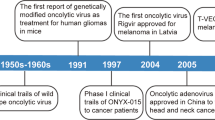
Abstract
In the wake of the success of modern immunotherapy, oncolytic viruses (OVs) are currently seen as a potential therapeutic option for patients with cancer who do not respond or fail to achieve durable responses following treatment with immune checkpoint inhibitors. OVs offer a multifaceted therapeutic platform because they preferentially replicate in tumour cells, can be engineered to express transgenes that augment their cytotoxic and immunostimulatory activities, and modulate the tumour microenvironment to optimize immune-mediated tumour eradication, both at locoregional and systemic sites of disease. Lysis of tumour cells releases tumour-specific antigens that trigger both the innate and adaptive immune systems. OVs also represent attractive combination partners with other systemically delivered agents by virtue of their highly favourable safety profiles. Rational combinations of OVs with different immune modifiers and/or antitumour agents, based on mechanisms of tumour resistance to immune-mediated attack, may benefit the large, currently underserved, population of patients who respond poorly to immune checkpoint inhibition.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
References
Robert, C. et al. Nivolumab in previously untreated melanoma without BRAF mutation. N. Engl. J. Med. 372, 320–330 (2015).
Motzer, R. J. et al. Nivolumab versus everolimus in advanced renal-cell carcinoma. N. Engl. J. Med. 373, 1803–1813 (2015).
Brahmer, J. et al. Nivolumab versus docetaxel in advanced squamous-cell non-small-cell lung cancer. N. Engl. J. Med. 373, 123–135 (2015).
Topalian, S. L. et al. Safety, activity, and immune correlates of anti-PD-1 antibody in cancer. N. Engl. J. Med. 366, 2443–2454 (2012).
Kather, J. N., Halama, N. & Jaeger, D. Genomics and emerging biomarkers for immunotherapy of colorectal cancer. Semin. Cancer Biol. 52, 189–197 (2018).
Khunger, M. et al. Programmed cell death 1 (PD1) ligand (PD-L1) expression in solid tumors as a predictive biomarker of benefit from PD-1/PD-L1 axis inhibitors: a systematic review and meta-analysis. JCO Precis. Oncol. https://doi.org/10.1200/PO.16.00030 (2017).
Rao, S. V., Moran, A. E. & Graff, J. N. Predictors of response and resistance to checkpoint inhibitors in solid tumors. Ann. Transl Med. 5, 468 (2017).
Gujar, S., Pol, J. G. & Kroemer, G. Heating it up: oncolytic viruses make tumors ‘hot’ and suitable for checkpoint blockade immunotherapies. OncoImmunology 7, e1442169 (2018).
Galon, J. et al. Type, density, and location of immune cells within human colorectal tumors predict clinical outcome. Science 313, 1960–1964 (2006). This study provides evidence for the clinical importance of immune cell infiltrates in determining outcomes for patients with a tumour type that is not classically viewed as immune-sensitive.
Le, D. T. et al. PD-1 blockade in tumors with mismatch-repair deficiency. N. Engl. J. Med. 372, 2509–2520 (2015).
Le, D. T. et al. Mismatch repair deficiency predicts response of solid tumors to PD-1 blockade. Science 357, 409–413 (2017).
Sharma, P., Hu-Lieskovan, S., Wargo, J. A. & Ribas, A. Primary, adaptive, and acquired resistance to cancer immunotherapy. Cell 168, 707–723 (2017).
Ribas, A. et al. Oncolytic virotherapy promotes intratumoral T cell infiltration and improves anti-PD-1 immunotherapy. Cell 170, 1109–1119 (2017). This study illustrates clinical benefit associated with the combination of T-VEC with PD-1 axis inhibition.
Jacquelot, N. et al. Predictors of responses to immune checkpoint blockade in advanced melanoma. Nat. Commun. 8, 592 (2017).
Gajewski, T. F., Schreiber, H. & Fu, Y. X. Innate and adaptive immune cells in the tumor microenvironment. Nat. Commun. 14, 1014–1022 (2013).
Tran Janco, J. M., Lamichhane, P., Karyampudi, L. & Knutson, K. L. Tumor-infiltrating dendritic cells in cancer pathogenesis. J. Immunol. 194, 2985–2991 (2015).
Lanitis, E., Dangaj, D., Irving, M. & Coukos, G. Mechanisms regulating T cell infiltration and activity in solid tumors. Ann. Oncol. 28 (Suppl. 12), xii18–xii32 (2017).
Bilir, C. & Sarisozen, C. Indoleamine 2, 3-dioxygenase (IDO): only an enzyme or a checkpoint controller? J. Onc. Sci. 3, 52–56 (2017).
Antonioli, L., Yegutkin, G. G., Pacher, P., Blandizzi, C. & Haskó, G. Anti-CD73 in cancer immunotherapy: awakening new opportunities. Trends Cancer 2, 95–109 (2016).
Schreiber, R. D., Old, L. J. & Smyth, M. J. Cancer immunoediting: integrating immunity’s roles in cancer suppression and promotion. Science 331, 1565–1570 (2011).
Linch, S. N., McNamara, M. J. & Redmond, W. L. OX40 agonists and combination immunotherapy: putting the pedal to the metal. Front. Oncol. 5, 34 (2015).
Lee, S.-W., Salek-Ardakani, S., Mittler, R. S. & Croft, M. Hypercostimulation through 4-1BB distorts homeostasis of immune cells. J. Immunol. 182, 6753 (2009).
Suntharalingam, G. et al. Cytokine storm in a phase 1 trial of the anti-CD28 monoclonal antibody TGN1412. N. Engl. J. Med. 355, 1018–1028 (2006).
Long, G. V. et al. Epacadostat (E) plus pembrolizumab (P) versus pembrolizumab alone in patients (pts) with unresectable or metastatic melanoma: results of the phase 3 ECHO-301/KEYNOTE-252 study [abstract]. J. Clin. Oncol. 36 (Suppl. 15), 108 (2018).
Lin, C. Z. et al. Advances in the mechanisms of action of cancer-targeting oncolytic viruses. Oncol. Lett. 15, 4053–4060 (2018).
Senior, M. Checkpoint inhibitors go viral. Nat. Biotechnol. 37, 12–17 (2019).
van Vloten, J. P., Workenhe, S. T., Wootton, S. K., Mossman, K. L. & Bridle, B. W. Critical interactions between immunogenic cancer cell death, oncolytic viruses, and the immune system define the rational design of combination immunotherapies. J. Immunol. 200, 450–458 (2018).
Chiocca, E. A. & Rabkin, S. D. Oncolytic viruses and their application to cancer immunotherapy. Cancer Immunol. Res. 2, 295–300 (2014).
Liu, B. L. et al. ICP34.5 deleted herpes simplex virus with enhanced oncolytic, immune stimulating, and anti-tumour properties. Gene Ther. 10, 292–303 (2003).
Toda, M., Martuza, R. L. & Rabkin, S. D. Tumor growth inhibition by intratumoral inoculation of defective herpes simplex virus vectors expressing granulocyte-macrophage colony-stimulating factor. Mol. Ther. 2, 324–329 (2000).
Andtbacka, R. H. et al. Talimogene laherparepvec improves durable response rate in patients with advanced melanoma. J. Clin. Oncol. 33, 2780–2788 (2015). This work was the first to demonstrate therapeutic benefit of an OV (T-VEC) in a randomized phase III clinical trial.
Harrington, K. J. et al. Phase I/II study of oncolytic HSV GM-CSF in combination with radiotherapy and cisplatin in untreated stage III/IV squamous cell cancer of the head and neck. Clin. Cancer Res. 16, 4005–4015 (2010). This study demonstrates the safety and efficacy of combining an OV (T-VEC) with full-dose chemoradiotherapy during curative treatment of head and neck cancer.
Heo, J. et al. Randomized dose-finding clinical trial of oncolytic immunotherapeutic vaccinia JX-594 in liver cancer. Nat. Med. 19, 329–336 (2013). This important study documents viral replication, transgene expression and immune stimulation in hepatocellular carcinoma patients treated with Pexa-Vec.
Mahalingam, D. et al. A phase II study of REOLYSIN® (pelareorep) in combination with carboplatin and paclitaxel for patients with advanced malignant melanoma. Cancer Chemother. Pharmacol. 79, 697–703 (2017).
Fukuhara, H., Ino, Y. & Todo, T. Oncolytic virus therapy: a new era of cancer treatment at dawn. Cancer Sci. 107, 1373–1379 (2016).
Bommareddy, P. K., Patel, A., Hossain, S. & Kaufman, H. L. Talimogene laherparepvec (T-VEC) and other oncolytic viruses for the treatment of melanoma. Am. J. Clin. Dermatol. 18, 1–15 (2017).
Russell, S. J. & Peng, K. W. Oncolytic virotherapy: a contest between apples and oranges. Mol. Ther. 25, 1107–1116 (2017).
Marabelle, A., Tselikas, L., de Baere, T. & Houot, R. Intratumoral immunotherapy: using the tumor as the remedy. Ann. Oncol. 28 (Suppl. 12), xii33–xii43 (2017).
de Graaf, J. F., de Vor, L., Fouchier, R. A. M. & van den Hoogen, B. G. Armed oncolytic viruses: a kick-start for anti-tumor immunity. Cytokine Growth Factor Rev. 41, 28–39 (2018).
Cattaneo, R. & Russell, S. J. How to develop viruses into anticancer weapons. PLOS Pathog. 13, e1006190 (2017).
Maroun, J. et al. Designing and building oncolytic viruses. Future Virol. 12, 193–213 (2017).
Stojdl, D. F. et al. Exploiting tumor-specific defects in the interferon pathway with a previously unknown oncolytic virus. Nat. Med. 6, 821–825 (2000). This paper outlines the rationale for leveraging tumour-specific interferon gene signatures for targeting OVs.
Strong, J. E., Coffey, M. C., Tang, D., Sabinin, P. & Lee, P. W. The molecular basis of viral oncolysis: usurpation of the Ras signaling pathway by reovirus. EMBO J. 17, 3351–3362 (1998).
Zhang, J. et al. A novel oncolytic adenovirus targeting Wnt signaling effectively inhibits cancer-stem like cell growth via metastasis, apoptosis and autophagy in HCC models. Biochem. Biophys. Res. Commun. 491, 469–477 (2017).
Fueyo, J. et al. Preclinical characterization of the antiglioma activity of a tropism-enhanced adenovirus targeted to the retinoblastoma pathway. J. Natl Cancer Inst. 95, 652–660 (2003).
Dhungel, B., Ramlogan-Steel, C. A. & Steel, J. C. MicroRNA-regulated gene delivery systems for research and therapeutic purposes. Molecules 23, E1500 (2018).
Leber, M. J. et al. Enhanced control of oncolytic measles virus using microRNA target sites. Mol. Ther. Oncolytics 9, 30–40 (2018).
Leber, M. J. et al. MicroRNA-sensitive oncolytic measles viruses for cancer-specific vector tropism. Mol. Ther. 19, 1097–1106 (2011).
Edge, R. E. et al. A let-7 microRNA-sensitive vesicular stomatitis virus demonstrates tumor-specific replication. Mol. Ther. 16, 1437–1443 (2008).
Ylösmäki, E. et al. Generation of a conditionally replicating adenovirus based on targeted destruction of E1A mRNA by a cell type-specific microRNA. J. Virol. 82, 11009–11015 (2008).
Deng, L. et al. Oncolytic efficacy of thymidine kinase-deleted vaccinia virus strain Guang9. Oncotarget 8, 40533–40543 (2017).
Han, J. et al. The E1B 19K protein blocks apoptosis by interacting with and inhibiting the p53-inducible and death-promoting Bax protein. Genes Dev. 10, 461–477 (1996).
Ogg, P. D., McDonell, P. J., Ryckman, B. J., Knudson, C. M. & Roller, R. J. The HSV-1 Us3 protein kinase is sufficient to block apoptosis induced by overexpression of a variety of Bcl-2 family members. Virology 319, 212–224 (2004).
Bommareddy, P. K., Shettigar, M. & Kaufman, H. L. Integrating oncolytic viruses in combination cancer immunotherapy. Nat. Rev. Immunol. 18, 498–513 (2018).
Breitbach, C. J. et al. Baseline neutralizing antibody status does not affect intravenous delivery of oncolytic vaccinia Pexa-Vec (JX-594) in liver cancer patients. Mol. Ther. 21, S7–S8 (2013). This important study demonstrates that the presence of nAbs does not necessarily prevent successful systemic delivery of an OV.
Haddad, D. Genetically engineered vaccinia viruses as agents for cancer treatment, imaging, and transgene delivery. Front. Oncol. 7, 96 (2017).
Ricca, J. M. et al. Pre-existing immunity to oncolytic virus potentiates its immunotherapeutic efficacy. Mol. Ther. 26, 1008–1019 (2018).
Baum, A. & Garcia-Sastre, A. Induction of type I interferon by RNA viruses: cellular receptors and their substrates. Amino Acids. 38, 1283–1299 (2010).
Sanjuan, R. & Domingo-Calap, P. Mechanisms of viral mutation. Cell. Mol. Life Sci. 73, 4433–4448 (2016).
Melcher, A., Parato, K., Rooney, C. M. & Bell, J. C. Thunder and lightning: immunotherapy and oncolytic viruses collide. Mol. Ther. 19, 1008–1016 (2011).
Meyers, D. E., Wang, A. A., Thirukkumaran, C. M. & Morris, D. G. Current immunotherapeutic strategies to enhance oncolytic virotherapy. Front. Oncol. 7, 114 (2017).
Choi, K. J. et al. Concurrent delivery of GM-CSF and B7-1 using an oncolytic adenovirus elicits potent antitumor effect. Gene Ther. 13, 1010–1020 (2006).
Breitbach, C. J. et al. Intravenous delivery of a multi-mechanistic cancer-targeted oncolytic poxvirus in humans. Nature 477, 99–102 (2011). This clinical study shows systemic delivery and therapeutic efficacy after intravenous delivery of an OV (JX-594).
Replimune. Immulytic platform. Replimune https://replimune.com/our-science/immulytic-platform/ (2019).
Doloff, J. C. & Waxman, D. J. Dual E1A oncolytic adenovirus: targeting tumor heterogeneity with two independent cancer-specific promoter elements, DF3/MUC1 and hTERT. Cancer Gene Ther. 18, 153 (2010).
Bartee, E., Bartee, M. Y., Bogen, B. & Yu, X. Z. Systemic therapy with oncolytic myxoma virus cures established residual multiple myeloma in mice. Mol. Ther. Oncolytics 3, 16032 (2016).
Marelli, G., Howells, A., Lemoine, N. R. & Wang, Y. Oncolytic viral therapy and the immune system: a double-edged sword against cancer. Front. Immunol. 9, 866 (2018).
Rehman, H., Silk, A. W., Kane, M. P. & Kaufman, H. L. Into the clinic: talimogene laherparepvec (T-VEC), a first-in-class intratumoral oncolytic viral therapy. J. Immunother. Cancer 4, 53 (2016).
Shen, Y. & Nemunaitis, J. Herpes simplex virus 1 (HSV-1) for cancer treatment. Cancer Gene Ther. 13, 975–992 (2006).
Masemann, D., Boergeling, Y. & Ludwig, S. Employing RNA viruses to fight cancer: novel insights into oncolytic virotherapy. Biol. Chem. 398, 891–909 (2017).
Durham, N. M. et al. Oncolytic VSV primes differential responses to immuno-oncology therapy. Mol. Ther. 25, 1917–1932 (2017).
Bridle, B. W. et al. Oncolytic vesicular stomatitis virus quantitatively and qualitatively improves primary CD8+ T cell responses to anticancer vaccines. OncoImmunology 2, e26013 (2013).
Zhu, J., Huang, X. & Yang, Y. Innate immune response to adenoviral vectors is mediated by both Toll-like receptor-dependent and -independent pathways. J. Virol. 81, 3170–3180 (2007).
Phillips, M. B. et al. Current understanding of reovirus oncolysis mechanisms. Oncolytic Virother. 7, 53–63 (2018).
Yount, J. S., Moran, T. M. & Lopez, C. B. Cytokine-independent upregulation of MDA5 in viral infection. J. Virol. 81, 7316–7319 (2007).
Smith, G. L., Symons, J. A. & Alcami, A. Poxviruses: interfering with interferon. Semin. Virol. 8, 409–418 (1998).
Sainz, B. Jr. & Halford, W. P. Alpha/beta interferon and gamma interferon synergize to inhibit the replication of herpes simplex virus type 1. J. Virol. 76, 11541–11550 (2002).
Corrales, L. & Gajewski, T. F. Molecular pathways: targeting the stimulator of interferon genes (STING) in the immunotherapy of cancer. Clin. Cancer Res. 21, 4774–4779 (2015).
Xia, T., Konno, H., Ahn, J. & Barber, G. N. Deregulation of STING signaling in colorectal carcinoma constrains DNA damage responses and correlates with tumorigenesis. Cell Rep. 14, 282–297 (2016).
Johnson, K. E., Song, B. & Knipe, D. M. Role for herpes simplex virus 1 ICP27 in the inhibition of type I interferon signaling. Virology 374, 487–494 (2008).
Patel, S. A. & Minn, A. J. Combination cancer therapy with immune checkpoint blockade: mechanisms and strategies. Immunity 48, 417–433 (2018).
Ghaffari, A. et al. STING agonist therapy in combination with PD-1 immune checkpoint blockade enhances response to carboplatin chemotherapy in high-grade serous ovarian cancer. Br. J. Cancer 119, 440–449 (2018).
Moesta, A. K. et al. Local delivery of OncoVEX (mGM-CSF) generates systemic antitumor immune responses enhanced by cytotoxic T-lymphocyte-associated protein blockade. Clin. Cancer Res. 23, 6190–6202 (2017).
Martin, N. T. & Bell, J. C. Oncolytic virus combination therapy: killing one bird with two stones. Mol. Ther. 26, 1414–1422 (2018).
Lavin, Y. et al. Innate immune landscape in early lung adenocarcinoma by paired single-cell analyses. Cell 169, 750–765 (2017).
LaRocca, C. J. & Warner, S. G. Oncolytic viruses and checkpoint inhibitors: combination therapy in clinical trials. Clin. Transl Med. 7, 35 (2018).
Harrington, K. J. et al. Safety and preliminary efficacy of talimogene laherparepvec (T-VEC) in combination (combo) with pembrolizumab (pembro) in patients (pts) with recurrent or metastatic squamous cell carcinoma of the head and neck (R/M HNSCC): a multicenter, phase 1b study (MASTERKEY-232) [abstract]. J. Clin. Oncol. 36 (Suppl. 15), 6036 (2018).
Chesney, J. et al. Randomized, open-label phase II study evaluating the efficacy and safety of talimogene laherparepvec in combination with ipilimumab versus ipilimumab alone in patients with advanced, unresectable melanoma. J. Clin. Oncol. 36, 1658–1667 (2018). This clinical study reports improved efficacy when combining an OV (T-VEC) with CTLA-4 immune checkpoint inhibition.
Gubin, M. M. et al. Checkpoint blockade cancer immunotherapy targets tumour-specific mutant antigens. Nature 515, 577–581 (2014).
Zaretsky, J. M. et al. Mutations associated with acquired resistance to PD-1 blockade in melanoma. N. Engl. J. Med. 375, 819–829 (2016).
Simpson, G. R. et al. Combination of a fusogenic glycoprotein, prodrug activation, and oncolytic herpes simplex virus for enhanced local tumor control. Cancer Res. 66, 4835–4842 (2006).
Currier, M. A. et al. Efficacy and safety of the oncolytic herpes simplex virus rRp450 alone and combined with cyclophosphamide. Mol. Ther. 16, 879–885 (2008).
Selman, M. et al. Dimethyl fumarate potentiates oncolytic virotherapy through NF-κB inhibition. Sci. Transl Med. 10, eaao1613 (2018).
Sharp, D. W. & Lattime, E. C. Recombinant poxvirus and the tumor microenvironment: oncolysis, immune regulation and immunization. Biomedicines 4, 19 (2016).
Pol, J. G. et al. Preclinical evaluation of a MAGE-A3 vaccination utilizing the oncolytic Maraba virus currently in first-in-human trials. Oncoimmunology 8, e1512329 (2018).
Zamarin, D. et al. Localized oncolytic virotherapy overcomes systemic tumor resistance to immune checkpoint blockade immunotherapy. Sci. Transl Med. 6, 226ra32 (2014). This work demonstrates that an oncolytic Newcastle disease virus can generate curative abscopal effects through virus-indirect mechanisms when given in combination with checkpoint inhibitors.
Benencia, F. et al. HSV oncolytic therapy upregulates interferon-inducible chemokines and recruits immune effector cells in ovarian cancer. Mol. Ther. 12, 789–802 (2005).
Li, J. et al. Expression of CCL19 from oncolytic vaccinia enhances immunotherapeutic potential while maintaining oncolytic activity. Neoplasia 14, 1115–1121 (2012).
Nishio, N. et al. Armed oncolytic virus enhances immune functions of chimeric antigen receptor-modified T cells in solid tumors. Cancer Res. 74, 5195–5205 (2014). This work demonstrates enhanced activity of CAR-T cell therapy by a combination with an armed OV expressing IL-15.
Rosewell Shaw, A. & Suzuki, M. Oncolytic viruses partner with T cell therapy for solid tumor treatment. Front. Immunol. 9, 2103 (2018).
Ajina, A. & Maher, J. Prospects for combined use of oncolytic viruses and CAR T cells. J. Immunother. Cancer 5, 90 (2017).
Saha, D., Martuza, R. L. & Rabkin, S. D. Macrophage polarization contributes to glioblastoma eradication by combination immunovirotherapy and immune checkpoint blockade. Cancer Cell 32, 253–267 (2017).
Chon, H. J. et al. Tumor microenvironment remodeling by intratumoral oncolytic vaccinia virus enhances the efficacy of immune-checkpoint blockade. Clin. Cancer Res. 25, 1612–1623 (2019).
Kowalsky, S. J. et al. Superagonist IL-15-armed oncolytic virus elicits potent antitumor immunity and therapy that are enhanced with PD-1 blockade. Mol. Ther. 26, 2476–2486 (2018).
June, C. H., O’Connor, R. S., Kawalekar, O. U., Ghassemi, S. & Milone, M. C. CAR T cell immunotherapy for human cancer. Science 359, 1361–1365 (2018).
Tanoue, K. et al. Armed oncolytic adenovirus-expressing PD-L1 mini-body enhances antitumor effects of chimeric antigen receptor T cells in solid tumors. Cancer Res. 77, 2040–2051 (2017).
Fajardo, C. A. et al. Oncolytic adenoviral delivery of an EGFR-targeting T cell engager improves antitumor efficacy. Cancer Res. 77, 2052–2063 (2017).
Freedman, J. D. et al. Oncolytic adenovirus expressing bispecific antibody targets T cell cytotoxicity in cancer biopsies. EMBO Mol. Med. 9, 1067–1087 (2017).
von Ahrens, D., Bhagat, T. D., Nagrath, D., Maitra, A. & Verma, A. The role of stromal cancer-associated fibroblasts in pancreatic cancer. J. Hematol. Oncol. 10, 76 (2017).
Ganesh, S., Gonzalez-Edick, M., Gibbons, D., Van Roey, M. & Jooss, K. Intratumoral coadministration of hyaluronidase enzyme and oncolytic adenoviruses enhances virus potency in metastatic tumor models. Clin. Cancer Res. 14, 3933–3941 (2008).
Choi, I. K. et al. Effect of decorin on overcoming the extracellular matrix barrier for oncolytic virotherapy. Gene Ther. 17, 190–201 (2010).
Kim, J. H. et al. Relaxin expression from tumor-targeting adenoviruses and its intratumoral spread, apoptosis induction, and efficacy. J. Natl Cancer Inst. 98, 1482–1493 (2006).
Vera, B. et al. Characterization of the antiglioma effect of the oncolytic adenovirus VCN-01. PLoS One 11, e0147211 (2016).
Tysome, J. R., Lemoine, N. R. & Wang, Y. Update on oncolytic viral therapy — targeting angiogenesis. Onco. Targets Ther. 6, 1031–1040 (2013).
Hou, W., Chen, H., Rojas, J., Sampath, P. & Thorne, S. H. Oncolytic vaccinia virus demonstrates antiangiogenic effects mediated by targeting of VEGF. Int. J. Cancer 135, 1238–1246 (2014).
Berkey, S. E., Thorne, S. H. & Bartlett, D. L. Oncolytic virotherapy and the tumor microenvironment. Adv. Exp. Med. Biol. 1036, 157–172 (2017).
Wojton, J. & Kaur, B. Impact of tumor microenvironment on oncolytic viral therapy. Cytokine Growth Factor Rev. 21, 127–134 (2010).
Koyama, S. et al. Adaptive resistance to therapeutic PD-1 blockade is associated with upregulation of alternative immune checkpoints. Nat. Commun. 7, 10501 (2016).
Marin-Acevedo, J. A., Soyano, A. E., Dholaria, B., Knutson, K. L. & Lou, Y. Cancer immunotherapy beyond immune checkpoint inhibitors. J. Hematol. Oncol. 11, 8 (2018).
Brown, M. C. et al. Cancer immunotherapy with recombinant poliovirus induces IFN-dominant activation of dendritic cells and tumor antigen-specific CTLs. Sci. Transl Med. 9, eaan4220 (2017).
Prestwich, R. J. et al. Tumor infection by oncolytic reovirus primes adaptive antitumor immunity. Clin. Cancer Res. 14, 7358–7366 (2008).
Hou, W., Sampath, P., Rojas, J. J. & Thorne, S. H. Oncolytic virus-mediated targeting of PGE2 in the tumor alters the immune status and sensitizes established and resistant tumors to immunotherapy. Cancer Cell 30, 108–119 (2016).
Engeland, C. E. et al. CTLA-4 and PD-L1 checkpoint blockade enhances oncolytic measles virus therapy. Mol. Ther. 22, 1949–1959 (2014). This early report describes the potential value of combining an OV (measles) with immune checkpoint inhibition.
Kleinpeter, P. et al. Vectorization in an oncolytic vaccinia virus of an antibody, a Fab and a scFv against programmed cell death-1 (PD-1) allows their intratumoral delivery and an improved tumor-growth inhibition. OncoImmunology 5, e1220467 (2016).
Passaro, C. et al. Arming an oncolytic herpes simplex virus type 1 with a single-chain fragment variable antibody against PD-1 for experimental glioblastoma therapy. Clin. Cancer Res. 25, 290–299 (2019).
Bartee, M. Y., Dunlap, K. M. & Bartee, E. Tumor-localized secretion of soluble PD1 enhances oncolytic virotherapy. Cancer Res. 77, 2952–2963 (2017).
Hamilton, J. R., Vijayakumar, G. & Palese, P. A recombinant antibody-expressing influenza virus delays tumor growth in a mouse model. Cell Rep. 22, 1–7 (2018).
Dong, C. et al. ICOS co-stimulatory receptor is essential for T cell activation and function. Nature 409, 97–101 (2001).
Zamarin, D. et al. Intratumoral modulation of the inducible co-stimulator ICOS by recombinant oncolytic virus promotes systemic anti-tumour immunity. Nat. Commun. 8, 14340 (2017).
Croft, M., So, T., Duan, W. & Soroosh, P. The significance of OX40 and OX40L to T cell biology and immune disease. Immunol. Rev. 229, 173–191 (2009).
Jiang, H. et al. Oncolytic adenovirus and tumor-targeting immune modulatory therapy improve autologous cancer vaccination. Cancer Res. 77, 3894–3907 (2017).
Wang, P. et al. Re-designing interleukin-12 to enhance its safety and potential as an anti-tumor immunotherapeutic agent. Nat. Commun. 8, 1395 (2017).
Poutou, J. et al. Safety and antitumor effect of oncolytic and helper-dependent adenoviruses expressing interleukin-12 variants in a hamster pancreatic cancer model. Gene Ther. 22, 696–706 (2015).
Barrett, J. A. et al. Regulated intratumoral expression of IL-12 using a RheoSwitch Therapeutic System®) (RTS®) gene switch as gene therapy for the treatment of glioma. Cancer Gene Ther. 25, 106–116 (2018).
Wollmann, G., Paglino, J. C., Maloney, P. R., Ahmadi, S. A. & van den Pol, A. N. Attenuation of vesicular stomatitis virus infection of brain using antiviral drugs and an adeno-associated virus-interferon vector. Virology 475, 1–14 (2015).
Cloughesy, T. F. et al. Durable complete responses in some recurrent high grade glioma patients treated with Toca 511 + Toca FC. Neuro-oncology 20, 1383–1392 (2018).
Chalikonda, S. et al. Oncolytic virotherapy for ovarian carcinomatosis using a replication-selective vaccinia virus armed with a yeast cytosine deaminase gene. Cancer Gene Ther. 15, 115–125 (2008).
Yamada, S. et al. Oncolytic herpes simplex virus expressing yeast cytosine deaminase: relationship between viral replication, transgene expression, prodrug bioactivation. Cancer Gene Ther. 19, 160–170 (2012).
Hermiston, T. Gene delivery from replication-selective viruses: arming guided missiles in the war against cancer. J. Clin. Invest. 105, 1169–1172 (2000).
The electronic Medicines Compendium (eMC). Imlygic — summary of product characteristics (SmPC). eMC https://www.medicines.org.uk/emc/product/5117/smpc (2019).
Wongthida, P. et al. Activating systemic T cell immunity against self tumor antigens to support oncolytic virotherapy with vesicular stomatitis virus. Hum. Gene Ther. 22, 1343–1353 (2011).
Vigil, A., Martinez, O., Chua, M. A. & Garcia-Sastre, A. Recombinant Newcastle disease virus as a vaccine vector for cancer therapy. Mol. Ther. 16, 1883–1890 (2008).
Näslund, T. I. et al. Comparative prime-boost vaccinations using semliki forest virus, adenovirus, and ALVAC vectors demonstrate differences in the generation of a protective central memory ctl response against the P815 tumor. J. Immunol. 178, 6761 (2007).
Jonker, D. J. et al. Phase I study of oncolytic virus (OV) MG1 maraba/MAGE-A3 (MG1MA3), with and without transgenic MAGE-A3 adenovirus vaccine (AdMA3) in incurable advanced/metastatic MAGE-A3-expressing solid tumours: CCTG IND.214 [abstract]. J. Clin. Oncol. 35 (Suppl. 15), e14637 (2017).
de Camargo, T. M. et al. Prime-boost vaccination with recombinant protein and adenovirus-vector expressing Plasmodium vivax circumsporozoite protein (CSP) partially protects mice against Pb/Pv sporozoite challenge. Sci. Rep. 8, 1118 (2018).
Chmielewska, A. M. et al. Combined adenovirus vector and hepatitis C virus envelope protein prime-boost regimen elicits T cell and neutralizing antibody immune responses. J. Virol. 88, 5502–5510 (2014).
Cuburu, N. et al. Adenovirus vector-based prime-boost vaccination via heterologous routes induces cervicovaginal CD8+ T cell responses against HPV16 oncoproteins. Int. J. Cancer 142, 1467–1479 (2018).
Le Boeuf, F. et al. Synergistic interaction between oncolytic viruses augments tumor killing. Mol. Ther. 18, 888–895 (2010). This paper shows the potential for combination of heterologous OVs to enhance therapeutic activity.
Turnbull, S. et al. Evidence for oncolytic virotherapy: where have we got to and where are we going? Viruses 7, 6291–6312 (2015).
Zhang, Y. Q., Tsai, Y. C., Monie, A., Wu, T. C. & Hung, C. F. Enhancing the therapeutic effect against ovarian cancer through a combination of viral oncolysis and antigen-specific immunotherapy. Mol. Ther. 18, 692–699 (2010).
Carrio, M. et al. Enhanced pancreatic tumor regression by a combination of adenovirus and retrovirus-mediated delivery of the herpes simplex virus thymidine kinase gene. Gene Ther. 6, 547–553 (1999).
Ribas, A. et al. Association of pembrolizumab with tumor response and survival among patients with advanced melanoma. JAMA 315, 1600–1609 (2016).
Puzanov, I. et al. Talimogene laherparepvec in combination with ipilimumab in previously untreated, unresectable stage IIIB–IV melanoma. J. Clin. Oncol. 34, 2619–2626 (2016). This study assesses the activity of T-VEC in combination with CTLA-4 blockade in patients with stage IIIB–IV melanoma.
Chesney, J. A. et al. Primary results from a randomized (1:1), open-label phase II study of talimogene laherparepvec (T) and ipilimumab (I) versus I alone in unresected stage IIIB–IV melanoma [abstract]. J. Clin. Oncol. 35 (Suppl. 15), 9509 (2017).
Long, G. V. et al. Efficacy analysis of MASTERKEY-265 phase 1b study of talimogene laherparepvec (T-VEC) and pembrolizumab (pembro) for unresectable stage IIIB–IV melanoma [abstract]. J. Clin. Oncol. 34 (Suppl. 15), 9568 (2016).
Adair, R. A. et al. Cell carriage, delivery, and selective replication of an oncolytic virus in tumor in patients. Sci. Transl Med. 4, 138ra77 (2012). This study establishes that cell carriers could be utilized to deliver an oncolytic reovirus to tumours while shielding it from nAbs.
Hadac, E. M., Kelly, E. J. & Russell, S. J. Myeloma xenograft destruction by a nonviral vector delivering oncolytic infectious nucleic acid. Mol. Ther. 19, 1041–1047 (2011). This paper illustrates that OVs can be delivered not just as purified viral particles, but through delivery of nucleic acid encoding the viral genome.
Louis Jeune, V., Joergensen, J. A., Hajjar, R. J. & Weber, T. Pre-existing anti-adeno-associated virus antibodies as a challenge in AAV gene therapy. Hum. Gene Ther. Methods. 24, 59–67 (2013).
Peng, K. W. et al. Pharmacokinetics of oncolytic measles virotherapy: eventual equilibrium between virus and tumor in an ovarian cancer xenograft model. Cancer Gene Ther. 13, 732 (2006).
Andtbacka, R. H. et al. Patterns of clinical response with talimogene laherparepvec (T-VEC) in patients with melanoma treated in the OPTIM phase III clinical trial. Ann. Surg. Oncol. 23, 4169–4177 (2016).
Kaufman, H. L. et al. Systemic versus local responses in melanoma patients treated with talimogene laherparepvec from a multi-institutional phase II study. J. Immunother. Cancer 4, 12 (2016).
Marabelle, A. et al. Starting the fight in the tumor: expert recommendatoins for the development of human intratumoral immunotherapy (HIT-IT). Ann. Oncol. 29, 2163–2174 (2018). This paper offers an elegant description of the opportunities and challenges associated with intratumoural and sets out suggestions for defining and measuring responses.
Liu, T. C. & Kirn, D. Systemic efficacy with oncolytic virus therapeutics: clinical proof-of-concept and future directions. Cancer Res. 67, 429–432 (2007).
Ferguson, M. S., Lemoine, N. R. & Wang, Y. Systemic delivery of oncolytic viruses: hopes and hurdles. Adv. Virol. 2012, 805629 (2012).
White, C. L. et al. Characterization of the adaptive and innate immune response to intravenous oncolytic reovirus (Dearing type 3) during a phase I clinical trial. Gene Ther. 15, 911–920 (2008).
Garcia-Carbonero, R. et al. Phase 1 study of intravenous administration of the chimeric adenovirus enadenotucirev in patients undergoing primary tumor resection. J. Immunother. Cancer 5, 71 (2017).
Kulu, Y. et al. Comparison of intravenous versus intraperitoneal administration of oncolytic herpes simplex virus 1 (HSV-1) for peritoneal carcinomatosis in mice. Cancer Gene Ther. 16, 291–297 (2009).
Galanis, E. et al. Oncolytic measles virus expressing the sodium iodide symporter to treat drug-resistant ovarian cancer. Cancer Res. 75, 22–30 (2015). This study shows that NIS, a reporter gene encoded in oncolytic measles virus, could be utilized for non-invasive monitoring of virus localization in patients receiving an OV.
Pencavel, T. D. et al. Isolated limb perfusion with melphalan, tumour necrosis factor-alpha and oncolytic vaccinia virus improves tumour targeting and prolongs survival in a rat model of advanced extremity sarcoma. Int. J. Cancer 136, 965–976 (2015).
Wilkinson, M. J. et al. Isolated limb perfusion with biochemotherapy and oncolytic virotherapy combines with radiotherapy and surgery to overcome treatment resistance in an animal model of extremity soft tissue sarcoma. Int. J. Cancer. 139, 1414–1422 (2016).
Rainov, N. G. & Ren, H. Oncolytic viruses for treatment of malignant brain tumours. Acta Neurochir. Suppl. 88, 113–123 (2003).
Yin, D. et al. Convection-enhanced delivery improves distribution and efficacy of tumor-selective retroviral replicating vectors in a rodent brain tumor model. Cancer Gene Ther. 20, 336–341 (2013).
Hong, S. H., Park, S. J., Lee, S., Cho, C. S. & Cho, M. H. Aerosol gene delivery using viral vectors and cationic carriers for in vivo lung cancer therapy. Expert Opin. Drug Deliv. 12, 977–991 (2015).
MVDP author group et al. Safety and immunogenicity of dry powder measles vaccine administered by inhalation: a randomized controlled phase I clinical trial. Vaccine 32, 6791–6797 (2014).
Low, N., Bavdekar, A., Jeyaseelan, L. & Hirve, S. A randomized controlled trial of an aerosolized vaccine against measles. N. Engl. J. Med. 372, 1519–1529 (2015).
Pencavel, T. et al. Locoregional intravascular viral therapy of cancer: precision guidance for Paris’s arrow? Gene Ther. 17, 949–960 (2010).
Geevarghese, S. K. et al. Phase I/II study of oncolytic herpes simplex virus NV1020 in patients with extensively pretreated refractory colorectal cancer metastatic to the liver. Hum. Gene Ther. 21, 1119–1128 (2010).
Carlisle, R. et al. Enhanced tumor uptake and penetration of virotherapy using polymer stealthing and focused ultrasound. J. Natl Cancer Inst. 105, 1701–1710 (2013).
Myers, R. et al. Polymeric cups for cavitation-mediated delivery of oncolytic vaccinia virus. Mol. Ther. 24, 1627–1633 (2016).
Wolchok, J. D. et al. Guidelines for the evaluation of immune therapy activity in solid tumors: immune-related response criteria. Clin. Cancer Res. 15, 7412–7420 (2009).
Kaufman, H. L. et al. Durable response rate as an endpoint in cancer immunotherapy: insights from oncolytic virus clinical trials. J. Immunother. Cancer 5, 72 (2017).
Anagnostou, V. et al. Immuno-oncology trial endpoints: capturing clinically meaningful activity. Clin. Cancer Res. 23, 4959–4969 (2017).
Harrington, K. J. et al. Clinical development of talimogene laherparepvec (T-VEC): a modified herpes simplex virus type-1-derived oncolytic immunotherapy. Expert Rev. Anticancer Ther. 15, 1389–1403 (2015).
Wolchok, J. D. et al. Nivolumab plus ipilimumab in advanced melanoma. N. Engl. J. Med. 369, 122–133 (2013).
Swift, S. L. & Stojdl, D. F. Big data offers novel insights for oncolytic virus immunotherapy. Viruses 8, 45 (2016).
Pitt, J. M. et al. Resistance mechanisms to immune-checkpoint blockade in cancer: tumor-intrinsic and -extrinsic factors. Immunity 44, 1255–1269 (2016).
Barber, D. L. et al. Restoring function in exhausted CD8 T cells during chronic viral infection. Nature 439, 682–687 (2006).
Shin, H. & Wherry, E. J. CD8 T cell dysfunction during chronic viral infection. Curr. Opin. Immunol. 19, 408–415 (2007).
Liu, Z., Ravindranathan, R., Kalinski, P., Guo, Z. S. & Bartlett, D. L. Rational combination of oncolytic vaccinia virus and PD-L1 blockade works synergistically to enhance therapeutic efficacy. Nat. Commun. 8, 14754 (2017).
Ma, Z. & Damania, B. The cGAS-STING defense pathway and its counteraction by viruses. Cell Host Microbe 19, 150–158 (2016).
Lyons, Y. A., Wu, S. Y., Overwijk, W. W., Baggerly, K. A. & Sood, A. K. Immune cell profiling in cancer: molecular approaches to cell-specific identification. NPJ Precis. Oncol. 1, 26 (2017).
Ansel, A., Rosenzweig, J. P., Zisman, P. D. & Gesundheit, B. Monitoring the efficacy of oncolytic viruses via gene expression. Front. Oncol. 7, 264 (2017).
Zloza, A. et al. Immunoglobulin-like transcript 2 (ILT2) is a biomarker of therapeutic response to oncolytic immunotherapy with vaccinia viruses. J. Immunother. Cancer 2, 1 (2014).
Terasawa, Y. et al. Activity levels of cathepsins B and L in tumor cells are a biomarker for efficacy of reovirus-mediated tumor cell killing. Cancer Gene Ther. 22, 188–197 (2015).
Liikanen, I. et al. Serum HMGB1 is a predictive and prognostic biomarker for oncolytic immunotherapy. OncoImmunology 4, e989771 (2015).
Tsoneva, D. et al. Drug-encoded biomarkers for monitoring biological therapies. PLoS One 10, e0137573 (2015).
Haddad, D. & Fong, Y. Molecular imaging of oncolytic viral therapy. Mol. Ther. Oncolytics 1, 14007 (2015).
Hastie, E. et al. Novel biomarkers of resistance of pancreatic cancer cells to oncolytic vesicular stomatitis virus. Oncotarget 7, 61601–61618 (2016).
Larson, C. et al. Going viral: a review of replication-selective oncolytic adenoviruses. Oncotarget 6, 19976–19989 (2015).
Breitbach, C. J., Moon, A., Burke, J., Hwang, T. H. & Kirn, D. H. A phase 2, open-label, randomized study of Pexa-Vec (JX-594) administered by intratumoral injection in patients with unresectable primary hepatocellular carcinoma. Methods Mol. Biol. 1317, 343–357 (2015).
Andtbacka, R. H. I. et al. Final data from CALM: a phase II study of coxsackievirus A21 (CVA21) oncolytic virus immunotherapy in patients with advanced melanoma [abstract]. J. Clin. Oncol. 33 (Suppl. 15), 9030 (2015). This study describes the activity of an oncolytic picornavirus, coxsackievirus A21, as a monotherapy in patients with melanoma.
Hotte, S. J. et al. An optimized clinical regimen for the oncolytic virus PV701. Clin. Cancer Res. 13, 977–985 (2007). This study illustrates the importance of the dose and schedule in OV delivery.
Laurie, S. A. et al. A phase 1 clinical study of intravenous administration of PV701, an oncolytic virus, using two-step desensitization. Clin. Cancer Res. 12, 2555–2562 (2006).
Pol, J. et al. Trial watch — oncolytic viruses and cancer therapy. Oncoimmunology 5, e1117740 (2015).
Kurokawa, C. et al. Constitutive interferon pathway activation in tumors as an efficacy determinant following oncolytic virotherapy. J. Natl Cancer Inst. 110, 1123–1132 (2018). This paper identifies constitutive interferon signalling as a resistance gene therapy signature for oncolytic measles viruses that predicts replication and response in patient tumours.
Ebrahimi, S. et al. Interferon-mediated tumor resistance to oncolytic virotherapy. J. Cell. Biochem. 118, 1994–1999 (2017).
Bazan-Peregrino, M., Carlisle, R. C., Purdie, L. & Seymour, L. W. Factors influencing retention of adenovirus within tumours following direct intratumoural injection. Gene Ther. 15, 688–694 (2008).
Kolodkin-Gal, D. et al. Herpes simplex virus type 1 preferentially targets human colon carcinoma: role of extracellular matrix. J. Virol. 82, 999–1010 (2008).
Thomas, M. A., Broughton, R. S., Goodrum, F. D. & Ornelles, D. A. E4orf1 limits the oncolytic potential of the E1B-55K deletion mutant adenovirus. J. Virol. 83, 2406–2416 (2009).
Zou, A., Atencio, I., Huang, W. M., Horn, M. & Ramachandra, M. Overexpression of adenovirus E3-11.6K protein induces cell killing by both caspase-dependent and caspase-independent mechanisms. Virology 326, 240–249 (2004).
Chai, L. et al. A novel conditionally replicating adenoviral vector with dual expression of IL-24 and arresten inserted in E1 and the region between E4 and fiber for improved melanoma therapy. Cancer Gene Ther. 19, 247–254 (2012).
Filley, A. C. & Dey, M. Immune system, friend or foe of oncolytic virotherapy? Front. Oncol. 7, 106 (2017).
Aurelian, L. Oncolytic viruses as immunotherapy: progress and remaining challenges. Onco. Targets Ther. 9, 2627–2637 (2016).
Moehler, M. et al. in 9th ILCA Annual Conference 24 (International Liver Cancer Association, 2015).
Desjardins, A. et al. Recurrent glioblastoma treated with recombinant poliovirus. N. Engl. J. Med. 379, 150–161 (2018). This study reports the phase I results of an oncolytic poliovirus chimera, PVSRIPO, for the treatment of high-grade glioma.
Lang, F. F. et al. Phase I study of DNX-2401 (Delta-24-RGD) oncolytic adenovirus: replication and immunotherapeutic effects in recurrent malignant glioma. J. Clin. Oncol. 36, 1419–1427 (2018).
Andtbacka, R. H. I. et al. Final results of a phase II multicenter trial of HF10, a replication-competent HSV-1 oncolytic virus, and ipilimumab combination treatment in patients with stage IIIB–IV unresectable or metastatic melanoma [abstract]. J. Clin. Oncol. 35 (Suppl. 15), 9510 (2017).
Acknowledgements
Medical writing support, which was in accordance with Good Publication Practice (GPP3) guidelines, was provided by S. Gilbert and H. Dbouk of Cirrus Communications (New York, NY, USA), an Ashfield company, and was funded by AstraZeneca.
Author information
Authors and Affiliations
Contributions
K.H. and D.J.F. provided research data for the article and a substantial contribution to discussion of content, and were involved in writing and reviewing/editing of the manuscript. B.K., J.H. and J.-C.S. provided research data for the article, wrote, reviewed and edited the manuscript before submission.
Corresponding author
Ethics declarations
Competing interests
D.J.F., B.K., J.H. and J.-C.S. are employees of and own stocks and shares in AstraZeneca. K.H. has received research funding from Oncolytics Biotech and Genelux GmbH, and honoraria from Amgen, Viralytics and Oncos Therapeutics. J.-C.S. has received consultancy fees from AstraZeneca, Astex, Clovis, GSK, GamaMabs, Eli Lilly, MSD, Mission Therapeutics, Merus, Pfizer, PharmaMar Pierre Fabre, Roche-Genentech, Sanofi, Servier, Symphogen and Takeda, and has been a full-time employee of AstraZeneca since September 2017.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Related links
ClinicalTrials.gov: https://www.clinicaltrials.gov
Glossary
- Vaccinia virus
-
Large, double-stranded DNA virus belonging to the poxvirus family.
- Neutralizing antibodies
-
(nAbs). Antibodies that bind to a virus and interfere with its ability to infect a cell.
- Fusogenic activity
-
The ability of a virus to fuse directly with target cells, resulting in immunogenic cell death by activation of the adaptive and innate immune systems.
- Seroconversion
-
The development of detectable IgG antibodies in the blood that are directed against an infectious agent.
- Chimeric antigen receptor (CAR)-T cells
-
T cells that have been genetically engineered to express an antigen receptor that recognizes an antigen present only on the surface of malignant cells. Upon recognition and binding of CAR-T cells to antigen-specific tumour cells, the tumour cells are killed.
- Extracellular matrix
-
A 3D network of extracellular macromolecules forming the non-cellular component of tissues that provides structural and biochemical support for surrounding cells.
- Prime-boost regimens
-
The use of multiple vaccinations, using the same (homologous) or different (heterologous) vaccines each time, in order to develop cell-mediated immunity.
- Chimeric adenovirus
-
A recombinant adenovirus containing a mixture of DNA from different adenoviral strains that may have different infection potency and activity to the original, ‘parental’ adenoviruses.
- Loculated
-
Characterized by fluid-filled cavities and divisions within an organ or body cavity that are separated from the remainder of the tissue with mucous, serous or fibrous membranes.
Rights and permissions
About this article
Cite this article
Harrington, K., Freeman, D.J., Kelly, B. et al. Optimizing oncolytic virotherapy in cancer treatment. Nat Rev Drug Discov 18, 689–706 (2019). https://doi.org/10.1038/s41573-019-0029-0
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41573-019-0029-0
This article is cited by
-
Recent advances in various adeno-associated viruses (AAVs) as gene therapy agents in hepatocellular carcinoma
Virology Journal (2024)
-
Competition-driven eco-evolutionary feedback reshapes bacteriophage lambda’s fitness landscape and enables speciation
Nature Communications (2024)
-
Mesenchymal stem cell-released oncolytic virus: an innovative strategy for cancer treatment
Cell Communication and Signaling (2023)
-
Dynamic profiling of medulloblastoma surfaceome
Acta Neuropathologica Communications (2023)
-
Improving the therapeutic efficacy of oncolytic viruses for cancer: targeting macrophages
Journal of Translational Medicine (2023)