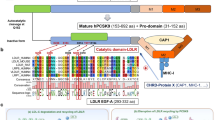
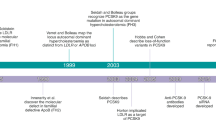
Abstract
Clinical trials have unequivocally shown that inhibition of proprotein convertase subtilisin/kexin type 9 (PCSK9) efficaciously and safely prevents cardiovascular events by lowering levels of LDL cholesterol. PCSK9 in the circulation is derived mainly from the liver, but the protein is also expressed in the pancreas, the kidney, the intestine and the central nervous system. Although PCSK9 modulates cholesterol metabolism by regulating LDL receptor expression in the liver, in vitro and in vivo studies have suggested that PCSK9 is involved in various other physiological processes. Although therapeutic PCSK9 inhibition could theoretically have undesired effects by interfering with these non-cholesterol-related processes, studies of individuals with genetically determined reduced PCSK9 function and clinical trials of PCSK9 inhibitors have not revealed clinically meaningful adverse consequences of almost completely eradicating PCSK9 from the circulation. The clinical implications of PCSK9 functions beyond lipid metabolism in terms of wanted or unwanted effects of therapeutic PCSK9 inhibition therefore appear to be limited. The objective of this Review is to describe the physiological role of PCSK9 beyond the LDL receptor to provide a rational basis for monitoring the effects of PCSK9 inhibition as these drugs gain traction in the clinic.
Key points
PCSK9 is expressed in several tissues other than the liver, including the pancreas, the kidney, the intestine and the brain.
Although PCSK9 might be involved in various pathophysiological and physiological processes in different organ systems, the clinical implications for therapeutic PCSK9 inhibition seem to be limited.
Clinical trials of PCSK9 inhibitors and studies of individuals with genetically determined reduced PCSK9 activity have provided reassurance regarding the safety of therapeutic PCSK9 inhibition.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout


Similar content being viewed by others
References
Abifadel, M. et al. Mutations in PCSK9 cause autosomal dominant hypercholesterolemia. Nat. Genet. 34, 154–156 (2003).
Cohen, J. et al. Low LDL cholesterol in individuals of African descent resulting from frequent nonsense mutations in PCSK9. Nat. Genet. 37, 161–165 (2005).
Cohen, J. C., Boerwinkle, E., Mosley Jr, T. H. & Hobbs, H. H. Sequence variations in PCSK9, low LDL, and protection against coronary heart disease. N. Engl. J. Med. 354, 1264–1272 (2006).
Stein, E. A. et al. Effect of a monoclonal antibody to PCSK9 on LDL cholesterol. N. Engl. J. Med. 366, 1108–1118 (2012).
Zhang, X.-L. et al. Safety and efficacy of anti-PCSK9 antibodies: a meta-analysis of 25 randomized, controlled trials. BMC Med. 13, 123 (2015).
Navarese, E. et al. Effects of proprotein convertase subtilisin/kexin type 9 antibodies in adults with hypercholesterolemia: a systematic review and meta-analysis. Ann. Intern. Med. 163, 40–51 (2015).
Steg, P. G. in Evaluation of cardiovascular outcomes after an acute coronary syndrome during treatment with alirocumab — ODYSSEY outcomes. Presented at American College of Cardiology Scientific Sessions 2018 in Orlando, USA (10 Mar 2018).
Sabatine, M. S. et al. Evolocumab and clinical outcomes in patients with cardiovascular disease. N. Engl. J. Med. 376, 1713–1722 (2017).
Fitzgerald, K. et al. A highly durable RNAi therapeutic inhibitor of PCSK9. N. Engl. J. Med. 376, 41–51 (2017).
Ray, K. K. et al. Inclisiran in patients at high cardiovascular risk with elevated LDL cholesterol. N. Engl. J. Med. 376, 1430–1440 (2017).
Dias, C. S. et al. Effects of AMG 145 on low-density lipoprotein cholesterol levels: results from 2 randomized, double-blind, placebo-controlled, ascending-dose phase 1 studies in healthy volunteers and hypercholesterolemic subjects on statins. J. Am. Coll. Cardiol. 60, 1888–1898 (2012).
US Department of Health and Human Services. Repatha (evolocumab) injection. FDA.gov https://www.accessdata.fda.gov/drugsatfda_docs/nda/2015/125522Orig1s000TOC.cfm (2015).
US Department of Health and Human Services. Praluent alirocumab. FDA.gov https://www.accessdata.fda.gov/drugsatfda_docs/nda/2015/125559Orig1s000TOC.cfm (2015).
Lambert, G., Sjouke, B., Choque, B., Kastelein, J. J. P. & Hovingh, G. K. The PCSK9 decade. J. Lipid Res. 53, 2515–2524 (2012).
Seidah, N. G. & Prat, A. The biology and therapeutic targeting of the proprotein convertases. Nat. Rev. Drug Discov. 11, 367–383 (2012).
Poirier, S. et al. Dissection of the endogenous cellular pathways of PCSK9-induced low density lipoprotein receptor degradation: evidence for an intracellular route. J. Biol. Chem. 284, 28856–28864 (2009).
Poirier, S., Mamarbachi, M., Chen, W. T., Lee, A. S. & Mayer, G. GRP94 regulates circulating cholesterol levels through blockade of PCSK9-induced LDLR degradation. Cell Rep. 13, 2064–2071 (2015).
Ray, K. K. et al. Effect of an siRNA therapeutic targeting PCSK9 on atherogenic lipoproteins: pre-specified secondary end points in ORION 1. Circulation https://doi.org/10.1161/CIRCULATIONAHA.118.034710 (2018).
Seidah, N. G., Awan, Z., Chrétien, M. & Mbikay, M. PCSK9: a key modulator of cardiovascular health. Circ. Res. 114, 1022–1036 (2014).
Roth, E. M., McKenney, J. M., Hanotin, C., Asset, G. & Stein, E. A. Atorvastatin with or without an antibody to PCSK9 in primary hypercholesterolemia. N. Engl. J. Med. 367, 1891–1900 (2012).
Sullivan, D. et al. Effect of a monoclonal antibody to PCSK9 on low-density lipoprotein cholesterol levels in statin-intolerant patients: the GAUSS randomized trial. JAMA 308, 2497–2506 (2012).
Raal, F. et al. Low-density lipoprotein cholesterol-lowering effects of AMG 145, a monoclonal antibody to proprotein convertase subtilisin/kexin type 9 serine protease in patients with heterozygous familial hypercholesterolemia: the reduction of LDL-C with PCSK9 inhibition in heterozygous familial hypercholesterolemia disorder (RUTHERFORD) randomized trial. Circulation 126, 2408–2417 (2012).
McKenney, J. M. et al. Safety and efficacy of a monoclonal antibody to proprotein convertase subtilisin/kexin type 9 serine protease, SAR236553/REGN727, in patients with primary hypercholesterolemia receiving ongoing stable atorvastatin therapy. J. Am. Coll. Cardiol. 59, 2344–2353 (2012).
Le May, C. et al. Proprotein convertase subtilisin kexin type 9 null mice are protected from postprandial triglyceridemia. Arterioscler. Thromb. Vasc. Biol. 29, 684–690 (2009).
Lambert, G. et al. Fasting induces hyperlipidemia in mice overexpressing proprotein convertase subtilisin kexin type 9: lack of modulation of very-low-density lipoprotein hepatic output by the low-density lipoprotein receptor. Endocrinology 147, 4985–4995 (2006).
Herbert, B. et al. Increased secretion of lipoproteins in transgenic mice expressing human D374Y PCSK9 under physiological genetic control. Arterioscler. Thromb. Vasc. Biol. 30, 1333–1339 (2010).
Reyes-Soffer, G. et al. Effects of PCSK9 inhibition with alirocumab on lipoprotein metabolism in healthy humans. Circulation 135, 352–362 (2017).
Ouguerram, K. et al. Apolipoprotein B100 metabolism in autosomal-dominant hypercholesterolemia related to mutations in PCSK9. Arterioscler. Thromb. Vasc. Biol. 24, 1448–1453 (2004).
Lambert, G. et al. The complexity of lipoprotein (a) lowering by PCSK9 monoclonal antibodies. Clin. Sci. 131, 261–268 (2017).
Kronenberg, F. & Utermann, G. Lipoprotein(a): resurrected by genetics. J. Intern. Med. 273, 6–30 (2013).
Tsimikas, S. et al. NHLBI Working Group recommendations to reduce lipoprotein(a)-mediated risk of cardiovascular disease and aortic stenosis. J. Am. Coll. Cardiol. 71, 177–192 (2018).
Tsimikas, S. A test in context: lipoprotein(a): diagnosis, prognosis, controversies, and emerging therapies. J. Am. Coll. Cardiol. 69, 692–711 (2017).
Yu, B. et al. Lipoprotein(a) induces human aortic valve interstitial cell calcification. JACC Basic Transl Sci. 2, 358–371 (2017).
Raal, F. J. et al. PCSK9 inhibition-mediated reduction in Lp(a) with evolocumab: an analysis of 10 clinical trials and the LDL receptor’s role. J. Lipid Res. 57, 1086–1096 (2016).
Ference, B. A. et al. Reduction of low density lipoprotein-cholesterol and cardiovascular events with proprotein convertase subtilisin-kexin type 9 (PCSK9) inhibitors and statins: an analysis of FOURIER, SPIRE, and the Cholesterol Treatment Trialists Collaboration. Eur. Heart J. 39, 2540–2545 (2018).
Gaudet, D. et al. Effect of alirocumab, a monoclonal proprotein convertase subtilisin/kexin 9 antibody, on lipoprotein(a) concentrations (a pooled analysis of 150 mg every two weeks dosing from phase 2 trials). Am. J. Cardiol. 114, 711–715 (2014).
Edmiston, J. B. et al. Discordant response of low-density lipoprotein cholesterol and lipoprotein(a) levels to monoclonal antibodies targeting proprotein convertase subtilisin/kexin type 9. J. Clin. Lipidol. 11, 667–673 (2017).
Raal, F. J. et al. Reduction in lipoprotein(a) with PCSK9 monoclonal antibody evolocumab (AMG 145): a pooled analysis of more than 1,300 patients in 4 phase II trials. J. Am. Coll. Cardiol. 63, 1278–1288 (2014).
Khera, A. V. et al. Lipoprotein(a) concentrations, rosuvastatin therapy, and residual vascular risk: an analysis from the JUPITER trial (justification for the use of statins in prevention: an intervention trial evaluating rosuvastatin). Circulation 129, 635–642 (2014).
Arsenault, B. J. et al. Effect of atorvastatin, cholesterol ester transfer protein inhibition, and diabetes mellitus on circulating proprotein subtilisin kexin type 9 and lipoprotein(a) levels in patients at high cardiovascular risk. J. Clin. Lipidol. 12, 130–136 (2018).
Stein, E. A. et al. Effect of the proprotein convertase subtilisin/kexin 9 monoclonal antibody, AMG 145, in homozygous familial hypercholesterolemia. Circulation 128, 2113–2120 (2013).
Thedrez, A. et al. Proprotein convertase subtilisin kexin type 9 inhibition for autosomal recessive hypercholesterolemia—brief report. Arterioscler. Thromb. Vasc. Biol. 36, 1647–1650 (2016).
Thedrez, A. et al. Homozygous familial hypercholesterolemia patients with identical mutations variably express the LDLR (low-density lipoprotein receptor): implications for the efficacy of evolocumab. Arterioscler. Thromb. Vasc. Biol. 38, 592–598 (2018).
Romagnuolo, R. et al. Lipoprotein(a) catabolism is regulated by proprotein convertase subtilisin/kexin type 9 through the low density lipoprotein receptor. J. Biol. Chem. 290, 11649–11662 (2015).
Romagnuolo, R. et al. Roles of the low density lipoprotein receptor and related receptors in inhibition of lipoprotein(a) internalization by proprotein convertase subtilisin/kexin type 9. PLOS ONE 12, e0180869 (2017).
Sharma, M., Redpath, G. M., Williams, M. J. & McCormick, S. P. Recycling of apolipoprotein(a) after PlgRKT-mediated endocytosis of lipoprotein(a). Circ. Res. 120, 1091–1102 (2017).
Villard, E. F. et al. PCSK9 modulates the secretion but not the cellular uptake of lipoprotein (a) ex vivo: an effect blunted by alirocumab. JACC Basic Transl Sci. 1, 419–427 (2016).
Kotani, K. & Banach, M. Lipoprotein(a) and inhibitors of proprotein convertase subtilisin/kexin type 9. J. Thorac. Dis. 9, E78–E82 (2017).
Rader, D. J., Cain, W., Zech, L. A., Usher, D. & Brewer, H. B. Jr Variation in lipoprotein(a) concentrations among individuals with the same apolipoprotein (a) isoform is determined by the rate of lipoprotein(a) production. J. Clin. Invest. 91, 443–447 (1993).
Croyal, M. et al. PCSK9 inhibition with alirocumab reduces lipoprotein(a) levels in non-human primates by lowering apolipoprotein(a) production rate. Clin. Sci. 132, 1075–1083 (2018).
Watts, G. F. et al. Factorial effects of evolocumab and atorvastatin on lipoprotein metabolism. Circulation 135, 338–351 (2017).
Jeong, H. J. et al. Sterol-dependent regulation of proprotein convertase subtilisin/kexin type 9 expression by sterol-regulatory element binding protein-2. J. Lipid Res. 49, 399–409 (2008).
Dubuc, G. et al. Statins upregulate PCSK9, the gene encoding the proprotein convertase neural apoptosis-regulated convertase-1 implicated in familial hypercholesterolemia. Arterioscler. Thromb. Vasc. Biol. 24, 1454–1459 (2004).
Dong, B. et al. Strong induction of PCSK9 gene expression through HNF1alpha and SREBP2: mechanism for the resistance to LDL-cholesterol lowering effect of statins in dyslipidemic hamsters. J. Lipid Res. 51, 1486–1495 (2010).
Costet, P. et al. Hepatic PCSK9 expression is regulated by nutritional status via insulin and sterol regulatory element-binding protein 1c. J. Biol. Chem. 281, 6211–6218 (2006).
Persson, L. et al. Circulating proprotein convertase subtilisin kexin type 9 has a diurnal rhythm synchronous with cholesterol synthesis and is reduced by fasting in humans. Arterioscler. Thromb. Vasc. Biol. 30, 2666–2672 (2010).
Persson, L. et al. Endogenous estrogens lower plasma PCSK9 and LDL cholesterol but not Lp(a) or bile acid synthesis in women. Arterioscler. Thromb. Vasc. Biol. 32, 810–814 (2012).
Ghosh, M., Galman, C., Rudling, M. & Angelin, B. Influence of physiological changes in endogenous estrogen on circulating PCSK9 and LDL cholesterol. J. Lipid Res. 56, 463–469 (2015).
Bonde, Y. et al. Thyroid hormone reduces PCSK9 and stimulates bile acid synthesis in humans. J. Lipid Res. 55, 2408–2415 (2014).
Cariou, B., Benoit, I. & Le May, C. Preserved adrenal function in fully PCSK9-deficient subject. Int. J. Cardiol. 176, 499–500 (2014).
Blom, D. J. et al. Effects of evolocumab on vitamin E and steroid hormone levels: results from the 52-week, phase 3, double-blind, randomized, placebo-controlled DESCARTES study. Circ. Res. 117, 731–741 (2015).
Illingworth, D. R., Kenny, T. A. & Orwoll, E. S. Adrenal function in heterozygous and homozygous hypobetalipoproteinemia. J. Clin. Endocrinol. Metab. 54, 27–33 (1982).
Robinson, J. G. Statins and diabetes risk: how real is it and what are the mechanisms? Curr. Opin. Lipidol. 26, 228–235 (2015).
Schmidt, A. F. et al. PCSK9 genetic variants and risk of type 2 diabetes: a mendelian randomisation study. Lancet Diabetes Endocrinol. 5, 97–105 (2017).
Ference, B. A. et al. Variation in PCSK9 and HMGCR and risk of cardiovascular disease and diabetes. N. Engl. J. Med. 375, 2144–2153 (2016).
Swerdlow, D. I. et al. HMG-coenzyme A reductase inhibition, type 2 diabetes, and bodyweight: evidence from genetic analysis and randomised trials. Lancet 385, 351–361 (2015).
Besseling, J., Kastelein, J. J., Defesche, J. C., Hutten, B. A. & Hovingh, G. K. Association between familial hypercholesterolemia and prevalence of type 2 diabetes mellitus. JAMA 313, 1029–1036 (2015).
Kruit, J. K., Brunham, L. R., Verchere, C. B. & Hayden, M. R. HDL and LDL cholesterol significantly influence beta-cell function in type 2 diabetes mellitus. Curr. Opin. Lipidol. 21, 178–185 (2010).
Rutti, S. et al. Low- and high-density lipoproteins modulate function, apoptosis, and proliferation of primary human and murine pancreatic beta-cells. Endocrinology 150, 4521–4530 (2009).
Mbikay, M. et al. PCSK9-deficient mice exhibit impaired glucose tolerance and pancreatic islet abnormalities. FEBS Lett. 584, 701–706 (2010).
Grupping, A. Y. et al. Low density lipoprotein binding and uptake by human and rat islet beta cells. Endocrinology 138, 4064–4068 (1997).
Cnop, M., Hannaert, J. C., Grupping, A. Y. & Pipeleers, D. G. Low density lipoprotein can cause death of islet beta-cells by its cellular uptake and oxidative modification. Endocrinology 143, 3449–3453 (2002).
Roehrich, M.-E. et al. Insulin-secreting beta-cell dysfunction induced by human lipoproteins. J. Biol. Chem. 278, 18368–18375 (2003).
Hao, M., Head, W. S., Gunawardana, S. C., Hasty, A. H. & Piston, D. W. Direct effect of cholesterol on insulin secretion: a novel mechanism for pancreatic beta-cell dysfunction. Diabetes 56, 2328–2338 (2007).
Langhi, C. et al. PCSK9 is expressed in pancreatic δ-cells and does not alter insulin secretion. Biochem. Biophys. Res. Commun. 390, 1288–1293 (2009).
Seidah, N. G. et al. The secretory proprotein convertase neural apoptosis-regulated convertase 1 (NARC-1): liver regeneration and neuronal differentiation. Proc. Natl Acad. Sci. USA 100, 928–933 (2003).
Brunham, L. R. et al. Beta-cell ABCA1 influences insulin secretion, glucose homeostasis and response to thiazolidinedione treatment. Nat. Med. 13, 340–347 (2007).
Baragetti, A. et al. PCSK9 deficiency results in increased ectopic fat accumulation in experimental models and in humans. Eur. J. Prev. Cardiol. 24, 1870–1877 (2017).
Roubtsova, A. et al. Circulating proprotein convertase subtilisin/kexin 9 (PCSK9) regulates VLDLR protein and triglyceride accumulation in visceral adipose tissue. Arterioscler. Thromb. Vasc. Biol. 31, 785–791 (2011).
Cariou, B. et al. Plasma PCSK9 concentrations during an oral fat load and after short term high-fat, high-fat high-protein and high-fructose diets. Nutr. Metab. 10, 4 (2013).
Lakoski, S. G., Lagace, T. A., Cohen, J. C., Horton, J. D. & Hobbs, H. H. Genetic and metabolic determinants of plasma PCSK9 levels. J. Clin. Endocrinol. Metab. 94, 2537–2543 (2009).
Baass, A. et al. Plasma PCSK9 is associated with age, sex, and multiple metabolic markers in a population-based sample of children and adolescents. Clin. Chem. 55, 1637–1645 (2009).
Levy, E. et al. PCSK9 plays a significant role in cholesterol homeostasis and lipid transport in intestinal epithelial cells. Atherosclerosis 227, 297–306 (2013).
Zaid, A. et al. Proprotein convertase subtilisin/kexin type 9 (PCSK9): hepatocyte-specific low-density lipoprotein receptor degradation and critical role in mouse liver regeneration. Hepatology 48, 646–654 (2008).
Le May, C. et al. Transintestinal cholesterol excretion is an active metabolic process modulated by PCSK9 and statin involving ABCB1. Arterioscler. Thromb. Vasc. Biol. 33, 1484–1493 (2013).
Rashid, S. et al. Proprotein convertase subtilisin kexin type 9 promotes intestinal overproduction of triglyceride-rich apolipoprotein B lipoproteins through both low-density lipoprotein receptor-dependent and -independent mechanisms. Circulation 130, 431–441 (2014).
Reeskamp, L. F., Meessen, E. C. E. & Groen, A. K. Transintestinal cholesterol excretion in humans. Curr. Opin. Lipidol. 29, 10–17 (2018).
Dugardin, C. et al. Retrograde cholesterol transport in the human Caco-2/TC7 cell line: a model to study trans-intestinal cholesterol excretion in atherogenic and diabetic dyslipidemia. Acta Diabetol. 54, 191–199 (2017).
Ooi, T. C. et al. The effect of PCSK9 loss-of-function variants on the postprandial lipid and ApoB-lipoprotein response. J. Clin. Endocrinol. Metab. 102, 3452–3460 (2017).
Boren, J., Matikainen, N., Adiels, M. & Taskinen, M.-R. Postprandial hypertriglyceridemia as a coronary risk factor. Clin. Chim. Acta 431, 131–142 (2014).
Veilleux, A. et al. Intestinal lipid handling: evidence and implication of insulin signaling abnormalities in human obese subjects. Arterioscler. Thromb. Vasc. Biol. 34, 644–653 (2014).
Grefhorst, A., McNutt, M. C., Lagace, T. A. & Horton, J. D. Plasma PCSK9 preferentially reduces liver LDL receptors in mice. J. Lipid Res. 49, 1303–1311 (2008).
Schmidt, R. J. et al. Secreted proprotein convertase subtilisin/kexin type 9 reduces both hepatic and extrahepatic low-density lipoprotein receptors in vivo. Biochem. Biophys. Res. Commun. 370, 634–640 (2008).
Sharotri, V., Collier, D. M., Olson, D. R., Zhou, R. & Snyder, P. M. Regulation of epithelial sodium channel trafficking by proprotein convertase subtilisin/kexin type 9 (PCSK9). J. Biol. Chem. 287, 19266–19274 (2012).
Berger, J.-M. et al. PCSK9-deficiency does not alter blood pressure and sodium balance in mouse models of hypertension. Atherosclerosis 239, 252–259 (2015).
Rogacev, K. S. et al. PCSK9 plasma concentrations are independent of GFR and do not predict cardiovascular events in patients with decreased GFR. PLOS ONE 11, e0146920 (2016).
Morena, M. et al. Plasma PCSK9 concentrations during the course of nondiabetic chronic kidney disease: relationship with glomerular filtration rate and lipid metabolism. J. Clin. Lipidol. 11, 87–93 (2017).
Konarzewski, M. et al. Elevated circulating PCSK-9 concentration in renal failure patients is corrected by renal replacement therapy. Am. J. Nephrol. 40, 157–163 (2014).
Haas, M. E. et al. The role of proprotein convertase subtilisin/kexin type 9 in nephrotic syndrome-associated hypercholesterolemia. Circulation 134, 61–72 (2016).
Poirier, S. et al. Implication of the proprotein convertase NARC-1/PCSK9 in the development of the nervous system. J. Neurochem. 98, 838–850 (2006).
Rashid, S. et al. Decreased plasma cholesterol and hypersensitivity to statins in mice lacking Pcsk9. Proc. Natl Acad. Sci. USA 102, 5374–5379 (2005).
An, D. et al. Identification of PCSK9 as a novel serum biomarker for the prenatal diagnosis of neural tube defects using iTRAQ quantitative proteomics. Sci. Rep. 5, 17559 (2015).
Rousselet, E. et al. PCSK9 reduces the protein levels of the LDL receptor in mouse brain during development and after ischemic stroke. J. Lipid Res. 52, 1383–1391 (2011).
Zimetti, F. et al. Increased PCSK9 cerebrospinal fluid concentrations in Alzheimer’s disease. J. Alzheimers. Dis. 55, 315–320 (2017).
Courtemanche, H. et al. PCSK9 concentrations in cerebrospinal fluid are not specifically increased in Alzheimer’s disease. J. Alzheimers Dis. 62, 1519–1525 (2018).
Bingham, B. et al. Proapoptotic effects of NARC 1 (=PCSK9), the gene encoding a novel serine proteinase. Cytometry A 69, 1123–1131 (2006).
Wu, Q. et al. The dual behavior of PCSK9 in the regulation of apoptosis is crucial in Alzheimer’s disease progression (review). Biomed. Rep. 2, 167–171 (2014).
Liu, M. et al. PCSK9 is not involved in the degradation of LDL receptors and BACE1 in the adult mouse brain. J. Lipid Res. 51, 2611–2618 (2010).
Benn, M., Nordestgaard, B. G., Frikke-Schmidt, R. & Tybjærg-Hansen, A. Low LDL cholesterol, PCSK9 and HMGCR genetic variation, and risk of Alzheimer’s disease and Parkinson’s disease: Mendelian randomisation study. BMJ 357, j1648 (2017).
Muldoon, M. F. et al. Effects of lovastatin on cognitive function and psychological well-being. Am. J. Med. 108, 538–546 (2000).
US Department of Health and Human Services. Important safety label changes to cholesterol-lowering statin drugs. FDA.gov https://www.fda.gov/Drugs/DrugSafety/ucm293101.htm (2012).
Ott, B. R. et al. Do statins impair cognition? A systematic review and meta-analysis of randomized controlled trials. J. Gen. Intern. Med. 30, 348–358 (2015).
Everett, B. M., Mora, S., Glynn, R. J., MacFadyen, J. & Ridker, P. M. Safety profile of subjects treated to very low low-density lipoprotein cholesterol levels (<30 mg/dl) with rosuvastatin 20 mg daily (from JUPITER). Am. J. Cardiol. 114, 1682–1689 (2014).
Collins, R. et al. Interpretation of the evidence for the efficacy and safety of statin therapy. Lancet 388, 2532–2561 (2016).
Giugliano, R. P. et al. Cognitive function in a randomized trial of evolocumab. N. Engl. J. Med. 377, 633–643 (2017).
Khan, A. R. et al. Increased risk of adverse neurocognitive outcomes with proprotein convertase subtilisin-kexin type 9 inhibitors. Circ. Cardiovasc. Qual. Outcomes 10, e003153 (2017).
Harvey, P. D. et al. No evidence of neurocognitive adverse events associated with alirocumab treatment in 3340 patients from 14 randomized phase 2 and 3 controlled trials: a meta-analysis of individual patient data. Eur. Heart J. 39, 374–381 (2018).
Mefford, M. T. et al. PCSK9 variants, LDL-cholesterol, and neurocognitive impairment: reasons for geographic and racial differences in stroke (REGARDS) study. Circulation 137, 1260–1269 (2018).
Zhao, Z. et al. Molecular characterization of loss-of-function mutations in PCSK9 and identification of a compound heterozygote. Am. J. Hum. Genet. 79, 514–523 (2006).
Reynolds, C. A. et al. Analysis of lipid pathway genes indicates association of sequence variation near SREBF1/TOM1L2/ATPAF2 with dementia risk. Hum. Mol. Genet. 19, 2068–2078 (2010).
Shibata, N. et al. No genetic association between PCSK9 polymorphisms and Alzheimer’s disease and plasma cholesterol level in Japanese patients. Psychiatr. Genet. 15, 239 (2005).
Filippatos, T. D., Christopoulou, E. C. & Elisaf, M. S. Pleiotropic effects of proprotein convertase subtilisin/kexin type 9 inhibitors? Curr. Opin. Lipidol. 29, 333–339 (2018).
Ding, Z. et al. Cross-talk between LOX-1 and PCSK9 in vascular tissues. Cardiovasc. Res. 107, 556–567 (2015).
Ilaria, G. et al. Local effects of human PCSK9 on the atherosclerotic lesion. J. Pathol. 238, 52–62 (2015).
Tang, Z.-H. et al. New role of PCSK9 in atherosclerotic inflammation promotion involving the TLR4/NF-kappaB pathway. Atherosclerosis 262, 113–122 (2017).
Yvan-Charvet, L. & Cariou, B. Poststatin era in atherosclerosis management: lessons from epidemiologic and genetic studies. Curr. Opin. Lipidol. 29, 246–258 (2018).
Proto, J. D. et al. Hypercholesterolemia induces T cell expansion in humanized immune mice. J. Clin. Invest. 128, 2370–2375 (2018).
Ridker, P. M. et al. Antiinflammatory therapy with canakinumab for atherosclerotic disease. N. Engl. J. Med. 377, 1119–1131 (2017).
Feingold, K. R., Moser, A. H., Shigenaga, J. K., Patzek, S. M. & Grunfeld, C. Inflammation stimulates the expression of PCSK9. Biochem. Biophys. Res. Commun. 374, 341–344 (2008).
Ruscica, M. et al. Suppressor of cytokine signaling-3 (SOCS-3) induces proprotein convertase subtilisin kexin type 9 (PCSK9) expression in hepatic HepG2 cell line. J. Biol. Chem. 291, 3508–3519 (2016).
Shende, V. R. et al. Reduction of circulating PCSK9 and LDL-C levels by liver-specific knockdown of HNF1α in normolipidemic mice. J. Lipid Res. 56, 801–809 (2015).
Boyd, J. H. et al. Increased plasma PCSK9 levels are associated with reduced endotoxin clearance and the development of acute organ failures during sepsis. J. Innate Immun. 8, 211–220 (2016).
Le Bras, M. et al. Plasma PCSK9 is a late biomarker of severity in patients with severe trauma injury. J. Clin. Endocrinol. Metab. 98, E732–E736 (2013).
Gencer, B. et al. Prognostic value of PCSK9 levels in patients with acute coronary syndromes. Eur. Heart J. 37, 546–553 (2016).
Cariou, B. et al. Circulating PCSK9 levels in acute coronary syndrome: results from the PC-SCA-9 prospective study. Diabetes Metab. 43, 529–535 (2017).
Li, S. et al. Association of plasma PCSK9 levels with white blood cell count and its subsets in patients with stable coronary artery disease. Atherosclerosis 234, 441–445 (2014).
Sahebkar, A. et al. Effect of monoclonal antibodies to PCSK9 on high-sensitivity C-reactive protein levels: a meta-analysis of 16 randomized controlled treatment arms. Br. J. Clin. Pharmacol. 81, 1175–1190 (2016).
Giunzioni, I. et al. Local effects of human PCSK9 on the atherosclerotic lesion. J. Pathol. 238, 52–62 (2016).
Ricci, C. et al. PCSK9 induces a pro-inflammatory response in macrophages. Sci. Rep. 8, 2267 (2018).
Tang, Z. et al. PCSK9 siRNA suppresses the inflammatory response induced by oxLDL through inhibition of NF-kappaB activation in THP-1-derived macrophages. Int. J. Mol. Med. 30, 931–938 (2012).
Topchiy, E. et al. Lipopolysaccharide is cleared from the circulation by hepatocytes via the low density lipoprotein receptor. PLOS ONE 11, e0155030 (2016).
Dwivedi, D. J. et al. Differential expression of PCSK9 modulates infection, inflammation, and coagulation in a murine model of sepsis. Shock 46, 672–680 (2016).
Walley, K. R. et al. PCSK9 is a critical regulator of the innate immune response and septic shock outcome. Sci. Transl Med. 6, 258ra143 (2014).
Berger, J.-M., Loza Valdes, A., Gromada, J., Anderson, N. & Horton, J. D. Inhibition of PCSK9 does not improve lipopolysaccharide-induced mortality in mice. J. Lipid Res. 58, 1661–1669 (2017).
Mazumdar, B., Banerjee, A., Meyer, K. & Ray, R. Hepatitis C virus E1 envelope glycoprotein interacts with apolipoproteins in facilitating entry into hepatocytes. Hepatology 54, 1149–1156 (2011).
Le, Q.-T., Blanchet, M., Seidah, N. G. & Labonte, P. Plasma membrane tetraspanin CD81 complexes with proprotein convertase subtilisin/kexin type 9 (PCSK9) and low density lipoprotein receptor (LDLR), and its levels are reduced by PCSK9. J. Biol. Chem. 290, 23385–23400 (2015).
Labonte, P. et al. PCSK9 impedes hepatitis C virus infection in vitro and modulates liver CD81 expression. Hepatology 50, 17–24 (2009).
Ramanathan, A., Gusarova, V., Stahl, N., Gurnett-Bander, A. & Kyratsous, C. A. Alirocumab, a therapeutic human antibody to PCSK9, does not affect CD81 levels or hepatitis C virus entry and replication into hepatocytes. PLOS ONE 11, e0154498 (2016).
Dai, C.-Y. et al. Associations between hepatitis C viremia and low serum triglyceride and cholesterol levels: a community-based study. J. Hepatol. 49, 9–16 (2008).
Gopal, K. et al. Correlation between beta-lipoprotein levels and outcome of hepatitis C treatment. Hepatology 44, 335–340 (2006).
Bridge, S. H. et al. PCSK9, apolipoprotein E and lipoviral particles in chronic hepatitis C genotype 3: evidence for genotype-specific regulation of lipoprotein metabolism. J. Hepatol. 62, 763–770 (2015).
Kohli, P. et al. HIV and hepatitis C-coinfected patients have lower low-density lipoprotein cholesterol despite higher proprotein convertase subtilisin kexin 9 (PCSK9): an apparent “PCSK9-lipid paradox”. J. Am. Heart Assoc. 5, e002683 (2016).
Boccara, F. et al. Impact of protease inhibitors on circulating PCSK9 levels in HIV-infected antiretroviral-naive patients from an ongoing prospective cohort. AIDS 31, 2367–2376 (2017).
Schlegel, V. et al. Low PCSK9 levels are correlated with mortality in patients with end-stage liver disease. PLOS ONE 12, e0181540 (2017).
Mbikay, M., Mayne, J., Seidah, N. G. & Chretien, M. Of PCSK9, cholesterol homeostasis and parasitic infections: possible survival benefits of loss-of-function PCSK9 genetic polymorphisms. Med. Hypotheses 69, 1010–1017 (2007).
Hooper, A. J., Marais, A. D., Tanyanyiwa, D. M. & Burnett, J. R. The C679X mutation in PCSK9 is present and lowers blood cholesterol in a Southern African population. Atherosclerosis 193, 445–448 (2007).
Arama, C. et al. Malaria severity: possible influence of the E670G PCSK9 polymorphism: a preliminary case-control study in Malian children. PLOS ONE 13, e0192850 (2018).
Lan, H. et al. Proprotein convertase subtilisin/kexin type 9 (PCSK9) affects gene expression pathways beyond cholesterol metabolism in liver cells. J. Cell. Physiol. 224, 273–281 (2010).
Lee, S. et al. Network analyses identify liver-specific targets for treating liver diseases. Mol. Syst. Biol. 13, 938 (2017).
Marimuthu, A. et al. SILAC-based quantitative proteomic analysis of gastric cancer secretome. Proteomics Clin. Appl. 7, 355–366 (2013).
Bhat, M. et al. Decreased PCSK9 expression in human hepatocellular carcinoma. BMC Gastroenterol. 15, 176 (2015).
Huang, J. et al. Tumor-induced hyperlipidemia contributes to tumor growth. Cell Rep. 15, 336–348 (2016).
Sun, X. et al. Proprotein convertase subtilisin/kexin type 9 deficiency reduces melanoma metastasis in liver. Neoplasia 14, 1122–1131 (2012).
Benn, M. et al. Low-density lipoprotein cholesterol levels, and risk of ischemic heart disease: 3 independent studies and meta-analyses. J. Am. Coll. Cardiol. 55, 2833–2842 (2010).
Langsted, A., Nordestgaard, B. G., Benn, M., Tybjærg-Hansen, A. & Kamstrup, P. R. PCSK9 R46L loss-of-function mutation reduces lipoprotein(a), LDL cholesterol, and risk of aortic valve stenosis. J. Clin. Endocrinol. Metab. 101, 3281–3287 (2016).
Fantus, D., Awan, Z., Seidah, N. G. & Genest, J. Aortic calcification: novel insights from familial hypercholesterolemia and potential role for the low-density lipoprotein receptor. Atherosclerosis 226, 9–15 (2013).
Awan, Z. et al. Vascular calcifications in homozygote familial hypercholesterolemia. Arterioscler. Thromb. Vasc. Biol. 28, 777–785 (2008).
Cowell, S. J. et al. A randomized trial of intensive lipid-lowering therapy in calcific aortic stenosis. N. Engl. J. Med. 352, 2389–2397 (2005).
Cariou, B. et al. PCSK9 dominant negative mutant results in increased LDL catabolic rate and familial hypobetalipoproteinemia. Arterioscler. Thromb. Vasc. Biol. 29, 2191–2197 (2009).
Acknowledgements
The authors are supported by grants from the Fondation LEDUCQ (13CVD03) to B.C. and by the French national project CHOPIN (Cholesterol Personalized Innovation) to B.C. and G.L., which was granted by the Agence Nationale de la Recherche (ANR-16-RHUS-0007) and coordinated by the Centre hospitalier universitaire de Nantes. G.K.H. is holder of a Vidi grant (016.156.445) from the Netherlands Organisation for Scientific Research (NWO), and is supported by a grant from the European Union (TransCard: FP7-603091-2).
Reviewer information
Nature Reviews Endocrinology thanks N. Seidah and other anonymous reviewers for their contribution to the peer review of this work.
Author information
Authors and Affiliations
Contributions
The authors contributed equally to all aspects of the article.
Corresponding author
Ethics declarations
Competing interests
G.L. has received research funding, honoraria and consulting fees from Affiris, Amgen, Pfizer, Regeneron and Sanofi. B.C. has received research funding from Pfizer, Regeneron and Sanofi and honoraria from Amgen, Merck Sharpe & Dohme (Merck & Co.), Regeneron and Sanofi. G.K.H.’s institution has received payment for conducting clinical trials from Aegerion, Amgen, AstraZeneca, Eli Lilly, Genzyme, Ionis Pharmaceuticals, Kowa, Pfizer, Regeneron, Roche, Sanofi and Synageva and for lectures and/or advisory panel participation from Amgen, Pfizer, Roche and Sanofi. R.M.S. declares no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Stoekenbroek, R.M., Lambert, G., Cariou, B. et al. Inhibiting PCSK9 — biology beyond LDL control. Nat Rev Endocrinol 15, 52–62 (2019). https://doi.org/10.1038/s41574-018-0110-5
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41574-018-0110-5
This article is cited by
-
Long-Term Efficacy and Tolerability of PCSK9 Targeted Therapy: A Review of the Literature
Drugs (2024)
-
Functional significance of cholesterol metabolism in cancer: from threat to treatment
Experimental & Molecular Medicine (2023)
-
Inhibition of PCSK9 Improves the Development of Pulmonary Arterial Hypertension Via Down-Regulating Notch3 Expression
Cardiovascular Drugs and Therapy (2023)
-
Anti-PCSK9 Treatment Attenuates Liver Fibrosis via Inhibiting Hypoxia-Induced Autophagy in Hepatocytes
Inflammation (2023)
-
Antitumor activity and molecular mechanism of proprotein convertase subtilisin/kexin type 9 (PCSK9) inhibition
Naunyn-Schmiedeberg's Archives of Pharmacology (2022)