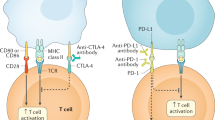
Abstract
The immune-related adverse events associated with treatment with immune checkpoint inhibitors result in significant morbidity for patients as well as considerable cost to the health-care system, and can limit the use of these beneficial drugs. Understanding the mechanisms of these side effects and how they can be separated from the antitumour effects of immune checkpoint inhibitors, as well as identifying biomarkers that predict the development of immune-related toxicities, will facilitate the conduct of trials to limit their onset and improve patient outcomes. In this Review, we discuss the different types of immune-related adverse events and how their treatment and identification of possible predictive biomarkers may shed light on their mechanisms, and describe possible strategies and targets for prophylactic and therapeutic intervention to mitigate them.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout

Similar content being viewed by others
References
Hodi, F. S. et al. Improved survival with ipilimumab in patients with metastatic melanoma. N. Engl. J. Med. 363, 711–723 (2010). This is the first article to show survival advantage in patients with melanoma treated with CTLA4 blockade.
Du, S. et al. PD-1 modulates radiation-induced cardiac toxicity through cytotoxic T lymphocytes. J. Thorac. Oncol. 13, 510–520 (2018).
Vogrig, A. et al. Central nervous system complications associated with immune checkpoint inhibitors. J. Neurol. Neurosurg. Psychiatry 91, 772–778 (2020).
Naidoo, J. et al. Autoimmune bullous skin disorders with immune checkpoint inhibitors targeting PD-1 and PD-L1. Cancer Immunol. Res. 4, 383–389 (2016).
Cortellini, A. et al. Correlations between the immune-related adverse events spectrum and efficacy of anti-PD1 immunotherapy in NSCLC patients. Clin. Lung Cancer 20, 237–247 (2019).
Maher, V. E. et al. Analysis of the association between adverse events and outcome in patients receiving a programmed death protein 1 or programmed death ligand 1 antibody. J. Clin. Oncol. 37, 2730–2737 (2019).
Weber, J. S. et al. Safety profile of nivolumab monotherapy: a pooled analysis of patients with advanced melanoma. J. Clin. Oncol. 35, 785–792 (2017).
Matsushita, H. et al. Cancer exome analysis reveals a T-cell-dependent mechanism of cancer immunoediting. Nature 482, 400–404 (2012).
DuPage, M., Mazumdar, C., Schmidt, L. M., Cheung, A. F. & Jacks, T. Expression of tumour-specific antigens underlies cancer immunoediting. Nature 482, 405–409 (2012).
Helmink, B. A. et al. B cells and tertiary lymphoid structures promote immunotherapy response. Nature 577, 549–555 (2020).
Cabrita, R. et al. Tertiary lymphoid structures improve immunotherapy and survival in melanoma. Nature 577, 561–565 (2020).
Dunn, G. P., Bruce, A. T., Ikeda, H., Old, L. J. & Schreiber, R. D. Cancer immunoediting: from immunosurveillance to tumor escape. Nat. Immunol. 3, 991–998 (2002).
Wei, S. C., Duffy, C. R. & Allison, J. P. Fundamental mechanisms of immune checkpoint blockade therapy. Cancer Discov. 8, 1069–1086 (2018). This is an important review of the mechanisms of ICI therapy.
Leach, D. R., Krummel, M. F. & Allison, J. P. Enhancement of antitumor immunity by CTLA-4 blockade. Science 271, 1734–1736 (1996).
Topalian, S. L. et al. Safety, activity, and immune correlates of anti-PD-1 antibody in cancer. N. Engl. J. Med. 2366, 2443–2454 (2012). This is the first documentation of the benefit of PD1 blockade in multiple cancers.
Hamid, O. et al. Safety and tumor responses with lambrolizumab (anti-PD-1) in melanoma. N. Engl. J. Med. 369, 134–144 (2013).
Weber, J. S. et al. Nivolumab versus chemotherapy in patients with advanced melanoma who progressed after anti-CTLA-4 treatment (CheckMate 037): a randomised, controlled, open-label, phase 3 trial. Lancet Oncol. 16, 375–384 (2015).
Sanderson, K. et al. Autoimmunity in a phase I trial of a fully human anti-cytotoxic T-lymphocyte antigen-4 monoclonal antibody with multiple melanoma peptides and Montanide ISA 51 for patients with resected stages III and IV melanoma. J. Clin. Oncol. 23, 741–750 (2005).
Weber, J. S., Kahler, K. C. & Hauschild, A. Management of immune-related adverse events and kinetics of response with ipilimumab. J. Clin. Oncol. 30, 2691–2697 (2012).
Pauken, K. E., Dougan, M., Rose, N. R., Lichtman, A. H. & Sharpe, A. H. Adverse events following cancer immunotherapy: obstacles and opportunities. Trends Immunol. 40, 511–523 (2019).
Boutros, C. et al. Safety profiles of anti-CTLA-4 and anti-PD-1 antibodies alone and in combination. Nat. Rev. Clin. Oncol. 13, 473–486 (2016).
Young, A., Quandt, Z. & Bluestone, J. A. The balancing act between cancer immunity and autoimmunity in response to immunotherapy. Cancer Immunol. Res. 6, 1445–1452 (2018).
Wang, Y. et al. Treatment-related adverse events of PD-1 and PD-L1 inhibitors in clinical trials: a systematic review and meta-analysis. JAMA Oncol. 5, 1008–1019 (2019).
Martins, F. et al. Adverse effects of immune-checkpoint inhibitors: epidemiology, management and surveillance. Nat. Rev. Clin. Oncol. 16, 563–580 (2019).
Ramos-Casals, M. et al. Immune-related adverse events of checkpoint inhibitors. Nat. Rev. Dis. Prim. 6, 38 (2020).
Brunet, J. F. et al. A new member of the immunoglobulin superfamily–CTLA-4. Nature 328, 267–270 (1987).
Linsley, P. S., Brady, W., Urnes, M., Grosmaire, L. S., Damle, N. K. & Ledbetter, J. A. CTLA-4 is a second receptor for the B cell activation antigen B7. J. Exp. Med. 174, 561–569 (1991).
Krummel, M. F. & Allison, J. P. CD28 and CTLA-4 have opposing effects on the response of T cells to stimulation. J. Exp. Med. 182, 459–465 (1995).
Yshii, L. M. et al. CTLA4 blockade elicits paraneoplastic neurological disease in a mouse model. Brain 139, 2923–2934 (2016).
Ascierto, P. A. et al. Ipilimumab 10 mg/kg versus ipilimumab 3 mg/kg in patients with unresectable or metastatic melanoma: a randomised, double-blind, multicentre, phase 3 trial. Lancet Oncol. 18, 611–622 (2017).
Eggermont, A. M. et al. Prolonged survival in stage III melanoma with ipilimumab adjuvant therapy. N. Engl. J. Med. 375, 1845–1855 (2016). This paper reports that IPI prolongs survival as adjuvant therapy in resected melanoma.
Tarhini, A. A. et al. Phase III study of adjuvant ipilimumab (3 or 10 mg/kg) versus high-dose interferon Alfa-2b for resected high-risk melanoma: North American intergroup E1609. J. Clin. Oncol. 38, 567–575 (2020).
Freeman, G. J. et al. Engagement of the PD-1 immunoinhibitory receptor by a novel B7 family member leads to negative regulation of lymphocyte activation. J. Exp. Med. 192, 1027–1034 (2000).
Latchman, Y. et al. PD-L2 is a second ligand for PD-1 and inhibits T cell activation. Nat. Immunol. 2, 261–268 (2001).
Curran, M. A., Montalvo, W., Yagita, H. & Allison, J. P. D-1 and CTLA-4 combination blockade expands infiltrating T cells and reduces regulatory T and myeloid cells within B16 melanoma tumors. Proc. Natl Acad. Sci. USA 107, 4275–4280 (2010).
Wolchok, J. D. et al. Nivolumab plus ipilimumab in advanced melanoma. N. Engl. J. Med. 369, 122–133 (2013). This paper provides the first description of the benefit of combination checkpoint inhibition.
Konishi, J., Yamazaki, K., Azuma, M., Kinoshita, I., Dosaka-Akita, H. & Nishimura, M. B7-H1 expression on non-small cell lung cancer cells and its relationship with tumor-infiltrating lymphocytes and their PD-1 expression. Clin. Cancer Res. 10, 5094–5100 (2004).
Herbst, R. S. et al. Predictive correlates of response to the anti-PD-L1 antibody MPDL3280A in cancer patients. Nature 515, 563–567 (2014).
Garcia-Diaz, A. et al. Interferon receptor signaling pathways regulating PD-L1 and PD-L2 expression. Cell Rep. 19, 1189–1201 (2017).
Brahmer, J. R. et al. Phase I study of single-agent anti-programmed death-1 (MDX-1106) in refractory solid tumors: safety, clinical activity, pharmacodynamics, and immunologic correlates. J. Clin. Oncol. 28, 3167–3175 (2010).
Robert, C. et al. Anti-programmed-death-receptor-1 treatment with pembrolizumab in ipilimumab-refractory advanced melanoma: a randomised dose-comparison cohort of a phase 1 trial. Lancet 384, 1109–1117 (2014). This article is the first to report the benefit of treatment with an antibody against PD1 in a randomized trial.
Robert, L. et al. CTLA4 blockade broadens the peripheral T-cell receptor repertoire. Clin. Cancer Res. 20, 2424–2432 (2014).
Cha, E. et al. Improved survival with T cell clonotype stability after anti-CTLA-4 treatment in cancer patients. Sci. Transl. Med. 6, 238ra70 (2014).
Harview, C. L. et al. Integrated molecular analysis of tumor biopsies on sequential CTLA-4 and PD-1 blockade reveals markers of response and resistance. Sci. Transl. Med. 9, eaah3560 (2017).
Johnson, D. B. et al. Fulminant myocarditis with combination immune checkpoint blockade. N. Engl. J. Med. 375, 1749–1755 (2016). This paper is the first to report checkpoint inhibition-related myocarditis.
Matson, D. R., Accola, M. A., Rehrauer, W. M. & Corliss, R. F. Fatal myocarditis following treatment with the PD-1 inhibitor nivolumab. J. Forensic Sci. 63, 954–957 (2018).
Zen, Y. & Yeh, M. M. Hepatotoxicity of immune checkpoint inhibitors: a histology study of seven cases in comparison with autoimmune hepatitis and idiosyncratic drug-induced liver injury. Mod. Pathol. 31, 965–973 (2018).
Belliere, J. et al. Hepatotoxicity of immune checkpoint inhibitors: a histology study of seven cases in comparison with autoimmune hepatitis and idiosyncratic drug-induced liver injury. Br. J. Cancer 115, 1457–1461 (2016).
Suresh, K. et al. The alveolar immune cell landscape is dysregulated in checkpoint inhibitor pneumonitis. J. Clin. Invest. 129, 4305–4315 (2019).
Luoma, A. M. et al. Molecular pathways of colon inflammation induced by cancer immunotherapy. Cell 182, 655–671.e22 (2020).
Petitprez, F. et al. B cells are associated with survival and immunotherapy response in sarcoma. Nature 577, 556–560 (2020).
Osorio, J. C. et al. Antibody-mediated thyroid dysfunction during T-cell checkpoint blockade in patients with non-small-cell lung cancer. Ann. Oncol. 28, 583–589 (2017).
Safa, H. et al. Immune checkpoint inhibitor related myasthenia gravis: single center experience and systematic review of the literature. J. Immunother. Cancer 7, 319 (2019).
Yang, J. C. et al. Ipilimumab (anti-CTLA4 antibody) causes regression of metastatic renal cell cancer associated with enteritis and hypophysitis. J. Immunother. 30, 825–830 (2007).
Maker, A. V. et al. Tumor regression and autoimmunity in patients treated with cytotoxic T lymphocyte-associated antigen 4 blockade and interleukin 2: a phase I/II study. Ann. Surg. Oncol. 12, 1005–1016 (2005). This article was the first to report immune-related adverse events with checkpoint inhibition.
Weber, J. S. et al. Phase I/II study of ipilimumab for patients with metastatic melanoma. J. Clin. Oncol. 26, 5950–5956 (2008).
Sibaud, V. Dermatologic reactions to immune checkpoint inhibitors: skin toxicities and immunotherapy. Am. J. Clin. Dermatol. 19, 345–361 (2018).
Freeman-Keller, M. et al. Nivolumab in resected and unresectable metastatic melanoma: characteristics of immune-related adverse events and association with outcomes. Clin. Cancer Res. 22, 886–894 (2016).
Hua, C. et al. Association of vitiligo with tumor response in patients with metastatic melanoma treated with pembrolizumab. JAMA Dermatol. 152, 45–51 (2016).
Wu, J. & Lacouture, M. E. Pruritus associated with targeted anticancer therapies and their management. Dermatol. Clin. 36, 315–324 (2018).
Zhang, M. L., Neyaz, A., Patil, D., Chen, J., Dougan, M. & Deshpande, V. Immune-related adverse events in the gastrointestinal tract: diagnostic utility of upper gastrointestinal biopsies. Histopathology 76, 233–243 (2020).
Wang, D. Y. et al. Clinical characterization of colitis arising from anti-PD-1 based therapy. Oncoimmunology 8, e1524695 (2018).
Badran, Y. R. et al. Immune checkpoint inhibitor-associated celiac disease. J. Immunother. Cancer 8, e000958 (2020).
Lang, N. et al. Clinical significance of signs of autoimmune colitis in 18F-fluorodeoxyglucose positron emission tomography-computed tomography of 100 stage-IV melanoma patients. Immunotherapy 1, 667–676 (2019).
Hughes, M. S. et al. Budesonide treatment for microscopic colitis from immune checkpoint inhibitors. J. Immunother. Cancer 7, 292 (2019).
Thompson, J. A. et al. Management of immunotherapy-related toxicities, version 1.2019. J. Natl Compr. Cancer Netw. 17, 255–289 (2019).
Horisberger, A. et al. A severe case of refractory esophageal stenosis induced by nivolumab and responding to tocilizumab therapy. J. Immunother. Cancer 6, 156 (2018).
Abu-Sbeih, H. et al. Outcomes of vedolizumab therapy in patients with immune checkpoint inhibitor-induced colitis: a multi-center study. J. Immunother. Cancer 6, 142 (2018).
Weber, J., Thompson, J. A., Hamid, O., Minor, D. & Amin, A. et al. A randomized, double-blind, placebo-controlled, phase II study comparing the tolerability and efficacy of ipilimumab administered with or without prophylactic budesonide in patients with unresectable stage III or IV melanoma. Clin. Cancer Res. 15, 5591–5598 (2009).
Barroso-Sousa, R. et al. Incidence of endocrine dysfunction following the use of different immune checkpoint inhibitor regimens: systematic review and meta-analysis. JAMA Oncol. 4, 173–182 (2018). This systematic review provides a detailed description of endocrinopathies with checkpoint inhibition.
Muir, C. A., Menzies, A. M., Clifton-Bligh, R. & Tsang, V. H. M. Thyroid toxicity following immune checkpoint inhibitor treatment in advanced cancer. Thyroid 30, 1458–1469 (2020).
Larkin, J. et al. Combined nivolumab and ipilimumab or monotherapy in untreated melanoma. N. Engl. J. Med. 373, 23–34 (2015).
Faje, A. T. et al. Ipilimumab-induced hypophysitis: a detailed longitudinal analysis in a large cohort of patients with metastatic melanoma. J. Clin. Endocrinol. Metab. 99, 4078–4085 (2014).
Faje, A. et al. Hypophysitis secondary to nivolumab and pembrolizumab is a clinical entity distinct from ipilimumab-associated hypophysitis. Eur. J. Endocrinol. 181, 211–219 (2019).
Iwama, S., De Remigis, A., Callahan, M. K., Slovin, S. F., Wolchok, J. D. & Caturegli, P. Pituitary expression of CTLA-4 mediates hypophysitis secondary to administration of CTLA-4 blocking antibody. Sci. Transl. Med. 6, 230ra45 (2014). This article describes the direct on-target effect of an antibody against CTLA4 in inducing hypophysitis.
Karamchandani, D. M. & Chetty, R. Immune checkpoint inhibitor-induced gastrointestinal and hepatic injury: pathologists’ perspective. J. Clin. Pathol. 71, 665–671 (2018).
Johncilla, M. et al. Ipilimumab-associated hepatitis: clinicopathologic characterization in a series of 11 cases. Am. J. Surg. Pathol. 39, 1075–1084 (2015).
Mahmood, S. S. et al. Myocarditis in patients treated with immune checkpoint inhibitors. J. Am. Coll. Cardiol. 71, 1755–1764 (2018).
Zhang, L. et al. Major adverse cardiovascular events and the timing and dose of corticosteroids in immune checkpoint inhibitor-associated myocarditis. Circulation 141, 2031–2034 (2020).
Puzanov, I. et al. Managing toxicities associated with immune checkpoint inhibitors: consensus recommendations from the Society for Immunotherapy of Cancer (SITC) Toxicity Management Working Group. J. Immunother. Cancer 5, 95 (2017).
Haanan, J. B. A. G. et al. Management of toxicities from immunotherapy: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann. Oncol. 29, iv264–iv266 (2018).
Jain, V., Mohebtash, M., Rodrigo, M. E., Ruiz, G., Atkins, M. B. & Barac, A. Autoimmune myocarditis caused by immune checkpoint inhibitors treated with antithymocyte globulin. J. Immunother. 41, 332–335 (2018).
Salem, J. E. et al. Abatacept for severe immune checkpoint inhibitor-associated myocarditis. N. Engl. J. Med. 380, 2377–2379 (2019).
Fellner, A. et al. Neurologic complications of immune checkpoint inhibitors. J. Neurooncol. 137, 601–609 (2018).
Johnson, D. B. et al. Neurologic toxicity associated with immune checkpoint inhibitors: a pharmacovigilance study. J. Immunother. Cancer 7, 134 (2019).
Michot, J. M. et al. Haematological immune-related adverse events with immune checkpoint inhibitors, how to manage? Eur. J. Cancer 122, 72–90 (2019).
Leaf, R. K. et al. Clinical and laboratory features of autoimmune hemolytic anemia associated with immunecheckpoint inhibitors. Am. J. Hematol. 94, 563–574 (2019).
Weber, J. S., Postow, M., Lao, C. D. & Schadendorf, D. Management of adverse events following treatment with anti-programmed death-1 agents. Oncologist 21, 1230–1240 (2016).
Wang, D. Y. et al. Fatal toxic effects associated with immune checkpoint inhibitors: a systematic review and meta-analysis. JAMA Oncol. 4, 1721–1728 (2018).
Routy, B. et al. Gut microbiome influences efficacy of PD-1-based immunotherapy against epithelial tumors. Science 359, 91–97 (2018).
Gopalakrishnan, V. et al. Gut microbiome modulates response to anti-PD-1 immunotherapy in melanoma patients. Science 359, 97–103 (2018).
Matson, V. et al. The commensal microbiome is associated with anti-PD-1 efficacy in metastatic melanoma patients. Science 359, 104–108 (2018).
Dubin, K. et al. Intestinal microbiome analyses identify melanoma patients at risk for checkpoint-blockade-induced colitis. Nat. Commun. 7, 10391 (2016). This is the first article to report that the microbiome may influence immune-related colitis.
Chaput, N. et al. Baseline gut microbiota predicts clinical response and colitis in metastatic melanoma patients treated with ipilimumab. Ann. Oncol. 28, 1368–1379 (2017).
Wang, Y. et al. Fecal microbiota transplantation for refractory immune checkpoint inhibitor-associated colitis. Nat. Med. 24, 1804–1808 (2018).
FDA. Fecal microbiota for transplantation: safety alert - risk of serious adverse events likely due to transmission of pathogenic organisms. FDA https://www.fda.gov/safety/medical-product-safety-information/fecal-microbiota-transplantation-safety-alert-risk-serious-adverse-events-likely-due-transmission?utm_campaign=FDA%20MedWatch%3A%20Fecal%20Microbiota%20for%20Transplantation&utm_medium=email&utm_source=Eloqua (2020).
Friedlander, P. et al. A whole-blood RNA transcript-based gene signature is associated with the development of CTLA-4 blockade-related diarrhea in patients with advanced melanoma treated with the checkpoint inhibitor tremelimumab. J. Immunother. Cancer 6, 90 (2018).
Subudhi, S. K. et al. Clonal expansion of CD8 T cells in the systemic circulation precedes development of ipilimumab-induced toxicities. Proc. Natl Acad. Sci. USA 113, 11919–11924 (2016). This paper provides evidence that expanded clonal T cells are associated with immune-related toxicities.
Iyoda, T., Kurita, N., Takada, A., Watanabe, H. & Ando, M. Resolution of infliximab-refractory nivolumab-induced acute severe enterocolitis after cyclosporine treatment in a patient with non-small cell lung cancer. Am. J. Case. Rep. 19, 360–364 (2018).
Nayar, N., Briscoe, K. & Fernandez Penas, P. Toxic epidermal necrolysis-like reaction with severe satellite cell necrosis associated with nivolumab in a patient with ipilimumab refractory metastatic melanoma. J. Immunother. 39, 149–152 (2016).
Toi, Y. et al. Profiling preexisting antibodies in patients treated with anti-PD-1 therapy for advanced non-small cell lung cancer. JAMA Oncol. 5, 376–383 (2019).
Giannicola, R. et al. Early blood rise in auto-antibodies to nuclear and smooth muscle antigens is predictive of prolonged survival and autoimmunity in metastatic-non-small cell lung cancer patients treated with PD-1 immune-check point blockade by nivolumab. Mol. Clin. Oncol. 11, 81–90 (2019).
de Moel, E. C. et al. Autoantibody development under treatment with immune-checkpoint inhibitors. Cancer Immunol. Res. 7, 6–11 (2019).
Hasan Ali, O. et al. BP180-specific IgG is associated with skin adverse events, therapy response, and overall survival in non-small cell lung cancer patients treated with checkpoint inhibitors. J. Am. Acad. Dermatol. 82, 854–861 (2020).
Tahir, S. A. et al. Autoimmune antibodies correlate with immune checkpoint therapy-induced toxicities. Proc. Natl Acad. Sci. USA 116, 22246–22251 (2019).
Gowen, M. F. et al. Baseline antibody profiles predict toxicity in melanoma patients treated with immune checkpoint inhibitors. J. Transl. Med. 16, 82 (2018).
Kurimoto, C. et al. Predictive and sensitive biomarkers for thyroid dysfunctions during treatment with immune-checkpoint inhibitors. Cancer Sci. 111, 1468–1477 (2020).
Khan, S. et al. Late-onset immunotherapy toxicity and delayed autoantibody changes: checkpoint inhibitor-induced Raynaud’s-like phenomenon. Oncologist 25, e753–e757 (2020).
Raibagkar, P., Ho, D., Gunturu, K. S. & Srinivasan, J. Worsening of anti-Hu paraneoplastic neurological syndrome related to anti-PD-1 treatment: Case report and review of literature. J. Neuroimmunol. 341, 577184 (2020).
Osaki, M. et al. Anti-transcriptional intermediary factor 1-γ antibody-positive dermatomyositis induced by nivolumab for lung adenocarcinoma: A case report. Invest. N. Drugs 39, 251–255 (2020).
Olson, D. J. et al. A case of dual-mechanism immune-related anaemia in a patient with metastatic melanoma treated with nivolumab and ipilimumab. J. Immunother. Cancer 8, e000380 (2020).
Hassel, J. C. et al. Autoantibodies as predictors for survival and immune-related adverse events in checkpoint inhibition therapy of metastasized melanoma. J. Clin. Oncol. 38 (Suppl.), Abstr. 10011 (2020). This article reports that autoantibodies are associated with immune-related toxicities.
Damin, D. C. et al. Effects of the gastrin-releasing peptide antagonist RC-3095 in a rat model of ulcerative colitis. Dig. Dis. Sci. 55, 2203–2210 (2010).
Das, R. et al. Early B cell changes predict autoimmunity following combination immune checkpoint blockade. J. Clin. Invest. 128, 715–720 (2018).
Bins, S. et al. Association between single-nucleotide polymorphisms and adverse events in nivolumab-treated non-small cell lung cancer patients. Br. J. Cancer 118, 1296–1301 (2018).
Refae, S. et al. Germinal Immunogenetics predict treatment outcome for PD-1/PD-L1 checkpoint inhibitors. Invest. N. Drugs 38, 160–171 (2020).
Simpson, D. et al. Anti-CTLA4 toxicity associates with genetic variation correlating with serum antibody diversity. Ann. Oncol. 29, viii421 (2018).
Yano, S. et al. Human leucocyte antigen DR15, a possible predictive marker for immune checkpoint inhibitor-induced secondary adrenal insufficiency. Eur. J. Cancer 130, 198–203 (2020).
Cortellini, A. et al. Clinical outcomes of patients with advanced cancer and pre-existing autoimmune diseases treated with anti-programmed death-1 immunotherapy: a real-world transverse study. Oncologist 24, e327–e337 (2019).
Menzies, A. M. et al. Anti-PD-1 therapy in patients with advanced melanoma and preexisting autoimmune disorders or major toxicity with ipilimumab. Ann. Oncol. 28, 368–376 (2017). This article describes that anti-PD1 therapy can be tolerated in patients with autoimmune disease.
Abu-Sbeih, H. et al. Immune checkpoint inhibitor therapy in patients with preexisting inflammatory bowel disease. J. Clin. Oncol. 38, 576–583 (2020).
Naqash, A. R. et al. Outcomes associated with immune-related adverse events in metastatic non-small cell lung cancer treated with nivolumab: a pooled exploratory analysis from a global cohort. Cancer Immunol. Immunother. 69, 1177–1187 (2020).
Weinstock, C. et al. An FDA analysis of the association between adverse events and outcome in patients with urothelial cancer receiving a programmed death protein 1 or programmed death ligand 1 (anti-PD-1/L1) antibody. J. Clin. Oncol. https://doi.org/10.1200/JCO.2019.37.15_suppl.4549 (2017).
Verzoni, E. et al. Real-world efficacy and safety of nivolumab in previously-treated metastatic renal cell carcinoma, and association between immune-related adverse events and survival: the Italian expanded access program. J. Immunother. Cancer 7, 99–108 (2019).
Abu-Sbeih, H. et al. The impact of immune checkpoint inhibitor-related adverse events and their immunosuppressive treatment on patients’ outcomes. J. Immunother. Precis. Oncol. 1, 7–18 (2018).
Robert, C., Hwu, W. J., Hamid, O., Ribas, A. & Weber, J. S. Long-term safety of pembrolizumab monotherapy and relationship with clinical outcome: a landmark analysis in patients with advanced melanoma. Eur. J. Cancer 144, 182–191 (2021).
Eggermont, A. M. M. et al. Association between immune-related adverse events and recurrence-free survival among patients with stage III melanoma randomized to receive pembrolizumab or placebo: a secondary analysis of a randomized clinical trial. JAMA Oncol. 6, 519–527 (2020). This article reports immune toxicities associated with positive outcome in resected melanoma.
Mandalà, M. et al. An analysis of nivolumab-mediated adverse events and association with clinical efficacy in resected stage III or IV melanoma (CheckMate 238). J. Clin. Oncol. https://doi.org/10.1200/JCO.2019.37.15_suppl.9584 (2019).
Hosoya, K. & et al. Association between early immune-related adverse events and clinical outcomes in patients with non-small cell lung cancer treated with immune checkpoint inhibitors. Clin. Lung Cancer https://doi.org/10.1016/j.cllc.2020.01.003 (2020).
Perez-Ruiz, E. et al. Prophylactic TNF blockade uncouples efficacy and toxicity in dual CTLA-4 and PD-1 immunotherapy. Nature 569, 428–432 (2019). This article reveals that TNF inhibition may reduce immune toxicity but not clinical efficacy.
Affolter, T. et al. Inhibition of immune checkpoints PD-1, CTLA-4, and IDO1 coordinately induces immune-mediated liver injury in mice. PLoS ONE 14, e0217276 (2019).
Liu, J. et al. Assessing immune-related adverse events of efficacious combination immunotherapies in preclinical models of cancer. Cancer Res. 76, 5288–5301 (2016).
Tsuruoka, K. et al. Exacerbation of autoimmune myocarditis by an immune checkpoint inhibitor is dependent on its time of administration in mice. Int. J. Cardiol. 313, 67–75 (2020).
Williams, T. J. et al. Association of autoimmune encephalitis with combined immune checkpoint inhibitor treatment for metastatic cancer. JAMA Neurol. 73, 928–933 (2016).
Marschner, D. et al. MicroRNA-146a regulates immune-related adverse events caused by immune checkpoint inhibitors. JCI Insight 5, e132334 (2020).
Song, M. Y. et al. Protective effects of Fc-fused PD-L1 on two different animal models of colitis. Gut 64, 260–271 (2015).
Wang, F., Yin, Q., Chen, L. & Davis, M. M. Bifidobacterium can mitigate intestinal immunopathology in the context of CTLA-4 blockade. Proc. Natl Acad. Sci. USA 115, 157–161 (2018).
Lebbé, C. et al. Evaluation of two dosing regimens for nivolumab in combination with ipilimumab in patients with advanced melanoma: results from the phase IIIb/IV Checkmate 511 trial. J. Clin. Oncol. 37, 867–875 (2019).
Hellmann, M. D. et al. Nivolumab plus ipilimumab in advanced non-small-cell lung cancer. N. Engl. J. Med. 381, 2020–2031 (2019).
Diab, A. et al. Bempegaldesleukin (NKTR-214) plus nivolumab in patients with advanced solid tumors: phase I dose-escalation study of safety, efficacy, and immune activation (PIVOT-02). Cancer Discov. 10, 1158–1173 (2020).
Autio, K. A., Boni, V., Humphrey, R. W. & Naing, A. Probody therapeutics: an emerging class of therapies designed to enhance on-target effects with reduced off-tumor toxicity for use in immuno-oncology. Clin. Cancer Res. 26, 984–989 (2020).
Doms, J., Prior, J. O., Peters, S. & Obeid, M. Tocilizumab for refractory severe immune checkpoint inhibitor-associated myocarditis. Ann. Oncol. 31, 1273–1275 (2020).
Haanen, J. et al. Autoimmune diseases and immune-checkpoint inhibitors for cancer therapy: review of the literature and personalized risk-based prevention strategy. Ann. Oncol. 21, 724–744 (2020).
Haanen, J. et al. Rechallenge patients with immune checkpoint inhibitors following severe immune-related adverse events: review of the literature and suggested prophylactic strategy. J. Immunother. Cancer 8, e000604 (2020).
Verheijden, R. J. et al. Association of anti-TNF with decreased survival in steroid refractory ipilimumab and anti-PD1-treated patients in the dutch melanoma treatment registry. Clin. Cancer Res. 26, 2268–2274 (2020).
Esfahani, K., Hudson, M. & Batist, G. N. Tofacitinib for refractory immune-related colitis from PD-1 therapy. N. Engl. J. Med. 382, 2374–2375 (2020).
Risbjerg, R. S., Hansen, M. V., Sørensen, A. S. & Kragstrup, T. W. The effects of B cell depletion on immune related adverse events associated with immune checkpoint inhibition. Exp. Hematol. Oncol. 9, 9 (2020).
Shiuan, E. et al. Thrombocytopenia in patients with melanoma receiving immune checkpoint inhibitor therapy. J. Immunother. Cancer 5, 8 (2017).
Damsky, W. et al. B cell depletion or absence does not impede anti-tumor activity of PD-1inhibitors. J. Immunother. Cancer 7, 153 (2019).
Ghosn, J. et al. A severe case of neuro-Sjögren’s syndrome induced by pembrolizumab. J. Immunother. Cancer 22, 110 (2018).
Anderson, A. C., Joller, N. & Kuchroo, V. K. Lag-3, Tim-3, and TIGIT: co-inhibitory receptors with specialized functions in immune regulation. Immunity 44, 989–1004 (2016).
Ascierto, P. A. et al. Initial efficacy of anti-lymphocyte activation gene-3 (anti–LAG-3; BMS-986016) in combination with nivolumab (nivo) in pts with melanoma (MEL) previously treated with anti–PD-1/PD-L1 therapy. J. Clin. Oncol. 35, 9520 (2017).
Siu, L. L. et al. Preliminary phase 1 profile of BMS-986179, an anti-CD73 antibody, in combination with nivolumab in patients with advanced solid tumors [abstract]. Cancer Res. 78 (Suppl.), CT180 (2018).
Goldman, J. W. et al. Safety and tolerability of MEDI0562 in combination with durvalumab or tremelimumab in patients with advanced solid tumors. J. Clin. Oncol. 38, 3003 (2020).
Angevin, E. et al. Updated analysis of the inducible T-cell co-stimulatory receptor (ICOS) agonist, GSK3359609 (GSK609), combination with pembrolizumab (PE) in patients (pts) with anti-PD-1/L1 treatment-naïve head and neck squamous cell carcinoma (HNSCC). J. Clin. Oncol. 38, 6517 (2020).
Abu-Sbeih, H. et al. Early introduction of selective immunosuppressive therapy associated with favorable clinical outcomes in patients with immune checkpoint inhibitor-induced colitis. J. Immunother. Cancer 7, 93 (2019).
Author information
Authors and Affiliations
Contributions
The authors contributed equally to all aspects of the article.
Corresponding author
Ethics declarations
Competing interests
J.S.W. is a consultant for Merck, Genentech, AstraZeneca, Pfizer, Regeneron, GSK, Alkermes, Novartis, Celldex, Incyte and EMD Serono. He is a member of the advisory board for BMS, and holds equity in CytoMx, Biond, Neximmune and Immunimax. He is also part of the scientific advisory boards for Celldex, CytoMx, Incyte, Biond, Neximmune and Sellas. J.S.W.’s laboratory has received research support from BMS, Merck, GSK, Novartis, Moderna and AstraZeneca. Moffitt Cancer Center filed a patent on an ipilimumab biomarker and a TIL growth method, in which J.S.W. has been named, as well as in a PD1 biomarker patent by Biodesix. R.J.S. declares no competing interests.
Additional information
Peer review information
Nature Reviews Drug Discovery thanks Manuel Ramos, Carolina Roberts and Ansuman Satpathy for their contribution to the peer review of this work.
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Glossary
- Clonotype
-
Unique sequence of a T cell receptor chain
- Tertiary lymphoid structures
-
Lymph node-like structures found within the stroma of some tumours.
- Bullous pemphigoid
-
An autoimmune skin disease marked by formation of bullae or blisters, urticarial lesions (hives) and itching due to an immune reaction against desmosomes responsible for anchoring the epidermis and the dermis.
- Stevens–Johnson syndrome/toxic epidermal necrolysis
-
A disorder of the skin and mucous membranes that causes the skin to peel off and die.
- Hashimoto’s thyroiditis
-
Also known as chronic lymphocytic thyroiditis. An autoimmune condition of the thyroid that presents initially with acute hyperthyroidism and then evolves to a ‘burnt-out’ thyroid with hypothyroidism.
- Cholestasis
-
Slowing of the flow of bile from the liver.
- ST segment
-
Flat segment of the electrocardiogram between the end of the S wave and the beginning of the T wave.
- Troponin
-
A complex of three regulatory proteins found in muscle that indicate heart damage when elevated.
- Guillain–Barré syndrome
-
An autoimmune condition in which there is ascending paralysis starting in the lower extremities.
- Autoantibodies
-
Antibodies that react with normal self-tissue and are often a marker for activity of various autoimmune diseases and themselves can cause pathology.
- Time-delay bias
-
An overestimation of survival or another parameter due to an excess of cases detected later in the course of a disease.
Rights and permissions
About this article
Cite this article
Sullivan, R.J., Weber, J.S. Immune-related toxicities of checkpoint inhibitors: mechanisms and mitigation strategies. Nat Rev Drug Discov 21, 495–508 (2022). https://doi.org/10.1038/s41573-021-00259-5
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41573-021-00259-5
This article is cited by
-
Predictive biomarkers for immune-related adverse events in cancer patients treated with immune-checkpoint inhibitors
BMC Immunology (2024)
-
Gut microbiome for predicting immune checkpoint blockade-associated adverse events
Genome Medicine (2024)
-
Mechanisms of immune checkpoint inhibitors: insights into the regulation of circular RNAS involved in cancer hallmarks
Cell Death & Disease (2024)
-
Immune-related serious adverse events with immune checkpoint inhibitors: Systematic review and network meta-analysis
European Journal of Clinical Pharmacology (2024)
-
Tislelizumab plus zanubrutinib for Richter transformation: the phase 2 RT1 trial
Nature Medicine (2024)