et al. Cancer-associated IDH1 mutations produce 2-hydroxyglutarate. Nature 462, 739–744 (2009).
Wallace, D. C. & Fan, W. Energetics, epigenetics, mitochondrial genetics. Mitochondrion 10, 12–31 (2010).
Letouze, E. et al. SDH mutations establish a hypermethylator phenotype in paraganglioma. Cancer Cell 23, 739–752 (2013).
Lu, C. et al. IDH mutation impairs histone demethylation and results in a block to cell differentiation. Nature 483, 474–478 (2012).
Picard, M. et al. Progressive increase in mtDNA 3243A>G heteroplasmy causes abrupt transcriptional reprogramming. Proc. Natl Acad. Sci. USA 111, E4033–E4042 (2014).
Kopinski, P. K. et al. Regulation of nuclear epigenome by mitochondrial DNA heteroplasmy. Proc. Natl Acad. Sci. USA 116, 16028–16035 (2019). This study is the first description of an mtDNA mutation causing changes in the nuclear epigenome.
MITOMAP. A Human Mitochondrial Genome Database. http://www.mitomap.org (2021).
Behar, D. M. et al. A “Copernican” reassessment of the human mitochondrial DNA tree from its root. Am. J. Hum. Genet. 90, 675–684 (2012).
Wallace, D. C. et al. Familial mitochondrial encephalomyopathy (MERRF): genetic, pathophysiological, and biochemical characterization of a mitochondrial DNA disease. Cell 55, 601–610 (1988).
Goto, Y., Nonaka, I. & Horai, S. A mutation in the tRNALeu(UUR) gene associated with the MELAS subgroup of mitochondrial encephalomyopathies. Nature 348, 651–653 (1990).
Holt, I. J., Harding, A. E. & Morgan-Hughes, J. A. Deletions of muscle mitochondrial DNA in patients with mitochondrial myopathies. Nature 331, 717–719 (1988).
Zeviani, M. et al. Deletions of mitochondrial DNA in Kearns-Sayre syndrome. Neurology 38, 1339–1346 (1988).
Shoffner, J. M. et al. Spontaneous Kearns-Sayre/chronic external ophthalmoplegia plus syndrome associated with a mitochondrial DNA deletion: a slip-replication model and metabolic therapy. Proc. Natl Acad. Sci. USA 86, 7952–7956 (1989).
Wallace, D. C., Ruiz-Pesini, E. & Mishmar, D. MtDNA variation, climatic adaptation, degenerative diseases, and longevity. Cold Spring Harb. Symp. Quant. Biol. 68, 479–486 (2003).
Mishmar, D., Ruiz-Pesini, E., Brandon, M. & Wallace, D. C. Mitochondrial DNA-like sequences in the nucleus (NUMTs): insights into our African origins and the mechanism of foreign DNA integration. Hum. Mutat. 23, 125–133 (2004).
Torroni, A. et al. MtDNA and the origin of Caucasians. Identification of ancient Caucasian-specific haplogroups, one of which is prone to a recurrent somatic duplication in the D-loop region. Am. J. Hum. Genet. 55, 760–776 (1994).
Torroni, A., Neel, J. V., Barrantes, R., Schurr, T. G. & Wallace, D. C. A mitochondrial DNA ‘clock’ for the Amerinds and its implication for timing their entry into North America. Proc. Natl Acad. Sci. USA 91, 1158–1162 (1994).
Torroni, A. et al. Classification of European mtDNAs from an analysis of three European populations. Genetics 144, 1835–1850 (1996).
Schurr, T. G. & Wallace, D. C. Mitochondrial DNA diversity in Southeast Asian populations. Hum. Biol. 74, 431–452 (2002).
Chen, Y. S., Torroni, A., Excoffier, L., Santachiara-Benerecetti, A. S. & Wallace, D. C. Analysis of mtDNA variation in African populations reveals the most ancient of all human continent-specific haplogroups. Am. J. Hum. Genet. 57, 133–149 (1995).
Chen, Y. S. et al. mtDNA variation in the South African Kung and Khwe and their genetic relationships to other African populations. Am. J. Hum. Genet. 66, 1362–1383 (2000).
Kazuno, A. A. et al. Mitochondrial DNA haplogroup analysis in patients with bipolar disorder. Am. J. Med. Genet. B Neuropsychiatr. Genet 150B, 243–247 (2009).
DeBerardinis, R. J. & Chandel, N. S. Fundamentals of cancer metabolism. Sci. Adv. 2, e1600200 (2016).
Courtney, K. D. et al. Isotope tracing of human clear cell renal cell carcinomas demonstrates suppressed glucose oxidation in vivo. Cell Metab. 28, 793–800 e792 (2018).
Stewart, T. A., Yapa, K. T. & Monteith, G. R. Altered calcium signaling in cancer cells. Biochim. Biophys. Acta. 1848, 2502–2511 (2015).
Huang, P., Feng, L., Oldham, E. A., Keating, M. J. & Plunkett, W. Superoxide dismutase as a target for the selective killing of cancer cells. Nature 407, 390–395 (2000).
Herrera-Cruz, M. S. & Simmen, T. Cancer: untethering mitochondria from the endoplasmic reticulum? Front. Oncol. 7, 105 (2017).
Verschoor, M. L. et al. Mitochondria and cancer: past, present, and future. Biomed. Res. Int. 2013, 612369 (2013).
Singh, K. K., Choudhury, A. R. & Tiwari, H. K. Numtogenesis as a mechanism for development of cancer. Semin. Cancer Biol. 47, 101–109 (2017).
Dayama, G., Emery, S. B., Kidd, J. M. & Mills, R. E. The genomic landscape of polymorphic human nuclear mitochondrial insertions. Nucleic Acids Res. 42, 12640–12649 (2014).
Leister, D. Origin, evolution and genetic effects of nuclear insertions of organelle DNA. Trends Genet. 21, 655–663 (2005).
Woischnik, M. & Moraes, C. T. Pattern of organization of human mitochondrial pseudogenes in the nuclear genome. Genome Res. 12, 885–893 (2002).
Hazkani-Covo, E., Zeller, R. M. & Martin, W. Molecular poltergeists: mitochondrial DNA copies (numts) in sequenced nuclear genomes. PLoS Genet. 6, e1000834 (2010).
Choudhury, A. R. & Singh, K. K. Mitochondrial determinants of cancer health disparities. Semin. Cancer Biol. 47, 125–146 (2017).
Ramos, A. et al. Nuclear insertions of mitochondrial origin: database updating and usefulness in cancer studies. Mitochondrion 11, 946–953 (2011).
Payne, B. A. et al. Universal heteroplasmy of human mitochondrial DNA. Hum. Mol. Genet. 22, 384–390 (2013).
Goto, H. et al. Dynamics of mitochondrial heteroplasmy in three families investigated via a repeatable re-sequencing study. Genome Biol. 12, R59 (2011).
Davis, R. E. et al. Mutations in mitochondrial cytochrome c oxidase genes segregate with late-onset Alzheimer disease. Proc. Natl Acad. Sci. USA 94, 4526–4531 (1997).
Wallace, D. C., Stugard, C., Murdock, D., Schurr, T. & Brown, M. D. Ancient mtDNA sequences in the human nuclear genome: a potential source of errors in identifying pathogenic mutations. Proc. Natl Acad. Sci. USA 94, 14900–14905 (1997).
Srinivasainagendra, V. et al. Migration of mitochondrial DNA in the nuclear genome of colorectal adenocarcinoma. Genome Med. 9, 31 (2017).
Ju, Y. S. et al. Frequent somatic transfer of mitochondrial DNA into the nuclear genome of human cancer cells. Genome Res. 25, 814–824 (2015). This study reports numtogenesis as a feature of cancer cells.
Shay, J. W., Baba, T., Zhan, Q. M., Kamimura, N. & Cuthbert, J. A. HeLaTG cells have mitochondrial DNA inserted into the c-myc oncogene. Oncogene 6, 1869–1874 (1991).
Gould, M. P. et al. PCR-free enrichment of mitochondrial DNA from human blood and cell lines for high quality Next-Generation DNA sequencing. PLoS ONE 10, e0139253 (2015).
Weerts, M. J. A. et al. Sensitive detection of mitochondrial DNA variants for analysis of mitochondrial DNA-enriched extracts from frozen tumor tissue. Sci. Rep. 8, 2261 (2018).
Kennedy, S. R. et al. Detecting ultralow-frequency mutations by duplex sequencing. Nat. Protoc. 9, 2586–2606 (2014).
Grandhi, S. et al. Heteroplasmic shifts in tumor mitochondrial genomes reveal tissue-specific signals of relaxed and positive selection. Hum. Mol. Genet. 26, 2912–2922 (2017). This study reports a survey of mtDNA variants in cancer.
Chinnery, P. F., Samuels, D. C., Elson, J. & Turnbull, D. M. Accumulation of mitochondrial DNA mutations in ageing, cancer, and mitochondrial disease: is there a common mechanism? Lancet 360, 1323–1325 (2002).
Copeland, W. C., Wachsman, J. T., Johnson, F. M. & Penta, J. S. Mitochondrial DNA alterations in cancer. Cancer Invest. 20, 557–569 (2002).
Gasparre, G. et al. Clonal expansion of mutated mitochondrial DNA is associated with tumor formation and complex I deficiency in the benign renal oncocytoma. Hum. Mol. Genet. 17, 986–995 (2008).
Bartoletti-Stella, A. et al. Mitochondrial DNA mutations in oncocytic adnexal lacrimal glands of the conjunctiva. Arch. Ophthalmol. 129, 664–666 (2011).
Pereira, L., Soares, P., Maximo, V. & Samuels, D. C. Somatic mitochondrial DNA mutations in cancer escape purifying selection and high pathogenicity mutations lead to the oncocytic phenotype: pathogenicity analysis of reported somatic mtDNA mutations in tumors. BMC Cancer 12, 53 (2012).
Gasparre, G. et al. An inherited mitochondrial DNA disruptive mutation shifts to homoplasmy in oncocytic tumor cells. Hum. Mutat. 30, 391–396 (2009). This study shows that mtDNA mutants are important in oncocytomas.
Gopal, R. K. et al. Early loss of mitochondrial complex I and rewiring of glutathione metabolism in renal oncocytoma. Proc. Natl Acad. Sci. USA 115, E6283–E6290 (2018).
Kalsbeek, A. M. F., Chan, E. K. F., Corcoran, N. M., Hovens, C. M. & Hayes, V. M. Mitochondrial genome variation and prostate cancer: a review of the mutational landscape and application to clinical management. Oncotarget 8, 71342–71357 (2017).
Kalsbeek, A. M. et al. Mutational load of the mitochondrial genome predicts pathological features and biochemical recurrence in prostate cancer. Aging 8, 2702–2712 (2016).
Hopkins, J. F. et al. Mitochondrial mutations drive prostate cancer aggression. Nat. Commun. 8, 656 (2017).
Yuan, Y. et al. Comprehensive molecular characterization of mitochondrial genomes in human cancers. Nat. Genet. 52, 342–352 (2020).
Bunn, C. L., Wallace, D. C. & Eisenstadt, J. M. Cytoplasmic inheritance of chloramphenicol resistance in mouse tissue culture cells. Proc. Natl Acad. Sci. USA 71, 1681–1685 (1974).
Wallace, D. C., Bunn, C. L. & Eisenstadt, J. M. Cytoplasmic transfer of chloramphenicol resistance in human tissue culture cells. J. Cell Biol. 67, 174–188 (1975).
Trounce, I. A., Kim, Y. L., Jun, A. S. & Wallace, D. C. Assessment of mitochondrial oxidative phosphorylation in patient muscle biopsies, lymphoblasts, and transmitochondrial cell lines. Methods Enzymol. 264, 484–509 (1996).
McCormick, E. M. et al. Specifications of the ACMG/AMP standards and guidelines for mitochondrial DNA variant interpretation. Hum. Mutat. 41, 2028–2057 (2020).
Ju, Y. S. et al. Origins and functional consequences of somatic mitochondrial DNA mutations in human cancer. eLife 3, e02935 (2014).
Anderson, A. P., Luo, X., Russell, W. & Yin, Y. W. Oxidative damage diminishes mitochondrial DNA polymerase replication fidelity. Nucleic Acids Res. 48, 817–829 (2020).
Trifunovic, A. et al. Premature ageing in mice expressing defective mitochondrial DNA polymerase. Nature 429, 417–423 (2004).
Kujoth, G. C. et al. Mitochondrial DNA mutations, oxidative stress, and apoptosis in mammalian aging. Science 309, 481–484 (2005).
Wallace, D. C. Mitochondria and cancer. Nat. Rev. Cancer 12, 685–698 (2012).
Xiao, J. et al. Mitochondrial biology and prostate cancer ethnic disparity. Carcinogenesis 39, 1311–1319 (2018).
Gopal, R. K. et al. Widespread chromosomal losses and mitochondrial DNA alterations as genetic drivers in Hurthle cell carcinoma. Cancer Cell 34, 242–255 e245 (2018).
Weerts, M. J. A., Smid, M., Foekens, J. A., Sleijfer, S. & Martens, J. W. M. Mitochondrial RNA expression and single nucleotide variants in association with clinical parameters in primary breast cancers. Cancers 10, E500 (2018).
Jimenez-Morales, S., Perez-Amado, C. J., Langley, E. & Hidalgo-Miranda, A. Overview of mitochondrial germline variants and mutations in human disease: focus on breast cancer (review). Int. J. Oncol. 53, 923–936 (2018).
Hardie, D. G. AMP-activated/SNF1 protein kinases: conserved guardians of cellular energy. Nat. Rev. Mol. Cell Biol. 8, 774–785 (2007).
Perrone, A. M. et al. Potential for mitochondrial DNA sequencing in the differential diagnosis of gynaecological malignancies. Int. J. Mol. Sci. 19, E2048 (2018).
Musicco, C. et al. Mitochondrial dysfunctions in type I endometrial carcinoma: exploring their role in oncogenesis and tumor progression. Int. J. Mol. Sci. 19, E2076 (2018).
Yuan, Y. et al. Nonsense and missense mutation of mitochondrial ND6 gene promotes cell migration and invasion in human lung adenocarcinoma. BMC Cancer 15, 346 (2015).
Li, N. et al. Dissecting the expression landscape of mitochondrial genes in lung squamous cell carcinoma and lung adenocarcinoma. Oncol. Lett. 16, 3992–4000 (2018).
Tyagi, A. et al. Pattern of mitochondrial D-loop variations and their relation with mitochondrial encoded genes in pediatric acute myeloid leukemia. Mutat. Res. 810, 13–18 (2018).
Kim, H. R. et al. Spectrum of mitochondrial genome instability and implication of mitochondrial haplogroups in Korean patients with acute myeloid leukemia. Blood Res. 53, 240–249 (2018).
Vidone, M. et al. A comprehensive characterization of mitochondrial DNA mutations in glioblastoma multiforme. Int. J. Biochem. Cell Biol. 63, 46–54 (2015).
Fischer, R. [On the histochemical demonstration of oxidative enzymes in oncocytes of different organs]. Virchows Arch. Pathol. Anat. Physiol. Klin. Med. 334, 445–452 (1961).
Gasparre, G. et al. Disruptive mitochondrial DNA mutations in complex I subunits are markers of oncocytic phenotype in thyroid tumors. Proc. Natl Acad. Sci. USA 104, 9001–9006 (2007).
Bonora, E. et al. Defective oxidative phosphorylation in thyroid oncocytic carcinoma is associated with pathogenic mitochondrial DNA mutations affecting complexes I and III. Cancer Res. 66, 6087–6096 (2006).
Kurschner, G. et al. Renal oncocytoma characterized by the defective complex I of the respiratory chain boosts the synthesis of the ROS scavenger glutathione. Oncotarget 8, 105882–105904 (2017).
Burdon, R. H. Superoxide and hydrogen peroxide in relation to mammalian cell proliferation. Free. Radic. Biol. Med. 18, 775–794 (1995).
Chouchani, E. T. et al. A unifying mechanism for mitochondrial superoxide production during ischemia-reperfusion injury. Cell Metab. 23, 254–263 (2016).
Arnold, R. S. et al. Bone metastasis in prostate cancer: recurring mitochondrial DNA mutation reveals selective pressure exerted by the bone microenvironment. Bone 78, 81–86 (2015). This study shows the recurrence of the adaptive 10398 variant in prostate cancer metastases.
Kalsbeek, A. M. F. et al. Altered mitochondrial genome content signals worse pathology and prognosis in prostate cancer. Prostate 78, 25–31 (2018).
Gupta, S. C. et al. Upsides and downsides of ROS for cancer: the roles of ROS in tumorigenesis, prevention, and therapy. Antioxid. Redox Signal. 16, 1295–1322 (2012).
Lander, H. M. An essential role for free radicals and derived species in signal transduction. FASEB J. 11, 118–124 (1997).
Chouchani, E. T. et al. Ischaemic accumulation of succinate controls reperfusion injury through mitochondrial ROS. Nature 515, 431–435 (2014).
Scialo, F. et al. Mitochondrial ROS produced via reverse electron transport extend animal lifespan. Cell Metab. 23, 725–734 (2016).
Slane, B. G. et al. Mutation of succinate dehydrogenase subunit C results in increased O2·−, oxidative stress, and genomic instability. Cancer Res. 66, 7615–7620 (2006).
Schriner, S. E. et al. Extension of murine life span by overexpression of catalase targeted to mitochondria. Science 308, 1909–1911 (2005).
Goh, J. et al. Mitochondrial targeted catalase suppresses invasive breast cancer in mice. BMC Cancer 11, 191 (2011). This study demonstrates that ROS can promote tumorigenesis.
Woo, D. K. et al. Mitochondrial genome instability and ROS enhance intestinal tumorigenesis in APC(Min/+) mice. Am. J. Pathol. 180, 24–31 (2012).
Sun, Q., Arnold, R. S., Sun, C. Q. & Petros, J. A. A mitochondrial DNA mutation influences the apoptotic effect of statins on prostate cancer. Prostate 75, 1916–1925 (2015).
Howell, A. N. & Sager, R. Tumorigenicity and its suppression in cybrids of mouse and Chinese hamster cell lines. Proc. Natl Acad. Sci. USA 75, 2358–2362 (1978).
Shidara, Y. et al. Positive contribution of pathogenic mutations in the mitochondrial genome to the promotion of cancer by prevention from apoptosis. Cancer Res. 65, 1655–1663 (2005).
Ishikawa, K. et al. ROS-generating mitochondrial DNA mutations can regulate tumor cell metastasis. Science 320, 661–664 (2008).
Sablina, A. A. et al. The antioxidant function of the p53 tumor suppressor. Nat. Med. 11, 1306–1313 (2005).
Nieborowska-Skorska, M. et al. Rac2-MRC-cIII-generated ROS cause genomic instability in chronic myeloid leukemia stem cells and primitive progenitors. Blood 119, 4253–4263 (2012).
Reznik, E. et al. Mitochondrial DNA copy number variation across human cancers. eLife 5, e10769 (2016).
West, A. P. & Shadel, G. S. Mitochondrial DNA in innate immune responses and inflammatory pathology. Nat. Rev. Immunol. 17, 363–375 (2017).
Ng, K. W., Marshall, E. A., Bell, J. C. & Lam, W. L. cGAS-STING and cancer: dichotomous roles in tumor immunity and development. Trends Immunol. 39, 44–54 (2018).
Guha, M. et al. Aggressive triple negative breast cancers have unique molecular signature on the basis of mitochondrial genetic and functional defects. Biochim. Biophys. Acta Mol. Basis Dis. 1864, 1060–1071 (2018).
Moreno-Loshuertos, R. et al. Differences in reactive oxygen species production explain the phenotypes associated with common mouse mitochondrial DNA variants. Nat. Genet. 38, 1261–1268 (2006).
Giordano, C. et al. Efficient mitochondrial biogenesis drives incomplete penetrance in Leber’s hereditary optic neuropathy. Brain 137, 335–353 (2014).
Ruiz-Pesini, E. & Wallace, D. C. Evidence for adaptive selection acting on the tRNA and rRNA genes of the human mitochondrial DNA. Hum. Mutat. 27, 1072–1081 (2006).
Canter, J. A., Kallianpur, A. R., Parl, F. F., Millikan, R. C. & Mitochondrial, D. N. A. G10398A polymorphism and invasive breast cancer in African-American women. Cancer Res. 65, 8028–8033 (2005).
Darvishi, K. et al. G10398A polymorphism imparts maternal haplogroup N a risk for breast and esophageal cancer. Cancer Lett. 249, 249–255 (2007).
Marom, S., Friger, M. & Mishmar, D. MtDNA meta-analysis reveals both phenotype specificity and allele heterogeneity: a model for differential association. Sci. Rep. 7, 43449 (2017).
Yu, Y. et al. Mitochondrial ND3 G10398A mutation: a biomarker for breast cancer. Genet. Mol. Res. 14, 17426–17431 (2015).
Fang, H. et al. Cancer type-specific modulation of mitochondrial haplogroups in breast, colorectal and thyroid cancer. BMC Cancer 10, 421 (2010).
Blein, S. et al. An original phylogenetic approach identified mitochondrial haplogroup T1a1 as inversely associated with breast cancer risk in BRCA2 mutation carriers. Breast Cancer Res. 17, 61 (2015).
Riley, J. S. et al. Mitochondrial inner membrane permeabilisation enables mtDNA release during apoptosis. EMBO J. 37, e99238 (2018).
Booker, L. M. et al. North American white mitochondrial haplogroups in prostate and renal cancer. J. Urol. 175, 468–472; discussion 472-473 (2006).
Cano, D. et al. Mitochondrial DNA haplogroups and susceptibility to prostate cancer in a Colombian population. ISRN Oncol. 2014, 530675 (2014).
Li, Y. et al. Association of genes, pathways, and haplogroups of the mitochondrial genome with the risk of colorectal cancer: the multiethnic cohort. PLoS ONE 10, e0136796 (2015).
Poynter, J. N. et al. Association between mitochondrial DNA haplogroup and myelodysplastic syndromes. Genes Chromosomes Cancer 55, 688–693 (2016).
Liu, V. W. et al. Mitochondrial DNA variant 16189T>C is associated with susceptibility to endometrial cancer. Hum. Mutat. 22, 173–174 (2003).
Zhang, J. et al. Strikingly higher frequency in centenarians and twins of mtDNA mutation causing remodeling of replication origin in leukocytes. Proc. Natl Acad. Sci. USA 100, 1116–1121 (2003).
Zhai, K., Chang, L., Zhang, Q., Liu, B. & Wu, Y. Mitochondrial C150T polymorphism increases the risk of cervical cancer and HPV infection. Mitochondrion 11, 559–563 (2011).
Kazuno, A. A. et al. Identification of mitochondrial DNA polymorphisms that alter mitochondrial matrix pH and intracellular calcium dynamics. PLoS Genet. 2, e128 (2006).
Ji, F. et al. Mitochondrial DNA variant associated with Leber hereditary optic neuropathy and high-altitude Tibetans. Proc. Natl Acad. Sci. USA 109, 7391–7396 (2012).
Vaupel, P. & Kelleher, D. K. Blood flow and oxygenation status of prostate cancers. Adv. Exp. Med. Biol. 765, 299–305 (2013).
Parrella, P. et al. Detection of mitochondrial DNA mutations in primary breast cancer and fine-needle aspirates. Cancer Res. 61, 7623–7626 (2001).
Kenney, M. C. et al. Molecular and bioenergetic differences between cells with African versus European inherited mitochondrial DNA haplogroups: Implications for population susceptibility to diseases. Biochim. Biophys. Acta. 1842, 208–219 (2014).
Zhong, Z. et al. New mitochondrial DNA synthesis enables NLRP3 inflammasome activation. Nature 560, 198–203 (2018).
Guha, M. et al. Mitochondrial retrograde signaling induces epithelial-mesenchymal transition and generates breast cancer stem cells. Oncogene 33, 5238–5250 (2014).
Amuthan, G. et al. Mitochondria-to-nucleus stress signaling induces phenotypic changes, tumor progression and cell invasion. EMBO J. 20, 1910–1920 (2001).
Guha, M., Srinivasan, S., Biswas, G. & Avadhani, N. G. Activation of a novel calcineurin-mediated insulin-like growth factor-1 receptor pathway, altered metabolism, and tumor cell invasion in cells subjected to mitochondrial respiratory stress. J. Biol. Chem. 282, 14536–14546 (2007).
Smith, A. L. et al. Age-associated mitochondrial DNA mutations cause metabolic remodelling that contributes to accelerated intestinal tumorigenesis. Nat. Cancer 1, 976–989 (2020).
Bardella, C., Pollard, P. J. & Tomlinson, I. SDH mutations in cancer. Biochim. Biophys. Acta. 1807, 1432–1443 (2011).
Picaud, S. et al. Structural basis of fumarate hydratase deficiency. J. Inherit. Metab. Dis. 34, 671–676 (2011).
Li, X., Egervari, G., Wang, Y., Berger, S. L. & Lu, Z. Regulation of chromatin and gene expression by metabolic enzymes and metabolites. Nat. Rev. Mol. Cell Biol. 19, 563–578 (2018).
Su, X., Wellen, K. E. & Rabinowitz, J. D. Metabolic control of methylation and acetylation. Curr. Opin. Chem. Biol. 30, 52–60 (2016).
Campbell, S. L. & Wellen, K. E. Metabolic signaling to the nucleus in cancer. Mol. Cell 71, 398–408 (2018).
Clapier, C. R., Iwasa, J., Cairns, B. R. & Peterson, C. L. Mechanisms of action and regulation of ATP-dependent chromatin-remodelling complexes. Nat. Rev. Mol. Cell Biol. 18, 407–422 (2017).
Balss, J. et al. Analysis of the IDH1 codon 132 mutation in brain tumors. Acta Neuropathol. 116, 597–602 (2008).
Watanabe, T., Nobusawa, S., Kleihues, P. & Ohgaki, H. IDH1 mutations are early events in the development of astrocytomas and oligodendrogliomas. Am. J. Pathol. 174, 1149–1153 (2009).
Yan, H. et al. IDH1 and IDH2 mutations in gliomas. N. Engl. J. Med. 360, 765–773 (2009).
Mardis, E. R. et al. Recurring mutations found by sequencing an acute myeloid leukemia genome. N. Engl. J. Med. 361, 1058–1066 (2009).
Losman, J. A. & Kaelin, W. G. Jr. What a difference a hydroxyl makes: mutant IDH, (R)-2-hydroxyglutarate, and cancer. Genes Dev. 27, 836–852 (2013).
Ward, P. S. et al. The common feature of leukemia-associated IDH1 and IDH2 mutations is a neomorphic enzyme activity converting alpha-ketoglutarate to 2-hydroxyglutarate. Cancer Cell 17, 225–234 (2010).
Xu, W. et al. Oncometabolite 2-hydroxyglutarate is a competitive inhibitor of alpha-ketoglutarate-dependent dioxygenases. Cancer Cell 19, 17–30 (2011).
Klose, R. J. et al. The transcriptional repressor JHDM3A demethylates trimethyl histone H3 lysine 9 and lysine 36. Nature 442, 312–316 (2006).
Shilatifard, A. Chromatin modifications by methylation and ubiquitination: implications in the regulation of gene expression. Annu. Rev. Biochem. 75, 243–269 (2006).
Turcan, S. et al. IDH1 mutation is sufficient to establish the glioma hypermethylator phenotype. Nature 483, 479–483 (2012).
Figueroa, M. E. et al. Leukemic IDH1 and IDH2 mutations result in a hypermethylation phenotype, disrupt TET2 function, and impair hematopoietic differentiation. Cancer Cell 18, 553–567 (2010). This study reports a link between IDH mutations and DNA methylation.
Losman, J. A. et al. (R)-2-hydroxyglutarate is sufficient to promote leukemogenesis and its effects are reversible. Science 339, 1621–1625 (2013).
Koivunen, P. et al. Transformation by the (R)-enantiomer of 2-hydroxyglutarate linked to EGLN activation. Nature 483, 484–488 (2012).
Rohle, D. et al. An inhibitor of mutant IDH1 delays growth and promotes differentiation of glioma cells. Science 340, 626–630 (2013).
Popovici-Muller, J. et al. Discovery of the first potent inhibitors of mutant IDH1 that lower tumor 2-HG in vivo. ACS Med. Chem. Lett. 3, 850–855 (2012).
Wang, F. et al. Targeted inhibition of mutant IDH2 in leukemia cells induces cellular differentiation. Science 340, 622–626 (2013).
Baysal, B. E. et al. Mutations in SDHD, a mitochondrial complex II gene, in hereditary paraganglioma. Science 287, 848–851 (2000). This study demonstrates the association of complex II mutations with hereditary paraganglioma.
Xiao, M. et al. Inhibition of alpha-KG-dependent histone and DNA demethylases by fumarate and succinate that are accumulated in mutations of FH and SDH tumor suppressors. Genes Dev. 26, 1326–1338 (2012).
Cervera, A. M., Bayley, J. P., Devilee, P. & McCreath, K. J. Inhibition of succinate dehydrogenase dysregulates histone modification in mammalian cells. Mol. Cancer 8, 89 (2009).
Epstein, A. C. et al. C. elegans EGL-9 and mammalian homologs define a family of dioxygenases that regulate HIF by prolyl hydroxylation. Cell 107, 43–54 (2001).
Yu, F., White, S. B., Zhao, Q. & Lee, F. S. HIF-1alpha binding to VHL is regulated by stimulus-sensitive proline hydroxylation. Proc. Natl Acad. Sci. USA 98, 9630–9635 (2001).
Enns, G. M. et al. Degree of glutathione deficiency and redox imbalance depend on subtype of mitochondrial disease and clinical status. PLoS ONE 9, e100001 (2014).
Selak, M. A. et al. Succinate links TCA cycle dysfunction to oncogenesis by inhibiting HIF-alpha prolyl hydroxylase. Cancer Cell 7, 77–85 (2005).
Bennett, B. D. et al. Absolute metabolite concentrations and implied enzyme active site occupancy in Escherichia coli. Nat. Chem. Biol. 5, 593–599 (2009).
Hirota, S. et al. Gain-of-function mutations of c-kit in human gastrointestinal stromal tumors. Science 279, 577–580 (1998).
Pantaleo, M. A. et al. SDHA loss-of-function mutations in KIT-PDGFRA wild-type gastrointestinal stromal tumors identified by massively parallel sequencing. J. Natl Cancer Inst. 103, 983–987 (2011).
Janeway, K. A. et al. Defects in succinate dehydrogenase in gastrointestinal stromal tumors lacking KIT and PDGFRA mutations. Proc. Natl Acad. Sci. USA 108, 314–318 (2011).
Miettinen, M. et al. Immunohistochemical loss of succinate dehydrogenase subunit A (SDHA) in gastrointestinal stromal tumors (GISTs) signals SDHA germline mutation. Am. J. Surg. Pathol. 37, 234–240 (2013).
Killian, J. K. et al. Succinate dehydrogenase mutation underlies global epigenomic divergence in gastrointestinal stromal tumor. Cancer Discov. 3, 648–657 (2013).
Cedar, H. & Bergman, Y. Linking DNA methylation and histone modification: patterns and paradigms. Nat. Rev. Genet. 10, 295–304 (2009).
Castro-Vega, L. J. et al. Germline mutations in FH confer predisposition to malignant pheochromocytomas and paragangliomas. Hum. Mol. Genet. 23, 2440–2446 (2014).
Wiese, M. & Bannister, A. J. Two genomes, one cell: mitochondrial-nuclear coordination via epigenetic pathways. Mol. Metab. 38, 100942 (2020).
Feinberg, A. P., Koldobskiy, M. A. & Gondor, A. Epigenetic modulators, modifiers and mediators in cancer aetiology and progression. Nat. Rev. Genet. 17, 284–299 (2016).
Smiraglia, D. J., Kulawiec, M., Bistulfi, G. L., Gupta, S. G. & Singh, K. K. A novel role for mitochondria in regulating epigenetic modification in the nucleus. Cancer Biol. Ther. 7, 1182–1190 (2008).
Meierhofer, D. et al. Mitochondrial DNA mutations in renal cell carcinomas revealed no general impact on energy metabolism. Br. J. Cancer 94, 268–274 (2006).
Wallace, D. C. Mitochondria and cancer: Warburg address. Cold Spring Harb. Symp. Quant. Biol. 70, 363–374 (2005).
Joshi, S. et al. The genomic landscape of renal oncocytoma identifies a metabolic barrier to tumorigenesis. Cell Rep. 13, 1895–1908 (2015).
Lott, M. T. et al. mtDNA variation and analysis using MITOMAP and MITOMASTER. Curr. Protoc. Bioinforma. https://doi.org/10.1002/0471250953.bi0123s44 (2013).
Attimonelli, M. et al. HmtDB, a human mitochondrial genomic resource based on variability studies supporting population genetics and biomedical research. BMC Bioinformatics 6 (Suppl. 4), S4 (2005).
Falk, M. J. et al. Mitochondrial Disease Sequence Data Resource (MSeqDR): A global grass-roots consortium to facilitate deposition, curation, annotation, and integrated analysis of genomic data for the mitochondrial disease clinical and research communities. Mol. Genet. Metab. 114, 388–396 (2015).
Landrum, M. J. et al. ClinVar: improving access to variant interpretations and supporting evidence. Nucleic Acids Res. 46, D1062–D1067 (2018).
Yao, Y. G., Kong, Q. P., Salas, A. & Bandelt, H. J. Pseudomitochondrial genome haunts disease studies. J. Med. Genet. 45, 769–772 (2008).
Schon, E. A., DiMauro, S. & Hirano, M. Human mitochondrial DNA: roles of inherited and somatic mutations. Nat. Rev. Genet. 13, 878–890 (2012).
Guo, Y., Li, J., Li, C. I., Shyr, Y. & Samuels, D. C. MitoSeek: extracting mitochondria information and performing high-throughput mitochondria sequencing analysis. Bioinformatics 29, 1210–1211 (2013).
Calabrese, C. et al. MToolBox: a highly automated pipeline for heteroplasmy annotation and prioritization analysis of human mitochondrial variants in high-throughput sequencing. Bioinformatics 30, 3115–3117 (2014).
Li, M., Schroeder, R., Ko, A. & Stoneking, M. Fidelity of capture-enrichment for mtDNA genome sequencing: influence of NUMTs. Nucleic Acids Res. 40, e137 (2012).
Gaidzik, V. I. et al. TET2 mutations in acute myeloid leukemia (AML): results from a comprehensive genetic and clinical analysis of the AML study group. J. Clin. Oncol. 30, 1350–1357 (2012).
Young, A. L., Baysal, B. E., Deb, A. & Young, W. F. Jr. Familial malignant catecholamine-secreting paraganglioma with prolonged survival associated with mutation in the succinate dehydrogenase B gene. J. Clin. Endocrinol. Metab. 87, 4101–4105 (2002).
Neumann, H. P. et al. Germ-line mutations in nonsyndromic pheochromocytoma. N. Engl. J. Med. 346, 1459–1466 (2002).
Maher, E. R. & Eng, C. The pressure rises: update on the genetics of phaeochromocytoma. Hum. Mol. Genet. 11, 2347–2354 (2002).
Stratakis, C. A. & Carney, J. A. The triad of paragangliomas, gastric stromal tumours and pulmonary chondromas (Carney triad), and the dyad of paragangliomas and gastric stromal sarcomas (Carney-Stratakis syndrome): molecular genetics and clinical implications. J. Intern. Med. 266, 43–52 (2009).
Belinsky, M. G., Rink, L. & von Mehren, M. Succinate dehydrogenase deficiency in pediatric and adult gastrointestinal stromal tumors. Front. Oncol. 3, 117 (2013).
Ricketts, C. J. et al. Succinate dehydrogenase kidney cancer: an aggressive example of the Warburg effect in cancer. J. Urol. 188, 2063–2071 (2012).
Schimke, R. N., Collins, D. L. & Stolle, C. A. Paraganglioma, neuroblastoma, and a SDHB mutation: Resolution of a 30-year-old mystery. Am. J. Med. Genet. A 152A, 1531–1535 (2010).
Grau, E. et al. There is no evidence that the SDHB gene is involved in neuroblastoma development. Oncol. Res. 15, 393–398 (2005).
Kim, S., Kim, D. H., Jung, W. H. & Koo, J. S. Succinate dehydrogenase expression in breast cancer. SpringerPlus 2, 299 (2013).
Pollard, P. J. et al. Accumulation of Krebs cycle intermediates and over-expression of HIF1alpha in tumours which result from germline FH and SDH mutations. Hum. Mol. Genet. 14, 2231–2239 (2005).
Lehtonen, R. et al. Biallelic inactivation of fumarate hydratase (FH) occurs in nonsyndromic uterine leiomyomas but is rare in other tumors. Am. J. Pathol. 164, 17–22 (2004).
Tomlinson, I. P. et al. Germline mutations in FH predispose to dominantly inherited uterine fibroids, skin leiomyomata and papillary renal cell cancer. Nat. Genet. 30, 406–410 (2002).
Wei, M. H. et al. Novel mutations in FH and expansion of the spectrum of phenotypes expressed in families with hereditary leiomyomatosis and renal cell cancer. J. Med. Genet. 43, 18–27 (2006).
Douwes Dekker, P. B. et al. SDHD mutations in head and neck paragangliomas result in destabilization of complex II in the mitochondrial respiratory chain with loss of enzymatic activity and abnormal mitochondrial morphology. J. Pathol. 201, 480–486 (2003).
Sciacovelli, M. et al. The mitochondrial chaperone TRAP1 promotes neoplastic growth by inhibiting succinate dehydrogenase. Cell Metab. 17, 988–999 (2013).
Isaacs, J. S. et al. HIF overexpression correlates with biallelic loss of fumarate hydratase in renal cancer: novel role of fumarate in regulation of HIF stability. Cancer Cell 8, 143–153 (2005).
Acknowledgements
This work was supported by a Howard Hughes Medical Institute Research Fellowship awarded to P.K.K., and US National Institutes of Health grants NS021328, MH108592 and OD010944, and US Department of Defense grants W81XWH-16-1-0401v and W81XWH-21-1-0128 awarded to D.C.W.
Author information
Authors and Affiliations
Contributions
P.K.K. and D.C.W. contributed to all aspects of the article. L.N.S., S.Z. and M.T.L. researched the literature and contributed to the discussion of content and reviewing or editing of the article.
Corresponding author
Ethics declarations
Competing interests
D.C.W. has associations with Pano Therapeutics and Medical Excellence Capital. The other authors declare no competing interests.
Additional information
Peer review information
Nature Reviews Cancer thanks L. Greaves and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Related links
ClinVar: https://www.ncbi.nlm.nih.gov/clinvar/
HmtDB: http://www.hmtdb.uniba.it
MITOMAP: https://mitomap.org
MSeqDR: https://MSeqDR.org
Glossary
- Heteroplasmy
-
The simultaneous presence of two or more mitochondrial DNA genotypes in one cell.
- Myoclonic epilepsy and ragged red fibre (MERRF) syndrome
-
A progressive disease including seizures, muscle spasm and weakness, lack of muscle control, abnormal sensations in limbs and fat growth (lipomas).
- Mitochondrial encephalomyopathy, lactic acidosis and stroke-like episodes (MELAS) syndrome
-
A heterogenous phenotype characterized by epilepsy or dementia, with characteristic elevated lactate levels in blood and cerebrospinal fluid, as well as episodes of selective loss of vision, sensation and speech.
- Leigh syndrome
-
Also known as subacute necrotizing encephalopathy, a disease of typically infantile onset with psychomotor retardation, floppiness, brainstem dysfunction and abnormal body movements.
- Homoplasmy
-
The presence of only one mitochondrial DNA genotype within one cell.
- Leber hereditary optic neuropathy
-
A disease of early adulthood with rapidly progressive central loss of vision sequentially in both eyes.
- Pearson syndrome
-
A disease of the newborn caused by mitochondrial DNA deletion presenting with anaemia, vomiting and failure to thrive.
- Kearns–Sayre syndrome
-
A disease with typical onset before the age of 20 years with abnormal pigmentation of the retina and progressive abnormal eye movements, as well as cardiac conduction abnormalities and cerebellar dysfunction.
- Chronic progressive external ophthalmoplegia
-
Slowly progressive weakness of extraocular eye muscles resulting in abnormal eye movements and lid lag.
- Numtogenesis
-
The transfer of mitochondrial DNA sequences into the nucleus.
- Transmitochondrial cybrid
-
A cell line in which the mitochondrial and mitochondrial DNA encompassed within an enucleated cytoplasmic fragment is fused to a recipient cell, resulting in a cytoplasmic hybrid (cybrid) with the nucleus of the recipient cell but the mitochondrial and mitochondrial DNA of the donor cell.
- Oncocytomas
-
Benign tumours harbouring a large numbers of cytoplasmic mitochondria.
Rights and permissions
About this article
Cite this article
Kopinski, P.K., Singh, L.N., Zhang, S. et al. Mitochondrial DNA variation and cancer. Nat Rev Cancer 21, 431–445 (2021). https://doi.org/10.1038/s41568-021-00358-w
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41568-021-00358-w
This article is cited by
-
Leveraging new methods for comprehensive characterization of mitochondrial DNA in esophageal squamous cell carcinoma
Genome Medicine (2024)
-
Role of reactive oxygen species in myelodysplastic syndromes
Cellular & Molecular Biology Letters (2024)
-
SUCLG1 restricts POLRMT succinylation to enhance mitochondrial biogenesis and leukemia progression
The EMBO Journal (2024)
-
OTUB1/NDUFS2 axis promotes pancreatic tumorigenesis through protecting against mitochondrial cell death
Cell Death Discovery (2024)
-
Construction and Analysis of a Mitochondrial Metabolism-Related Prognostic Model for Breast Cancer to Evaluate Survival and Immunotherapy
The Journal of Membrane Biology (2024)