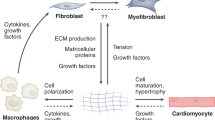
Abstract
The term ‘mechanosensation’ describes the capacity of cells to translate mechanical stimuli into the coordinated regulation of intracellular signals, cellular function, gene expression and epigenetic programming. This capacity is related not only to the sensitivity of the cells to tissue motion, but also to the decryption of tissue geometric arrangement and mechanical properties. The cardiac stroma, composed of fibroblasts, has been historically considered a mechanically passive component of the heart. However, the latest research suggests that the mechanical functions of these cells are an active and necessary component of the developmental biology programme of the heart that is involved in myocardial growth and homeostasis, and a crucial determinant of cardiac repair and disease. In this Review, we discuss the general concept of cell mechanosensation and force generation as potent regulators in heart development and pathology, and describe the integration of mechanical and biohumoral pathways predisposing the heart to fibrosis and failure. Next, we address the use of 3D culture systems to integrate tissue mechanics to mimic cardiac remodelling. Finally, we highlight the potential of mechanotherapeutic strategies, including pharmacological treatment and device-mediated left ventricular unloading, to reverse remodelling in the failing heart.
Key points
-
The sensing of mechanical tissue properties is a process related to cell differentiation, maturation and pathology in multicellular organs such as the heart.
-
Remodelling of the cardiac extracellular matrix, which occurs as a consequence of a pathological stimulus, induces changes in the mechanical properties of the myocardium.
-
Variations in the mechanical properties of the myocardium are related to the activation of pro-fibrotic cells (so-called myofibroblasts).
-
Mechanical cues can potentiate pro-fibrotic humoral signalling.
-
The identification of molecular pathways involved in mechanosensation of myofibroblasts facilitates the identification of therapeutic targets that can reverse mechanically induced pathological activation.
-
The possibility that interfering with mechanical cues in vivo might result in cardiac regeneration opens new therapeutic avenues in cardioprotection.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
References
Pinto, A. R. et al. Revisiting cardiac cellular composition. Circ. Res. 118, 400–409 (2016).
Litvinukova, M. et al. Cells of the adult human heart. Nature 588, 466–472 (2020).
Herum, K. M., Choppe, J., Kumar, A., Engler, A. J. & McCulloch, A. D. Mechanical regulation of cardiac fibroblast profibrotic phenotypes. Mol. Biol. Cell 28, 1871–1882 (2017).
Berry, M. F. et al. Mesenchymal stem cell injection after myocardial infarction improves myocardial compliance. Am. J. Physiol. Heart Circ. Physiol. 290, H2196–H2203 (2006).
Discher, D. E., Janmey, P. & Wang, Y. L. Tissue cells feel and respond to the stiffness of their substrate. Science 310, 1139–1143 (2005).
Downing, T. L. et al. Biophysical regulation of epigenetic state and cell reprogramming. Nat. Mater. 12, 1154–1162 (2013).
Van Linthout, S., Miteva, K. & Tschope, C. Crosstalk between fibroblasts and inflammatory cells. Cardiovasc. Res. 102, 258–269 (2014).
Steffens, S. et al. Stimulating pro-reparative immune responses to prevent adverse cardiac remodelling: consensus document from the joint 2019 meeting of the ESC Working Groups of Cellular Biology of the Heart and Myocardial Function. Cardiovasc. Res. 116, 1850–1862 (2020).
van Putten, S., Shafieyan, Y. & Hinz, B. Mechanical control of cardiac myofibroblasts. J. Mol. Cell. Cardiol. 93, 133–142 (2016).
Yu, J. et al. Topological arrangement of cardiac fibroblasts regulates cellular plasticity. Circ. Res. 123, 73–85 (2018).
Bracco Gartner, T. C. L. et al. Advanced in vitro modeling to study the paradox of mechanically induced cardiac fibrosis. Tissue Eng. Part. C. Methods 27, 100–114 (2021).
Majkut, S., Dingal, P. C. & Discher, D. E. Stress sensitivity and mechanotransduction during heart development. Curr. Biol. 24, R495–R501 (2014).
Andres-Delgado, L. & Mercader, N. Interplay between cardiac function and heart development. Biochim. Biophys. Acta 1863, 1707–1716 (2016).
Happe, C. L. & Engler, A. J. Mechanical forces reshape differentiation cues that guide cardiomyogenesis. Circ. Res. 118, 296–310 (2016).
Courchaine, K., Rykiel, G. & Rugonyi, S. Influence of blood flow on cardiac development. Prog. Biophys. Mol. Biol. 137, 95–110 (2018).
Tallquist, M. D. Developmental pathways of cardiac fibroblasts. Cold Spring Harb. Perspect. Biol. 12, a037184 (2020).
Majkut, S. et al. Heart-specific stiffening in early embryos parallels matrix and myosin expression to optimize beating. Curr. Biol. 23, 2434–2439 (2013).
Chiou, K. K. et al. Mechanical signaling coordinates the embryonic heartbeat. Proc. Natl Acad. Sci. USA 113, 8939–8944 (2016).
Matrone, G., Tucker, C. S. & Denvir, M. A. Cardiomyocyte proliferation in zebrafish and mammals: lessons for human disease. Cell. Mol. Life Sci. 74, 1367–1378 (2017).
Eulalio, A. et al. Functional screening identifies miRNAs inducing cardiac regeneration. Nature 492, 376–381 (2012).
Gabisonia, K. et al. MicroRNA therapy stimulates uncontrolled cardiac repair after myocardial infarction in pigs. Nature 569, 418–422 (2019).
Kennedy-Lydon, T. & Rosenthal, N. Cardiac regeneration: all work and no repair? Sci. Transl Med. 9, eaad9019 (2017).
Garcia-Gonzalez, C. & Morrison, J. I. Cardiac regeneration in non-mammalian vertebrates. Exp. Cell Res. 321, 58–63 (2014).
Poss, K. D., Wilson, L. G. & Keating, M. T. Heart regeneration in zebrafish. Science 298, 2188–2190 (2002).
Sanz-Morejon, A. & Mercader, N. Recent insights into zebrafish cardiac regeneration. Curr. Opin. Genet. Dev. 64, 37–43 (2020).
Yu, J. K. et al. Cardiac regeneration following cryoinjury in the adult zebrafish targets a maturation-specific biomechanical remodeling program. Sci. Rep. 8, 15661 (2018).
Sanchez-Iranzo, H. et al. Transient fibrosis resolves via fibroblast inactivation in the regenerating zebrafish heart. Proc. Natl Acad. Sci. USA 115, 4188–4193 (2018).
Ito, K. et al. Differential reparative phenotypes between zebrafish and medaka after cardiac injury. Dev. Dyn. 243, 1106–1115 (2014).
Porrello, E. R. et al. Transient regenerative potential of the neonatal mouse heart. Science 331, 1078–1080 (2011).
Chen, W. C. et al. Decellularized zebrafish cardiac extracellular matrix induces mammalian heart regeneration. Sci. Adv. 2, e1600844 (2016).
Wang, Z. et al. Decellularized neonatal cardiac extracellular matrix prevents widespread ventricular remodeling in adult mammals after myocardial infarction. Acta Biomater. 87, 140–151 (2019).
Missinato, M. A., Tobita, K., Romano, N., Carroll, J. A. & Tsang, M. Extracellular component hyaluronic acid and its receptor Hmmr are required for epicardial EMT during heart regeneration. Cardiovasc. Res. 107, 487–498 (2015).
Wang, J., Karra, R., Dickson, A. L. & Poss, K. D. Fibronectin is deposited by injury-activated epicardial cells and is necessary for zebrafish heart regeneration. Dev. Biol. 382, 427–435 (2013).
Kuhn, B. et al. Periostin induces proliferation of differentiated cardiomyocytes and promotes cardiac repair. Nat. Med. 13, 962–969 (2007).
Garcia-Puig, A. et al. Proteomics analysis of extracellular matrix remodeling during zebrafish heart regeneration. Mol. Cell Proteom. 18, 1745–1755 (2019).
Notari, M. et al. The local microenvironment limits the regenerative potential of the mouse neonatal heart. Sci. Adv. 4, eaao5553 (2018).
Yahalom-Ronen, Y., Rajchman, D., Sarig, R., Geiger, B. & Tzahor, E. Reduced matrix rigidity promotes neonatal cardiomyocyte dedifferentiation, proliferation and clonal expansion. eLife 4, e07455 (2015).
Wang, X. et al. Microenvironment stiffness requires decellularized cardiac extracellular matrix to promote heart regeneration in the neonatal mouse heart. Acta Biomater. 113, 380–392 (2020).
Bassat, E. et al. The extracellular matrix protein agrin promotes heart regeneration in mice. Nature 547, 179–184 (2017).
van der Pol, A. & Bouten, C. V. C. A brief history in cardiac regeneration, and how the extra cellular matrix may turn the tide. Front. Cardiovasc. Med. 8, 682342 (2021).
Gaetani, R. et al. When stiffness matters: mechanosensing in heart development and disease. Front. Cell Dev. Biol. 8, 334 (2020).
Perestrelo, A. R. et al. Multiscale analysis of extracellular matrix remodeling in the failing heart. Circ. Res. 128, 24–38 (2021).
Civitarese, R. A. et al. The α11 integrin mediates fibroblast-extracellular matrix-cardiomyocyte interactions in health and disease. Am. J. Physiol. Heart Circ. Physiol. 311, H96–H106 (2016).
Gullberg, D. et al. Analysis of alpha 1 beta 1, alpha 2 beta 1 and alpha 3 beta 1 integrins in cell–collagen interactions: identification of conformation dependent alpha 1 beta 1 binding sites in collagen type I. EMBO J. 11, 3865–3873 (1992).
Balasubramanian, S. et al. β3 Integrin in cardiac fibroblast is critical for extracellular matrix accumulation during pressure overload hypertrophy in mouse. PLoS ONE 7, e45076 (2012).
Schiller, H. B. & Fassler, R. Mechanosensitivity and compositional dynamics of cell-matrix adhesions. EMBO Rep. 14, 509–519 (2013).
Sit, B., Gutmann, D. & Iskratsch, T. Costameres, dense plaques and podosomes: the cell matrix adhesions in cardiovascular mechanosensing. J. Muscle Res. Cell Motil. 40, 197–209 (2019).
Chen, Y., Lee, H., Tong, H., Schwartz, M. & Zhu, C. Force regulated conformational change of integrin αVβ3. Matrix Biol. 60-61, 70–85 (2017).
Shemesh, T., Geiger, B., Bershadsky, A. D. & Kozlov, M. M. Focal adhesions as mechanosensors: a physical mechanism. Proc. Natl Acad. Sci. USA 102, 12383–12388 (2005).
Kong, F., Garcia, A. J., Mould, A. P., Humphries, M. J. & Zhu, C. Demonstration of catch bonds between an integrin and its ligand. J. Cell Biol. 185, 1275–1284 (2009).
Kaushik, G. et al. Vinculin network-mediated cytoskeletal remodeling regulates contractile function in the aging heart. Sci. Transl Med. 7, 292ra299 (2015).
Zhang, J. et al. Targeted inhibition of focal adhesion kinase attenuates cardiac fibrosis and preserves heart function in adverse cardiac remodeling. Sci. Rep. 7, 43146 (2017).
Manso, A. M. et al. Loss of mouse cardiomyocyte talin-1 and talin-2 leads to β-1 integrin reduction, costameric instability, and dilated cardiomyopathy. Proc. Natl Acad. Sci. USA 114, E6250–E6259 (2017).
Civitarese, R. A., Kapus, A., McCulloch, C. A. & Connelly, K. A. Role of integrins in mediating cardiac fibroblast-cardiomyocyte cross talk: a dynamic relationship in cardiac biology and pathophysiology. Basic. Res. Cardiol. 112, 6 (2017).
Hinz, B., Pittet, P., Smith-Clerc, J., Chaponnier, C. & Meister, J. J. Myofibroblast development is characterized by specific cell–cell adherens junctions. Mol. Biol. Cell 15, 4310–4320 (2004).
Schroer, A. K. & Merryman, W. D. Mechanobiology of myofibroblast adhesion in fibrotic cardiac disease. J. Cell Sci. 128, 1865–1875 (2015).
Rakshit, S., Zhang, Y., Manibog, K., Shafraz, O. & Sivasankar, S. Ideal, catch, and slip bonds in cadherin adhesion. Proc. Natl Acad. Sci. USA 109, 18815–18820 (2012).
Yao, M. et al. Force-dependent conformational switch of α-catenin controls vinculin binding. Nat. Commun. 5, 4525 (2014).
Buckley, C. D. et al. Cell adhesion. The minimal cadherin-catenin complex binds to actin filaments under force. Science 346, 1254211 (2014).
Vermij, S. H., Abriel, H. & van Veen, T. A. Refining the molecular organization of the cardiac intercalated disc. Cardiovasc. Res. 113, 259–275 (2017).
Baddam, S. R. et al. The desmosomal cadherin desmoglein-2 experiences mechanical tension as demonstrated by a FRET-based tension biosensor expressed in living cells. Cells 7, 66 (2018).
Samarel, A. M. Costameres, focal adhesions, and cardiomyocyte mechanotransduction. Am. J. Physiol. Heart Circ. Physiol. 289, H2291–H2301 (2005).
Galie, P. A., Khalid, N., Carnahan, K. E., Westfall, M. V. & Stegemann, J. P. Substrate stiffness affects sarcomere and costamere structure and electrophysiological function of isolated adult cardiomyocytes. Cardiovasc. Pathol. 22, 219–227 (2013).
Fancher, I. S. in Cellular Mechanotransduction Mechanisms in Cardiovascular and Fibrotic Diseases Ch. 2 (ed. Fang, Y.) 47–95 (Academic, 2021).
Alonso-Carbajo, L. et al. Muscling in on TRP channels in vascular smooth muscle cells and cardiomyocytes. Cell Calcium 66, 48–61 (2017).
Jakob, D. et al. Piezo1 and BKCa channels in human atrial fibroblasts: interplay and remodelling in atrial fibrillation. J. Mol. Cell. Cardiol. 158, 49–62 (2021).
Adapala, R. K. et al. TRPV4 channels mediate cardiac fibroblast differentiation by integrating mechanical and soluble signals. J. Mol. Cell. Cardiol. 54, 45–52 (2013).
Harada, M. et al. Transient receptor potential canonical-3 channel-dependent fibroblast regulation in atrial fibrillation. Circulation 126, 2051–2064 (2012).
Du, J. et al. TRPM7-mediated Ca2+ signals confer fibrogenesis in human atrial fibrillation. Circ. Res. 106, 992–1003 (2010).
Davis, J., Burr, A. R., Davis, G. F., Birnbaumer, L. & Molkentin, J. D. A TRPC6-dependent pathway for myofibroblast transdifferentiation and wound healing in vivo. Dev. Cell 23, 705–715 (2012).
Dupont, S. & Wickstrom, S. A. Mechanical regulation of chromatin and transcription. Nat. Rev. Genet. 23, 624–643 (2022).
Roper, J. C. et al. The major β-catenin/E-cadherin junctional binding site is a primary molecular mechano-transductor of differentiation in vivo. eLife 7, e33381 (2018).
Zhao, X. H. et al. Force activates smooth muscle α-actin promoter activity through the Rho signaling pathway. J. Cell Sci. 120, 1801–1809 (2007).
Ho, C. Y., Jaalouk, D. E., Vartiainen, M. K. & Lammerding, J. Lamin A/C and emerin regulate MKL1-SRF activity by modulating actin dynamics. Nature 497, 507–511 (2013).
Arsenovic, P. T. et al. Nesprin-2G, a component of the nuclear LINC complex, is subject to myosin-dependent tension. Biophys. J. 110, 34–43 (2016).
Elosegui-Artola, A. et al. Force triggers YAP nuclear entry by regulating transport across nuclear pores. Cell 171, 1397–1410.e14 (2017).
Denais, C. M. et al. Nuclear envelope rupture and repair during cancer cell migration. Science 352, 353–358 (2016).
Nava, M. M. et al. Heterochromatin-driven nuclear softening protects the genome against mechanical stress-induced damage. Cell 181, 800–817 (2020).
Tajik, A. et al. Transcription upregulation via force-induced direct stretching of chromatin. Nat. Mater. 15, 1287–1296 (2016).
Sun, J., Chen, J., Mohagheghian, E. & Wang, N. Force-induced gene up-regulation does not follow the weak power law but depends on H3K9 demethylation. Sci. Adv. 6, eaay9095 (2020).
Swift, J. et al. Nuclear lamin-A scales with tissue stiffness and enhances matrix-directed differentiation. Science 341, 1240104 (2013).
Lomakin, A. J. et al. The nucleus acts as a ruler tailoring cell responses to spatial constraints. Science 370, eaba2894 (2020).
Venturini, V. et al. The nucleus measures shape changes for cellular proprioception to control dynamic cell behavior. Science 370, eaba2644 (2020).
Schuller, A. P. et al. The cellular environment shapes the nuclear pore complex architecture. Nature 598, 667–671 (2021).
Zimmerli, C. E. et al. Nuclear pores dilate and constrict in cellulo. Science 374, eabd9776 (2021).
Andreu, I. et al. Mechanical force application to the nucleus regulates nucleocytoplasmic transport. Nat. Cell Biol. 24, 896–905 (2022).
Piccolo, S., Dupont, S. & Cordenonsi, M. The biology of YAP/TAZ: hippo signaling and beyond. Physiol. Rev. 94, 1287–1312 (2014).
Wei, S. C. et al. Matrix stiffness drives epithelial-mesenchymal transition and tumour metastasis through a TWIST1-G3BP2 mechanotransduction pathway. Nat. Cell Biol. 17, 678–688 (2015).
Zhang, K. et al. Mechanical signals regulate and activate SNAIL1 protein to control the fibrogenic response of cancer-associated fibroblasts. J. Cell. Sci. 129, 1989–2002 (2016).
Infante, E. et al. The mechanical stability of proteins regulates their translocation rate into the cell nucleus. Nat. Phys. 15, 973–981 (2019).
Ugolini, G. S. et al. On-chip assessment of human primary cardiac fibroblasts proliferative responses to uniaxial cyclic mechanical strain. Biotechnol. Bioeng. 113, 859–869 (2016).
Niu, L. et al. Matrix stiffness controls cardiac fibroblast activation through regulating YAP via AT1 R. J. Cell. Physiol. 235, 8345–8357 (2020).
Heallen, T. et al. Hippo signaling impedes adult heart regeneration. Development 140, 4683–4690 (2013).
Xin, M. et al. Hippo pathway effector Yap promotes cardiac regeneration. Proc. Natl Acad. Sci. USA 110, 13839–13844 (2013).
Flinn, M. A., Link, B. A. & O’Meara, C. C. Upstream regulation of the Hippo-Yap pathway in cardiomyocyte regeneration. Semin. Cell Dev. Biol. 100, 11–19 (2020).
Weichhart, T., Hengstschlager, M. & Linke, M. Regulation of innate immune cell function by mTOR. Nat. Rev. Immunol. 15, 599–614 (2015).
Castillo, E. A., Lane, K. V. & Pruitt, B. L. Micromechanobiology: focusing on the cardiac cell-substrate interface. Annu. Rev. Biomed. Eng. 22, 257–284 (2020).
Ieda, M. et al. Cardiac fibroblasts regulate myocardial proliferation through β1 integrin signaling. Dev. Cell 16, 233–244 (2009).
Wu, C. C., Jeratsch, S., Graumann, J. & Stainier, D. Y. R. Modulation of mammalian cardiomyocyte cytokinesis by the extracellular matrix. Circ. Res. 127, 896–907 (2020).
Heallen, T. et al. Hippo pathway inhibits Wnt signaling to restrain cardiomyocyte proliferation and heart size. Science 332, 458–461 (2011).
Halder, G. & Johnson, R. L. Hippo signaling: growth control and beyond. Development 138, 9–22 (2011).
Del Re, D. P. et al. Yes-associated protein isoform 1 (Yap1) promotes cardiomyocyte survival and growth to protect against myocardial ischemic injury. J. Biol. Chem. 288, 3977–3988 (2013).
Hou, N. et al. Activation of Yap1/Taz signaling in ischemic heart disease and dilated cardiomyopathy. Exp. Mol. Pathol. 103, 267–275 (2017).
Monroe, T. O. et al. YAP partially reprograms chromatin accessibility to directly induce adult cardiogenesis in vivo. Dev. Cell 48, 765–779.e7 (2019).
Xiao, Y. et al. Hippo pathway deletion in adult resting cardiac fibroblasts initiates a cell state transition with spontaneous and self-sustaining fibrosis. Genes Dev. 33, 1491–1505 (2019).
Mosqueira, D. et al. Hippo pathway effectors control cardiac progenitor cell fate by acting as dynamic sensors of substrate mechanics and nanostructure. ACS Nano 8, 2033–2047 (2014).
Morikawa, Y. et al. Actin cytoskeletal remodeling with protrusion formation is essential for heart regeneration in Hippo-deficient mice. Sci. Signal. 8, ra41 (2015).
Morikawa, Y., Heallen, T., Leach, J., Xiao, Y. & Martin, J. F. Dystrophin-glycoprotein complex sequesters Yap to inhibit cardiomyocyte proliferation. Nature 547, 227–231 (2017).
Aharonov, A. et al. ERBB2 drives YAP activation and EMT-like processes during cardiac regeneration. Nat. Cell Biol. 22, 1346–1356 (2020).
Frangogiannis, N. G. Cardiac fibrosis. Cardiovasc. Res. 117, 1450–1488 (2020).
Zile, M. R. et al. Myocardial stiffness in patients with heart failure and a preserved ejection fraction: contributions of collagen and titin. Circulation 131, 1247–1259 (2015).
Thomas, D. P., Cotter, T. A., Li, X., McCormick, R. J. & Gosselin, L. E. Exercise training attenuates aging-associated increases in collagen and collagen crosslinking of the left but not the right ventricle in the rat. Eur. J. Appl. Physiol. 85, 164–169 (2001).
Asif, M. et al. An advanced glycation endproduct cross-link breaker can reverse age-related increases in myocardial stiffness. Proc. Natl Acad. Sci. USA 97, 2809–2813 (2000).
Hutchinson, K. R., Lord, C. K., West, T. A. & Stewart, J. A. Jr Cardiac fibroblast-dependent extracellular matrix accumulation is associated with diastolic stiffness in type 2 diabetes. PLoS ONE 8, e72080 (2013).
Angelini, A., Trial, J., Ortiz-Urbina, J. & Cieslik, K. A. Mechanosensing dysregulation in the fibroblast: a hallmark of the aging heart. Ageing Res. Rev. 63, 101150 (2020).
Villemain, O. et al. Myocardial stiffness evaluation using noninvasive shear wave imaging in healthy and hypertrophic cardiomyopathic adults. JACC Cardiovasc. Imaging 12, 1135–1145 (2019).
Engler, A. J. et al. Embryonic cardiomyocytes beat best on a matrix with heart-like elasticity: scar-like rigidity inhibits beating. J. Cell Sci. 121, 3794–3802 (2008).
Pandey, P. et al. Cardiomyocytes sense matrix rigidity through a combination of muscle and non-muscle myosin contractions. Dev. Cell 44, 326–336.e3 (2018).
Nishimura, M. et al. A dual role for integrin-linked kinase and β1-integrin in modulating cardiac aging. Aging Cell 13, 431–440 (2014).
Nawata, J. et al. Differential expression of α1, α3 and α5 integrin subunits in acute and chronic stages of myocardial infarction in rats. Cardiovasc. Res. 43, 371–381 (1999).
Hein, S., Kostin, S., Heling, A., Maeno, Y. & Schaper, J. The role of the cytoskeleton in heart failure. Cardiovasc. Res. 45, 273–278 (2000).
Heling, A. et al. Increased expression of cytoskeletal, linkage, and extracellular proteins in failing human myocardium. Circ. Res. 86, 846–853 (2000).
Sessions, A. O. & Engler, A. J. Mechanical regulation of cardiac aging in model systems. Circ. Res. 118, 1553–1562 (2016).
Neff, L. S. & Bradshaw, A. D. Cross your heart? Collagen cross-links in cardiac health and disease. Cell Signal. 79, 109889 (2021).
Al-U’datt, D., Allen, B. G. & Nattel, S. Role of the lysyl oxidase enzyme family in cardiac function and disease. Cardiovasc. Res. 115, 1820–1837 (2019).
Gonzalez-Santamaria, J. et al. Matrix cross-linking lysyl oxidases are induced in response to myocardial infarction and promote cardiac dysfunction. Cardiovasc. Res. 109, 67–78 (2016).
Grilo, G. A. et al. Age- and sex-dependent differences in extracellular matrix metabolism associate with cardiac functional and structural changes. J. Mol. Cell. Cardiol. 139, 62–74 (2020).
Yang, J. et al. Targeting LOXL2 for cardiac interstitial fibrosis and heart failure treatment. Nat. Commun. 7, 13710 (2016).
Bhana, B. et al. Influence of substrate stiffness on the phenotype of heart cells. Biotechnol. Bioeng. 105, 1148–1160 (2010).
Forte, G. et al. Substrate stiffness modulates gene expression and phenotype in neonatal cardiomyocytes in vitro. Tissue Eng. Part A 18, 1837–1848 (2012).
McCain, M. L., Yuan, H., Pasqualini, F. S., Campbell, P. H. & Parker, K. K. Matrix elasticity regulates the optimal cardiac myocyte shape for contractility. Am. J. Physiol. Heart Circ. Physiol. 306, H1525–H1539 (2014).
Jacot, J. G., McCulloch, A. D. & Omens, J. H. Substrate stiffness affects the functional maturation of neonatal rat ventricular myocytes. Biophys. J. 95, 3479–3487 (2008).
Ribeiro, A. J. et al. Contractility of single cardiomyocytes differentiated from pluripotent stem cells depends on physiological shape and substrate stiffness. Proc. Natl Acad. Sci. USA 112, 12705–12710 (2015).
Katz, A. M. & Rolett, E. L. Heart failure: when form fails to follow function. Eur. Heart J. 37, 449–454 (2016).
Fomovsky, G. M., Rouillard, A. D. & Holmes, J. W. Regional mechanics determine collagen fiber structure in healing myocardial infarcts. J. Mol. Cell. Cardiol. 52, 1083–1090 (2012).
Garoffolo, G. et al. Reduction of cardiac fibrosis by interference with YAP-dependent transactivation. Circ. Res. 131, 239–257 (2022).
Khalil, H. et al. Fibroblast-specific TGF-β–Smad2/3 signaling underlies cardiac fibrosis. J. Clin. Invest. 127, 3770–3783 (2017).
Dang, S. et al. Blockade of β-adrenergic signaling suppresses inflammasome and alleviates cardiac fibrosis. Ann. Transl Med. 8, 127 (2020).
Miteva, K. et al. Human endomyocardial biopsy specimen-derived stromal cells modulate angiotensin II-induced cardiac remodeling. Stem Cell Transl Med. 5, 1707–1718 (2016).
Tschope, C. et al. Modulation of the acute defence reaction by eplerenone prevents cardiac disease progression in viral myocarditis. ESC Heart Fail. 7, 2838–2852 (2020).
Xia, Y. et al. Endogenous thrombospondin 1 protects the pressure-overloaded myocardium by modulating fibroblast phenotype and matrix metabolism. Hypertension 58, 902–911 (2011).
Lorenzen, J. M. et al. Osteopontin is indispensible for AP1-mediated angiotensin II-related miR-21 transcription during cardiac fibrosis. Eur. Heart J. 36, 2184–2196 (2015).
Van Linthout, S. & Tschope, C. The quest for antiinflammatory and immunomodulatory strategies in heart failure. Clin. Pharmacol. Ther. 106, 1198–1208 (2019).
Wu, Y. et al. S100a8/a9 released by CD11b+Gr1+ neutrophils activates cardiac fibroblasts to initiate angiotensin II-Induced cardiac inflammation and injury. Hypertension 63, 1241–1250 (2014).
Lindner, D. et al. Cardiac fibroblasts support cardiac inflammation in heart failure. Basic. Res. Cardiol. 109, 428 (2014).
Pappritz, K. et al. Cardiac (myo)fibroblasts modulate the migration of monocyte subsets. Sci. Rep. 8, 5575 (2018).
Matz, I., Pappritz, K., Springer, J. & Van Linthout, S. Left ventricle- and skeletal muscle-derived fibroblasts exhibit a differential inflammatory and metabolic responsiveness to interleukin-6. Front. Immunol. 13, 947267 (2022).
Sandanger, O. et al. The NLRP3 inflammasome is up-regulated in cardiac fibroblasts and mediates myocardial ischaemia-reperfusion injury. Cardiovasc. Res. 99, 164–174 (2013).
Mia, M. et al. Loss of Yap/Taz in cardiac fibroblasts attenuates adverse remodeling and improves cardiac function. Cardiovasc. Res. 118, 1785–1804 (2022).
Wong, V. W. et al. Mechanical force prolongs acute inflammation via T-cell-dependent pathways during scar formation. FASEB J. 25, 4498–4510 (2011).
Li, C. et al. Mineralocorticoid receptor deficiency in T cells attenuates pressure overload-induced cardiac hypertrophy and dysfunction through modulating T-cell activation. Hypertension 70, 137–147 (2017).
Sun, X. J. et al. Deletion of interleukin 1 receptor-associated kinase 1 (Irak1) improves glucose tolerance primarily by increasing insulin sensitivity in skeletal muscle. J. Biol. Chem. 292, 12339–12350 (2017).
Huang, H. W., Fang, X. X., Wang, X. Q., Peng, Y. P. & Qiu, Y. H. Regulation of differentiation and function of helper T cells by lymphocyte-derived catecholamines via α1- and β2-adrenoceptors. Neuroimmunomodulation 22, 138–151 (2015).
Woodall, M. C., Woodall, B. P., Gao, E., Yuan, A. & Koch, W. J. Cardiac fibroblast GRK2 deletion enhances contractility and remodeling following ischemia/reperfusion injury. Circ. Res. 119, 1116–1127 (2016).
Travers, J. G. et al. Pharmacological and activated fibroblast targeting of Gβγ-GRK2 after myocardial ischemia attenuates heart failure progression. J. Am. Coll. Cardiol. 70, 958–971 (2017).
Sorrentino, G. et al. Metabolic control of YAP and TAZ by the mevalonate pathway. Nat. Cell Biol. 16, 357–366 (2014).
Iaccarino, G. et al. Elevated myocardial and lymphocyte GRK2 expression and activity in human heart failure. Eur. Heart J. 26, 1752–1758 (2005).
Mia, M. M. et al. YAP/TAZ deficiency reprograms macrophage phenotype and improves infarct healing and cardiac function after myocardial infarction. PLoS Biol. 18, e3000941 (2020).
Wang, D. et al. YAP promotes the activation of NLRP3 inflammasome via blocking K27-linked polyubiquitination of NLRP3. Nat. Commun. 12, 2674 (2021).
Wu, Y. et al. Helicobacter pylori-induced YAP1 nuclear translocation promotes gastric carcinogenesis by enhancing IL-1β expression. Cancer Med. 8, 3965–3980 (2019).
Ramjee, V. et al. Epicardial YAP/TAZ orchestrate an immunosuppressive response following myocardial infarction. J. Clin. Invest. 127, 899–911 (2017).
Tomasek, J. J., Gabbiani, G., Hinz, B., Chaponnier, C. & Brown, R. A. Myofibroblasts and mechano-regulation of connective tissue remodelling. Nat. Rev. Mol. Cell Biol. 3, 349–363 (2002).
Negmadjanov, U. et al. TGF-β1-mediated differentiation of fibroblasts is associated with increased mitochondrial content and cellular respiration. PLoS ONE 10, e0123046 (2015).
Emelyanova, L. et al. Impact of statins on cellular respiration and de-differentiation of myofibroblasts in human failing hearts. ESC Heart Fail. 6, 1027–1040 (2019).
Van Linthout, S. et al. Anti-inflammatory effects of atorvastatin improve left ventricular function in experimental diabetic cardiomyopathy. Diabetologia 50, 1977–1986 (2007).
Van Linthout, S. et al. Human apolipoprotein A-I gene transfer reduces the development of experimental diabetic cardiomyopathy. Circulation 117, 1563–1573 (2008).
Van Linthout, S. et al. Reduced MMP-2 activity contributes to cardiac fibrosis in experimental diabetic cardiomyopathy. Basic. Res. Cardiol. 103, 319–327 (2008).
Spillmann, F. et al. High-density lipoproteins reduce palmitate-induced cardiomyocyte apoptosis in an AMPK-dependent manner. Biochem. Biophys. Res. Commun. 466, 272–277 (2015).
Beauloye, C., Bertrand, L., Horman, S. & Hue, L. AMPK activation, a preventive therapeutic target in the transition from cardiac injury to heart failure. Cardiovasc. Res. 90, 224–233 (2011).
Wang, W. et al. AMPK modulates Hippo pathway activity to regulate energy homeostasis. Nat. Cell Biol. 17, 490–499 (2015).
Vinci, M. C., Polvani, G. & Pesce, M. Epigenetic programming and risk: the birthplace of cardiovascular disease? Stem Cell Rev. Rep. 9, 241–253 (2013).
Felisbino, M. B. & McKinsey, T. A. Epigenetics in cardiac fibrosis: emphasis on inflammation and fibroblast activation. JACC Basic. Transl Sci. 3, 704–715 (2018).
Yang, C., Tibbitt, M. W., Basta, L. & Anseth, K. S. Mechanical memory and dosing influence stem cell fate. Nat. Mater. 13, 645–652 (2014).
Stewart-Morgan, K. R., Petryk, N. & Groth, A. Chromatin replication and epigenetic cell memory. Nat. Cell Biol. 22, 361–371 (2020).
Ferrari, S. & Pesce, M. Cell-based mechanosensation, epigenetics, and non-coding RNAs in progression of cardiac fibrosis. Int. J. Mol. Sci. 21, 28 (2019).
Santinon, G., Pocaterra, A. & Dupont, S. Control of YAP/TAZ activity by metabolic and nutrient-sensing pathways. Trends Cell Biol. 26, 289–299 (2016).
Lin, Z. et al. Acetylation of VGLL4 regulates Hippo-YAP signaling and postnatal cardiac growth. Dev. Cell 39, 466–479 (2016).
Levy, L. et al. Acetylation of β-catenin by p300 regulates β-catenin-Tcf4 interaction. Mol. Cell Biol. 24, 3404–3414 (2004).
Yang, Y., Li, Z., Guo, J. & Xu, Y. Deacetylation of MRTF-A by SIRT1 defies senescence induced down-regulation of collagen type I in fibroblast cells. Biochim. Biophys. Acta Mol. Basis Dis. 1866, 165723 (2020).
Bradshaw, P. C. Acetyl-CoA metabolism and histone acetylation in the regulation of aging and lifespan. Antioxidants 10, 572 (2021).
Francois, A., Canella, A., Marcho, L. M. & Stratton, M. S. Protein acetylation in cardiac aging. J. Mol. Cell. Cardiol. 157, 90–97 (2021).
Kimball, T. H. & Vondriska, T. M. Metabolism, epigenetics, and causal inference in heart failure. Trends Endocrinol. Metab. 31, 181–191 (2020).
Ferrari, S. & Pesce, M. Stiffness and aging in cardiovascular diseases: the dangerous relationship between force and senescence. Int. J. Mol. Sci. 22, 3404 (2021).
Osmanagic-Myers, S., Dechat, T. & Foisner, R. Lamins at the crossroads of mechanosignaling. Genes Dev. 29, 225–237 (2015).
Hampoelz, B. & Lecuit, T. Nuclear mechanics in differentiation and development. Curr. Opin. Cell Biol. 23, 668–675 (2011).
Maeshima, K., Tamura, S. & Shimamoto, Y. Chromatin as a nuclear spring. Biophys. Physicobiol. 15, 189–195 (2018).
Kupfer, M. E. et al. In situ expansion, differentiation, and electromechanical coupling of human cardiac muscle in a 3D bioprinted, chambered organoid. Circ. Res. 127, 207–224 (2020).
Drakhlis, L. et al. Human heart-forming organoids recapitulate early heart and foregut development. Nat. Biotechnol. 39, 737–746 (2021).
Tiburcy, M. et al. Defined engineered human myocardium with advanced maturation for applications in heart failure modeling and repair. Circulation 135, 1832–1847 (2017).
Ott, H. C. et al. Perfusion-decellularized matrix: using nature’s platform to engineer a bioartificial heart. Nat. Med. 14, 213–221 (2008).
Kanoldt, V., Fischer, L. & Grashoff, C. Unforgettable force – crosstalk and memory of mechanosensitive structures. Biol. Chem. 400, 687–698 (2019).
Norman, M. D. A., Ferreira, S. A., Jowett, G. M., Bozec, L. & Gentleman, E. Measuring the elastic modulus of soft culture surfaces and three-dimensional hydrogels using atomic force microscopy. Nat. Protoc. 16, 2418–2449 (2021).
Sadeghi, A. H. et al. Engineered 3D cardiac fibrotic tissue to study fibrotic remodeling. Adv. Healthc. Mater. 6, 1601434 (2017).
Bracco Gartner, T. C. L. et al. Anti-fibrotic effects of cardiac progenitor cells in a 3D-model of human cardiac fibrosis. Front. Cardiovasc. Med. 6, 52 (2019).
Ragazzini, S. et al. Mechanosensor YAP cooperates with TGF-β1 signaling to promote myofibroblast activation and matrix stiffening in a 3D model of human cardiac fibrosis. Acta Biomater. 152, 300–312 (2022).
Wang, H., Haeger, S. M., Kloxin, A. M., Leinwand, L. A. & Anseth, K. S. Redirecting valvular myofibroblasts into dormant fibroblasts through light-mediated reduction in substrate modulus. PLoS ONE 7, e39969 (2012).
Milani-Nejad, N. & Janssen, P. M. Small and large animal models in cardiac contraction research: advantages and disadvantages. Pharmacol. Ther. 141, 235–249 (2014).
Abramochkin, D. V., Lozinsky, I. T. & Kamkin, A. Influence of mechanical stress on fibroblast–myocyte interactions in mammalian heart. J. Mol. Cell. Cardiol. 70, 27–36 (2014).
Li, X., Garcia-Elias, A., Benito, B. & Nattel, S. The effects of cardiac stretch on atrial fibroblasts: analysis of the evidence and potential role in atrial fibrillation. Cardiovasc. Res. 118, 440–460 (2022).
Guo, Y. et al. Extracellular matrix of mechanically stretched cardiac fibroblasts improves viability and metabolic activity of ventricular cells. Int. J. Med. Sci. 10, 1837–1845 (2013).
Papakrivopoulou, J., Lindahl, G. E., Bishop, J. E. & Laurent, G. J. Differential roles of extracellular signal-regulated kinase 1/2 and p38MAPK in mechanical load-induced procollagen α1(I) gene expression in cardiac fibroblasts. Cardiovasc. Res. 61, 736–744 (2004).
Watson, C. J. et al. Mechanical stretch up-regulates the B-type natriuretic peptide system in human cardiac fibroblasts: a possible defense against transforming growth factor-β mediated fibrosis. Fibrogenes. Tissue Repair 5, 9 (2012).
Watson, C. J. et al. Extracellular matrix sub-types and mechanical stretch impact human cardiac fibroblast responses to transforming growth factor beta. Connect. Tissue Res. 55, 248–256 (2014).
Li, Y., Asfour, H. & Bursac, N. Age-dependent functional crosstalk between cardiac fibroblasts and cardiomyocytes in a 3D engineered cardiac tissue. Acta Biomater. 55, 120–130 (2017).
Kreutzer, F. P. et al. Development and characterization of anti-fibrotic natural compound similars with improved effectivity. Basic. Res. Cardiol. 117, 9 (2022).
Perbellini, F. & Thum, T. Living myocardial slices: a novel multicellular model for cardiac translational research. Eur. Heart J. 41, 2405–2408 (2020).
Valls-Margarit, M. et al. Engineered macroscale cardiac constructs elicit human myocardial tissue-like functionality. Stem Cell Rep. 13, 207–220 (2019).
Salvi, M. et al. Automated segmentation of fluorescence microscopy images for 3D cell detection in human-derived cardiospheres. Sci. Rep. 9, 6644 (2019).
Neuber, S., Nazari-Shafti, T. Z., Nugraha, B., Falk, V. & Emmert, M. Y. The link between regeneration and extracellular matrix in the heart – can three-dimensional in vitro models uncover it? Eur. Heart J. 42, 2518–2522 (2021).
de Boer, R. A. et al. Towards better definition, quantification and treatment of fibrosis in heart failure. A scientific roadmap by the Committee of Translational Research of the Heart Failure Association (HFA) of the European Society of Cardiology. Eur. J. Heart Fail. 21, 272–285 (2019).
Santos, G. L., Hartmann, S., Zimmermann, W. H., Ridley, A. & Lutz, S. Inhibition of Rho-associated kinases suppresses cardiac myofibroblast function in engineered connective and heart muscle tissues. J. Mol. Cell. Cardiol. 134, 13–28 (2019).
Francisco, J. et al. Blockade of fibroblast YAP attenuates cardiac fibrosis and dysfunction through MRTF-A inhibition. JACC Basic. Transl Sci. 5, 931–945 (2020).
Nagaraju, C. K. et al. Myofibroblast phenotype and reversibility of fibrosis in patients with end-stage heart failure. J. Am. Coll. Cardiol. 73, 2267–2282 (2019).
Tzahor, E. & Poss, K. D. Cardiac regeneration strategies: staying young at heart. Science 356, 1035–1039 (2017).
Leach, J. P. et al. Hippo pathway deficiency reverses systolic heart failure after infarction. Nature 550, 260–264 (2017).
Wang, J., Liu, S., Heallen, T. & Martin, J. F. The Hippo pathway in the heart: pivotal roles in development, disease, and regeneration. Nat. Rev. Cardiol. 15, 672–684 (2018).
Bouvet, M. et al. Anti-integrin αv therapy improves cardiac fibrosis after myocardial infarction by blunting cardiac PW1+ stromal cells. Sci. Rep. 10, 11404 (2020).
Esposito, M. L. et al. Left ventricular unloading before reperfusion promotes functional recovery after acute myocardial infarction. J. Am. Coll. Cardiol. 72, 501–514 (2018).
Spillmann, F. et al. Mode-of-action of the PROPELLA concept in fulminant myocarditis. Eur. Heart J. 40, 2164–2169 (2019).
Burkhoff, D., Topkara, V. K., Sayer, G. & Uriel, N. Reverse remodeling with left ventricular assist devices. Circ. Res. 128, 1594–1612 (2021).
Levin, H. R. et al. Reversal of chronic ventricular dilation in patients with end-stage cardiomyopathy by prolonged mechanical unloading. Circulation 91, 2717–2720 (1995).
Mann, D. L., Barger, P. M. & Burkhoff, D. Myocardial recovery and the failing heart: myth, magic, or molecular target? J. Am. Coll. Cardiol. 60, 2465–2472 (2012).
Birks, E. J. et al. Prospective multicenter study of myocardial recovery using left ventricular assist devices (RESTAGE-HF [Remission from Stage D Heart Failure]): medium-term and primary end point results. Circulation 142, 2016–2028 (2020).
Diakos, N. A. et al. Myocardial atrophy and chronic mechanical unloading of the failing human heart: implications for cardiac assist device-induced myocardial recovery. J. Am. Coll. Cardiol. 64, 1602–1612 (2014).
Terracciano, C. M. et al. Clinical recovery from end-stage heart failure using left-ventricular assist device and pharmacological therapy correlates with increased sarcoplasmic reticulum calcium content but not with regression of cellular hypertrophy. Circulation 109, 2263–2265 (2004).
Diakos, N. A. et al. Evidence of glycolysis up-regulation and pyruvate mitochondrial oxidation mismatch during mechanical unloading of the failing human heart: implications for cardiac reloading and conditioning. JACC Basic Transl Sci. 1, 432–444 (2016).
Vatta, M. et al. Molecular remodelling of dystrophin in patients with end-stage cardiomyopathies and reversal in patients on assistance-device therapy. Lancet 359, 936–941 (2002).
Canseco, D. C. et al. Human ventricular unloading induces cardiomyocyte proliferation. J. Am. Coll. Cardiol. 65, 892–900 (2015).
Symons, J. D. et al. Effect of continuous-flow left ventricular assist device support on coronary artery endothelial function in ischemic and nonischemic cardiomyopathy. Circ. Heart Fail. 12, e006085 (2019).
Castillero, E. et al. Structural and functional cardiac profile after prolonged duration of mechanical unloading: potential implications for myocardial recovery. Am. J. Physiol. Heart Circ. Physiol. 315, H1463–H1476 (2018).
Klotz, S. et al. Left ventricular assist device support normalizes left and right ventricular beta-adrenergic pathway properties. J. Am. Coll. Cardiol. 45, 668–676 (2005).
Bruckner, B. A. et al. Degree of cardiac fibrosis and hypertrophy at time of implantation predicts myocardial improvement during left ventricular assist device support. J. Heart Lung Transpl. 23, 36–42 (2004).
Segura, A. M., Frazier, O. H., Demirozu, Z. & Buja, L. M. Histopathologic correlates of myocardial improvement in patients supported by a left ventricular assist device. Cardiovasc. Pathol. 20, 139–145 (2011).
Pan, S. et al. Incidence and predictors of myocardial recovery on long-term left ventricular assist device support: results from the United Network for Organ Sharing database. J. Heart Lung Transpl. 34, 1624–1629 (2015).
Topkara, V. K. et al. Myocardial recovery in patients receiving contemporary left ventricular assist devices: results from the Interagency Registry for Mechanically Assisted Circulatory Support (INTERMACS). Circ. Heart Fail. 9, e003157 (2016).
Wever-Pinzon, O. et al. Cardiac recovery during long-term left ventricular assist device support. J. Am. Coll. Cardiol. 68, 1540–1553 (2016).
Margulies, K. B. et al. Mixed messages: transcription patterns in failing and recovering human myocardium. Circ. Res. 96, 592–599 (2005).
Yang, K. C. et al. Deep RNA sequencing reveals dynamic regulation of myocardial noncoding RNAs in failing human heart and remodeling with mechanical circulatory support. Circulation 129, 1009–1021 (2014).
Tschope, C. et al. Mechanical unloading by fulminant myocarditis: LV-IMPELLA, ECMELLA, BI-PELLA, and PROPELLA concepts. J. Cardiovasc. Transl Res. 12, 116–123 (2019).
Weinheimer, C. J. et al. Load-dependent changes in left ventricular structure and function in a pathophysiologically relevant murine model of reversible heart failure. Circ. Heart Fail. 11, e004351 (2018).
Oriyanhan, W. et al. Determination of optimal duration of mechanical unloading for failing hearts to achieve bridge to recovery in a rat heterotopic heart transplantation model. J. Heart Lung Transpl. 26, 16–23 (2007).
Webber, M., Jackson, S. P., Moon, J. C. & Captur, G. Myocardial fibrosis in heart failure: anti-fibrotic therapies and the role of cardiovascular magnetic resonance in drug trials. Cardiol. Ther. 9, 363–376 (2020).
Pezel, T. et al. Imaging interstitial fibrosis, left ventricular remodeling, and function in stage A and B heart failure. JACC Cardiovasc. Imaging 14, 1038–1052 (2021).
Khalique, Z. et al. Diffusion tensor cardiovascular magnetic resonance in cardiac amyloidosis. Circ. Cardiovasc. Imaging 13, e009901 (2020).
Tschope, C. et al. Cardiac contractility modulation: mechanisms of action in heart failure with reduced ejection fraction and beyond. Eur. J. Heart Fail. 21, 14–22 (2019).
Daneshgar, A. et al. The human liver matrisome – proteomic analysis of native and fibrotic human liver extracellular matrices for organ engineering approaches. Biomaterials 257, 120247 (2020).
Moriel, N. et al. NovoSpaRc: flexible spatial reconstruction of single-cell gene expression with optimal transport. Nat. Protoc. 16, 4177–4200 (2021).
Aghajanian, H. et al. Targeting cardiac fibrosis with engineered T cells. Nature 573, 430–433 (2019).
Rurik, J. G. et al. CAR T cells produced in vivo to treat cardiac injury. Science 375, 91–96 (2022).
Croft, A. P. et al. Distinct fibroblast subsets drive inflammation and damage in arthritis. Nature 570, 246–251 (2019).
Nguyen, P. D., de Bakker, D. E. M. & Bakkers, J. Cardiac regenerative capacity: an evolutionary afterthought. Cell. Mol. Life Sci. 78, 5107–5122 (2021).
Silva, A. C., Pereira, C., Fonseca, A., Pinto-do, O. P. & Nascimento, D. S. Bearing my heart: the role of extracellular matrix on cardiac development, homeostasis, and injury response. Front. Cell Dev. Biol. 8, 621644 (2020).
Pesce, M., Messina, E., Chimenti, I. & Beltrami, A. P. Cardiac mechanoperception: a life-long story from early beats to aging and failure. Stem Cell Dev. 26, 77–90 (2017).
del Rio, A. et al. Stretching single talin rod molecules activates vinculin binding. Science 323, 638–641 (2009).
Acknowledgements
M.P. is supported by institutional grants from the Italian Ministry of Health (Ricerca Corrente, Ricerca 5 per mille). G.N.D. is supported by the Deutsche Forschungsgemeinschaft (SFB 1444). G.F. is supported by the European Regional Development Fund – Project ENOCH (CZ.02.1.01/0.0/0.0/16_019/0000868) and Project MAGNET (CZ.02.1.01/0.0/0.0/15_003/0000492). H.G. is supported by the European Regional Development Fund through the Operational Program for Competitiveness Factors (under the projects HealthyAging2020 CENTRO-01-0145-FEDER-000012-N2323, CENTRO-01-0145-FEDER-032179, CENTRO-01-0145-FEDER-032414, POCI-01-0145-FEDER-022122, UIDB/04539/2020 and UIDP/04539/2020). A.R. is supported by the Spanish Ministry of Economy and Competitiveness (RTI2018-095377-B-100), Instituto de Salud Carlos III-ISCIII/FEDER (TerCel RD16/0011/0024), AGAUR (2017-SGR-899) and CERCA Programme Generalitat de Catalunya. P.R.-C. is supported by the Spanish Ministry of Science and Innovation (PID2019-110298GB-I00), the European Commission (H2020-FETPROACT-01-2016-731957), the ICREA Academia prize for excellence in research, Fundació la Marató de TV3 (201936-30-31) and la Caixa Foundation (agreement LCF/PR/HR20/52400004). J.P.G.S. is supported by a European Union H2020 programme grant EVICARE (725229) and BRAV∃ (874827), and the Gravitation Program (Materials Driven Regeneration 024.003.013) by the Netherlands Organization for Scientific Research. C.T. and S.V.L. are supported by the Deutsche Forschungsgemeinschaft (SFB 1470).
Author information
Authors and Affiliations
Contributions
M.P. and S.V.L. contributed to the discussion of manuscript content. All the authors contributed to writing, reviewing and editing the manuscript before submission.
Corresponding authors
Ethics declarations
Competing interests
C.T. has received speaker fees and has contributed to congresses organized by Abbott, Abiomed, AstraZeneca, Bayer, Boehringer-Ingelheim, Novartis, Pfizer and Servier. The other authors declare no competing interests.
Peer review
Peer review information
Nature Reviews Cardiology thanks Nikolaos Frangogiannis and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Glossary
- Costameres
-
Submembranous, Z-line-associated structures found in striated muscle that have important roles in force transmission from the sarcomeres to the sarcolemma and the extracellular matrix, maintenance of mechanical integrity of the sarcolemma, and orchestration of mechanically related signalling.
- Dense plaques
-
Also known as dense bodies. Intercellular adhesion complexes that are functional equivalents of the Z-discs in the striated muscle and fulfil a mechanical function that allows the coordinated contraction of smooth muscle cells.
- Dystroglycan complex
-
A large multicomponent complex that is composed of transmembrane, cytoplasmic and extracellular proteins, including dystrophin, sarcoglycans, dystroglycan, dystrobrevins, syntrophins, sarcospan, caveolin 3 and nitric oxide synthase, and has both mechanical stabilizing and signalling roles in mediating interactions between the cytoskeleton, plasma membrane and extracellular matrix.
- Euchromatin
-
A less condensed chromatin than heterochromatin and more accessible to transcription factors.
- Heterochromatin
-
A densely packed chromatin that is inaccessible to transcription factors and has an important role in maintaining the structural and functional integrity of specific chromosomal regions, such as centromeres and telomeres.
- Podosomes
-
Actin-based dynamic protrusions of the plasma membrane that act as sites of attachment to, and degradation of, the extracellular matrix.
- Rigidity
-
The capacity of a material to withstand deformation when subjected to mechanical loading (such as tension).
- Strain
-
Strain (ε) is the deformation of a material or tissue when subjected to stress; stress (σ) is an internal loading on a material caused by an external force.
- Stiffness
-
The extent to which a material resists deformation in response to an applied load, and is the inverse of compliance.
- Teleost
-
Teleost fish are the most species-rich vertebrate clade, roughly making up half of the existing vertebrate species on the planet, and have extensive genetic and phenotypic variation, resulting in their use in biodiversity and genome evolution studies.
- Young’s modulus
-
(E). Also referred to as modulus of elasticity, it is a property of the material that indicates how easily it can stretch and deform, and is defined as the ratio of tensile stress (σ) to tensile strain (ε).
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Pesce, M., Duda, G.N., Forte, G. et al. Cardiac fibroblasts and mechanosensation in heart development, health and disease. Nat Rev Cardiol 20, 309–324 (2023). https://doi.org/10.1038/s41569-022-00799-2
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41569-022-00799-2
This article is cited by
-
Single-molecule magnetic tweezers to probe the equilibrium dynamics of individual proteins at physiologically relevant forces and timescales
Nature Protocols (2024)
-
Cardiovascular adverse effects and mechanistic insights of arsenic exposure: a review
Environmental Chemistry Letters (2024)
-
Electrically conductive scaffolds mimicking the hierarchical structure of cardiac myofibers
Scientific Reports (2023)