Abstract
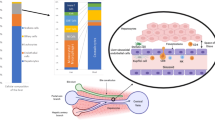
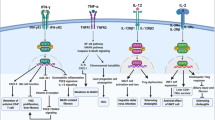
The term cirrhosis-associated immune dysfunction (CAID) comprises the distinctive spectrum of immune alterations associated with the course of end-stage liver disease. Systemic inflammation and immune deficiency are the key components of CAID. Their severity is highly dynamic and progressive, paralleling cirrhosis stage. CAID involves two different immune phenotypes: the low-grade systemic inflammatory phenotype and the high-grade systemic inflammatory phenotype. The low-grade systemic inflammatory phenotype can be found in patients with compensated disease or clinical decompensation with no organ failure. In this phenotype, there is an exaggerated immune activation but the effector response is not markedly compromised. The high-grade systemic inflammatory phenotype is present in patients with acute-on-chronic liver failure, a clinical situation characterized by decompensation, organ failure and high short-term mortality. Along with high-grade inflammation, this CAID phenotype includes intense immune paralysis that critically increases the risk of infections and worsens prognosis. The intensity of CAID has important consequences on cirrhosis progression and correlates with the severity of liver insufficiency, bacterial translocation and organ failure. Therapies targeting the modulation of the dysfunctional immune response are currently being evaluated in preclinical and clinical studies.
Key points
-
Systemic inflammation and immune deficiency are the key components of cirrhosis-associated immune dysfunction (CAID) and their intensity varies according to the stage of cirrhosis and the presence of incidental events.
-
The low-grade systemic inflammatory phenotype is present in patients with cirrhosis with no organ failure. It contributes to worsening systemic circulatory dysfunction, precipitating complications and acute decompensation.
-
The high-grade systemic inflammatory phenotype is the pathogenic driver of organ failure in acute-on-chronic liver failure.
-
A crucial component of the high-grade systemic inflammatory phenotype is an intense functional paralysis of immune system cells that critically increases the risk of infections.
-
An abnormal gut–liver axis, causing intestinal dysbiosis, a disrupted intestinal barrier and increased bacterial translocation, has a leading pathogenic role in systemic inflammation.
-
Treatment of CAID should involve strategies to modulate, rather than inhibit, the immune response as the abrogation or stimulation of the inflammatory response could respectively increase the infection risk or worsen immunopathology.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout







Similar content being viewed by others
References
Bonnel, A. R., Bunchorntavakul, C. & Reddy, K. R. Immune dysfunction and infections in patients with cirrhosis. Clin. Gastroenterol. Hepatol. 9, 727–738 (2011).
Albillos, A., Lario, M. & Álvarez-Mon, M. Cirrhosis-associated immune dysfunction: distinctive features and clinical relevance. J. Hepatol. 61, 1385–1396 (2014).
Moreau, R. et al. Acute-on-chronic liver failure is a distinct syndrome that develops in patients with acute decompensation of cirrhosis. Gastroenterology 144, 1426–1437 (2013).
Martin-Mateos, R., Alvarez-Mon, M. & Albillos, A. Dysfunctional immune response in acute-on-chronic liver failure: it takes two to tango. Front. Immunol. 10, 973 (2019).
Albillos, A. et al. Increased lipopolysaccharide binding protein in cirrhotic patients with marked immune and hemodynamic derangement. Hepatology 37, 208–217 (2003).
Tilg, H. et al. Serum levels of cytokines in chronic liver diseases. Gastroenterology 103, 264–274 (1992).
Albillos, A. et al. Tumour necrosis factor-alpha expression by activated monocytes and altered T-cell homeostasis in ascitic alcoholic cirrhosis: amelioration with norfloxacin. J. Hepatol. 40, 624–631 (2004).
Trebicka, J. et al. The PREDICT study uncovers three clinical courses of acutely decompensated cirrhosis that have distinct pathophysiology. J. Hepatol. 73, 842–854 (2020).
Tritto, G. et al. Evidence of neutrophil functional defect despite inflammation in stable cirrhosis. J. Hepatol. 55, 574–581 (2011).
Rajkovic, I. A. & Williams, R. Abnormalities of neutrophil phagocytosis, intracellular killing and metabolic activity in alcoholic cirrhosis and hepatitis. Hepatology 6, 252–262 (1986).
Clària, J. et al. Systemic inflammation in decompensated cirrhosis: characterization and role in acute-on-chronic liver failure. Hepatology 64, 1249–1264 (2016).
Wasmuth, H. E. et al. Patients with acute on chronic liver failure display ‘sepsis-like’ immune paralysis. J. Hepatol. 42, 195–201 (2005).
Korf, H. et al. Inhibition of glutamine synthetase in monocytes from patients with acute-on-chronic liver failure resuscitates their antibacterial and inflammatory capacity. Gut 68, 1872–1883 (2019).
Albillos, A., de Gottardi, A. & Rescigno, M. The gut-liver axis in liver disease: pathophysiological basis for therapy. J. Hepatol. 72, 558–577 (2020).
Wiest, R., Albillos, A., Trauner, M., Bajaj, J. S. & Jalan, R. Targeting the gut-liver axis in liver disease. J. Hepatol. 67, 1084–1103 (2017).
Wiest, R., Lawson, M. & Geuking, M. Pathological bacterial translocation in liver cirrhosis. J. Hepatol. 60, 197–209 (2014).
Zhao, J. et al. Single-cell RNA sequencing reveals the heterogeneity of liver-resident immune cells in human. Cell Discov. 6, 22 (2020).
Franco, A. et al. Expression of class I and class II major histocompatibility complex antigens on human hepatocytes. Hepatology 8, 449–454 (1988).
Gao, B., Jeong, W.-I. & Tian, Z. Liver: an organ with predominant innate immunity. Hepatology 47, 729–736 (2007).
Kubes, P., Jenne, C. & Snyder, J. Immune responses in the liver. Annu. Rev. Immunol. 36, 1–31 (2018).
Hossain, M. & Kubes, P. Innate immune cells orchestrate the repair of sterile injury in the liver and beyond. Eur. J. Immunol. 49, 831–841 (2019).
Knolle, P. A. Staying local-antigen presentation in the liver. Curr. Opin. Immunol. 40, 36–42 (2016).
Muñoz, L. et al. Intestinal immune dysregulation driven by dysbiosis promotes barrier disruption and bacterial translocation in rats with cirrhosis. Hepatology 70, 925–938 (2019).
Mörbe, U. M. et al. Human gut-associated lymphoid tissues (GALT); diversity, structure, and function. Mucosal Immunol. 14, 793–802 (2021).
Tripathi, A. et al. The gut–liver axis and the intersection with the microbiome. Nat. Rev. Gastroenterol. Hepatol. 15, 397–411 (2018).
Mowat, A. M. Anatomical basis of tolerance and immunity to intestinal antigens. Nat. Rev. Immunol. 3, 331–341 (2003).
MacPherson, G. et al. Uptake of antigens from the intestine by dendritic cells. Ann. N. Y. Acad. Sci. 1029, 75–82 (2004).
Esplugues, E. et al. Control of TH17 cells occurs in the small intestine. Nature 475, 514–518 (2011).
Ismail, A. S. et al. Gamma/Delta intraepithelial lymphocytes are essential mediators of host-microbial homeostasis at the intestinal mucosal surface. Proc. Natl Acad. Sci. USA 108, 8743–8748 (2011).
Slack, E., Balmer, M. L. & Macpherson, A. J. B cells as a critical node in the microbiota-host immune system network. Immunol. Rev. 260, 50–66 (2014).
Kirkland, D. et al. B cell-intrinsic MyD88 signaling prevents the lethal dissemination of commensal bacteria during colonic damage. Immunity 36, 228–238 (2012).
Medzhitov, R. Origin and physiological roles of inflammation. Nature 454, 428–435 (2008).
Kubes, P. & Mehal, W. Z. Sterile Inflammation in the Liver. Gastroenterology 143, 1158–1172 (2012).
Kumar, H., Kawai, T. & Akira, S. Pathogen recognition by the innate immune system. Int. Rev. Immunol. 30, 16–34 (2011).
Úbeda, M. et al. Critical role of the liver in the induction of systemic inflammation in rats with preascitic cirrhosis. Hepatology 52, 2086–2095 (2010).
Wang, H. et al. Hepatoprotective versus oncogenic functions of STAT3 in liver tumorigenesis. Am. J. Pathol. 179, 714–724 (2011).
Muñoz, L. et al. Mesenteric Th1 polarization and monocyte TNF-α production: first steps to systemic inflammation in rats with cirrhosis. Hepatology 42, 411–419 (2005).
Guarner, C. et al. Increased serum nitrite and nitrate levels in patients with cirrhosis: relationship to endotoxemia. Hepatology 18, 1139–1143 (1993).
La Mura, V. et al. Von Willebrand factor levels predict clinical outcome in patients with cirrhosis and portal hypertension. Gut 60, 1133–1138 (2011).
Buck, M. et al. Novel inflammatory biomarkers of portal pressure in compensated cirrhosis patients. Hepatology 59, 1052–1059 (2014).
Grünhage, F. et al. Elevated soluble tumor necrosis factor receptor 75 concentrations identify patients with liver cirrhosis at risk of death. Clin. Gastroenterol. Hepatol. 6, 1255–1262 (2008).
Fiuza, C., Salcedo, M., Clemente, G. & Tellado, J. M. Granulocyte colony-stimulating factor improves deficient in vitro neutrophil transendothelial migration in patients with advanced liver disease. Clin. Vaccin. Immunol. 9, 433–439 (2002).
Girón, J. A. et al. Increased spontaneous and lymphokine-conditioned IgA and IgG synthesis by B cells from alcoholic cirrhotic patients. Hepatology 16, 664–670 (1992).
Sun, H. Q. et al. Increased Th17 cells contribute to disease progression in patients with HBV-associated liver cirrhosis. J. Viral Hepat. 19, 396–403 (2012).
Trebicka, J. et al. Addressing profiles of systemic inflammation across the different clinical phenotypes of acutely decompensated cirrhosis. Front. Immunol. 10, 476 (2019).
Solé, C. et al. Characterization of inflammatory response in acute-on-chronic liver failure and relationship with prognosis. Sci. Rep. 6, 32341 (2016).
Grønbæk, H. et al. The soluble macrophage activation markers sCD163 and mannose receptor (sMR) predict mortality in patients with liver cirrhosis without or with acute-on-chronic liver failure (ACLF). J. Hepatol. 64, 813–822 (2016).
Chen, P., Stärkel, P., Turner, J. R., Ho, S. B. & Schnabl, B. Dysbiosis-induced intestinal inflammation activates tumor necrosis factor receptor I and mediates alcoholic liver disease in mice. Hepatology 61, 883–894 (2015).
Viganò, E. et al. Human caspase-4 and caspase-5 regulate the one-step non-canonical inflammasome activation in monocytes. Nat. Commun. 6, 8761 (2015).
Christgen, S., Place, D. E. & Kanneganti, T.-D. Toward targeting inflammasomes: insights into their regulation and activation. Cell Res. 30, 315–327 (2020).
Monteiro, S. et al. Differential inflammasome activation predisposes to acute-on-chronic liver failure in human and experimental cirrhosis with and without previous decompensation. Gut 70, 379–387 (2021).
Spadoni, I. et al. A gut-vascular barrier controls the systemic dissemination of bacteria. Science 350, 830–834 (2015).
Jenne, C. N. & Kubes, P. Immune surveillance by the liver. Nat. Immunol. 14, 996–1006 (2013).
Yang, A.-M. et al. Intestinal fungi contribute to development of alcoholic liver disease. J. Clin. Invest. 127, 2829–2841 (2017).
Crispe, I. N. Immune tolerance in liver disease. Hepatology 60, 2109–2117 (2014).
Navasa, M. et al. Tumor necrosis factor and interleukin-6 in spontaneous bacterial peritonitis in cirrhosis: relationship with the development of renal impairment and mortality. Hepatology 27, 1227–1232 (1998).
Heller, J. et al. Effects of lipopolysaccharide on TNF-α production, hepatic NOS2 activity, and hepatic toxicity in rats with cirrhosis. J. Hepatol. 33, 376–381 (2000).
Tazi, K. A. et al. In vivo altered unfolded protein response and apoptosis in livers from lipopolysaccharide-challenged cirrhotic rats. J. Hepatol. 46, 1075–1088 (2007).
Tazi, K. A. et al. Upregulation of TNF-alpha production signaling pathways in monocytes from patients with advanced cirrhosis: possible role of Akt and IRAK-M. J. Hepatol. 45, 280–289 (2006).
Thompson, K. et al. Interleukin-10 expression and function in experimental murine liver inflammation and fibrosis. Hepatology 28, 1597–1606 (1998).
Coant, N. et al. Glycogen synthase kinase 3 involvement in the excessive proinflammatory response to LPS in patients with decompensated cirrhosis. J. Hepatol. 55, 784–793 (2011).
Szabo, G. & Saha, B. Alcohol’s effect on host defense. Alcohol. Res. 37, 159–170 (2015).
Gao, B. et al. Innate immunity in alcoholic liver disease. Am. J. Physiol. Gastrointest. Liver Physiol. 300, G516–G525 (2011).
Bajaj, J. S. Alcohol, liver disease and the gut microbiota. Nat. Rev. Gastroenterol. Hepatol. 16, 235–246 (2019).
Schuster, S., Cabrera, D., Arrese, M. & Feldstein, A. E. Triggering and resolution of inflammation in NASH. Nat. Rev. Gastroenterol. Hepatol. 15, 349–364 (2018).
Spruss, A. et al. Toll-like receptor 4 is involved in the development of fructose-induced hepatic steatosis in mice. Hepatology 50, 1094–1104 (2009).
Liu, J. et al. Toll-like receptor-4 signalling in the progression of non-alcoholic fatty liver disease induced by high-fat and high-fructose diet in mice. Clin. Exp. Pharmacol. Physiol. 41, 482–488 (2014).
Stanton, M. C. et al. Inflammatory signals shift from adipose to liver during high fat feeding and influence the development of steatohepatitis in mice. J. Inflamm. 8, 8 (2011).
Guo, J. & Friedman, S. L. Toll-like receptor 4 signaling in liver injury and hepatic fibrogenesis. Fibrogenes. Tissue Repair. 3, 21 (2010).
Petrasek, J. et al. Interferon regulatory factor 3 and type I interferons are protective in alcoholic liver injury in mice by way of crosstalk of parenchymal and myeloid cells. Hepatology 53, 649–660 (2011).
Yan, A. W. et al. Enteric dysbiosis associated with a mouse model of alcoholic liver disease. Hepatology 53, 96–105 (2011).
Mutlu, E. A. et al. Colonic microbiome is altered in alcoholism. Am. J. Physiol. Liver Physiol. 302, G966–G978 (2012).
Parlesak, A., Schäfer, C., Schütz, T., Bode, J. C. & Bode, C. Increased intestinal permeability to macromolecules and endotoxemia in patients with chronic alcohol abuse in different stages of alcohol-induced liver disease. J. Hepatol. 32, 742–747 (2000).
Zhou, Z., Sun, X. & Kang, Y. J. Ethanol-induced apoptosis in mouse liver. Am. J. Pathol. 159, 329–338 (2001).
Lu, Y., Zhuge, J., Wang, X., Bai, J. & Cederbaum, A. I. Cytochrome P450 2E1 contributes to ethanol-induced fatty liver in mice. Hepatology 47, 1483–1494 (2008).
Macdonald, S. et al. Cell death markers in patients with cirrhosis and acute decompensation. Hepatology 67, 989–1002 (2018).
Zheng, S.-J. et al. Prognostic value of M30/M65 for outcome of hepatitis B virus-related acute-on-chronic liver failure. World J. Gastroenterol. 20, 2403–2411 (2014).
Dear, J. W. et al. Cyclophilin A is a damage-associated molecular pattern molecule that mediates acetaminophen-induced liver injury. J. Immunol. 187, 3347–3352 (2011).
Guan, Z. et al. Extracellular gp96 is a crucial mediator for driving immune hyperactivation and liver damage. Sci. Rep. 10, 12596 (2020).
Yotti, R. et al. Left ventricular systolic function is associated with sympathetic nervous activity and markers of inflammation in cirrhosis. Hepatology 65, 2019–2030 (2017).
Tazi, K. A. et al. Norfloxacin reduces aortic no synthases and proinflammatory cytokine up-regulation in cirrhotic rats: role of Akt signaling. Gastroenterology 129, 303–314 (2005).
Téllez, L. et al. Non-selective beta-blockers impair global circulatory homeostasis and renal function in cirrhotic patients with refractory ascites. J. Hepatol. 73, 1404–1414 (2020).
Wiest, R. et al. Bacterial translocation in cirrhotic rats stimulates eNOS-derived NO production and impairs mesenteric vascular contractility. J. Clin. Invest. 104, 1223–1233 (1999).
Bellot, P. et al. Bacterial DNA translocation is associated with systemic circulatory abnormalities and intrahepatic endothelial dysfunction in patients with cirrhosis. Hepatology 52, 2044–2052 (2010).
Waidmann, O. et al. Macrophage activation is a prognostic parameter for variceal bleeding and overall survival in patients with liver cirrhosis. J. Hepatol. 58, 956–961 (2013).
Grønbæk, H. et al. Macrophage activation markers predict mortality in patients with liver cirrhosis without or with acute-on-chronic liver failure (ACLF). J. Hepatol. 64, 813–822 (2016).
Arroyo, V. et al. The systemic inflammation hypothesis: towards a new paradigm of acute decompensation and multiorgan failure in cirrhosis. J. Hepatol. 74, 670–685 (2021).
Shah, N. et al. Increased renal expression and urinary excretion of TLR4 in acute kidney injury associated with cirrhosis. Liver Int. 33, 398–409 (2013).
Shawcross, D. L., Davies, N. A., Williams, R. & Jalan, R. Systemic inflammatory response exacerbates the neuropsychological effects of induced hyperammonemia in cirrhosis. J. Hepatol. 40, 247–254 (2004).
Wright, G. et al. Reduction in hyperammonaemia by ornithine phenylacetate prevents lipopolysaccharide-induced brain edema and coma in cirrhotic rats. Liver Int. 32, 410–419 (2012).
D’Mello, C., Le, T. & Swain, M. G. Cerebral microglia recruit monocytes into the brain in response to tumor necrosis factora signaling during peripheral organ inflammation. J. Neurosci. 29, 2089–2102 (2009).
Kerfoot, S. M. et al. TNF-α-secreting monocytes are recruited into the brain of cholestatic mice. Hepatology 43, 154–162 (2006).
Bajaj, J. S. et al. Linkage of gut microbiome with cognition in hepatic encephalopathy. Am. J. Physiol. Liver Physiol. 302, G168–G175 (2012).
Liu, R. et al. Neuroinflammation in murine cirrhosis is dependent on the gut microbiome and is attenuated by fecal transplant. Hepatology 71, 611–626 (2020).
Yang, Y. M., Kim, S. Y. & Seki, E. Inflammation and liver cancer: molecular mechanisms and therapeutic targets. Semin. Liver Dis. 39, 26–42 (2019).
Naugler, W. E. et al. Gender disparity in liver cancer due to sex differences in MyD88-dependent IL-6 production. Science 317, 121–124 (2007).
Inokuchi, S. et al. Disruption of TAK1 in hepatocytes causes hepatic injury, inflammation, fibrosis, and carcinogenesis. Proc. Natl Acad. Sci. USA 107, 844–849 (2010).
Pikarsky, E. et al. NF-κB functions as a tumour promoter in inflammation-associated cancer. Nature 431, 461–466 (2004).
Jalan, R. et al. Development and validation of a prognostic score to predict mortality in patients with acute-on-chronic liver failure. J. Hepatol. 61, 1038–1047 (2014).
Hotchkiss, R. S. et al. Apoptotic cell death in patients with sepsis, shock, and multiple organ dysfunction. Crit. Care Med. 27, 1230–1251 (1999).
Bernardi, M., Moreau, R., Angeli, P., Schnabl, B. & Arroyo, V. Mechanisms of decompensation and organ failure in cirrhosis: From peripheral arterial vasodilation to systemic inflammation hypothesis. J. Hepatol. 63, 1272–1284 (2015).
Iwasaki, A. & Medzhitov, R. Control of adaptive immunity by the innate immune system. Nat. Immunol. 16, 343–353 (2015).
Gomez, H. et al. A unified theory of sepsis-induced acute kidney injury. Shock 41, 3–11 (2014).
Alobaidi, R., Basu, R. K., Goldstein, S. L. & Bagshaw, S. M. Sepsis-associated acute kidney injury. Semin. Nephrol. 35, 2–11 (2015).
Piano, S. et al. Association between grade of acute on chronic liver failure and response to terlipressin and albumin in patients with hepatorenal syndrome. Clin. Gastroenterol. Hepatol. 16, 1792–1800 (2018).
Rodrigo, R. et al. Hyperammonemia induces neuroinflammation that contributes to cognitive impairment in rats with hepatic encephalopathy. Gastroenterology 139, 675–684 (2010).
Romero-Gómez, M., Montagnese, S. & Jalan, R. Hepatic encephalopathy in patients with acute decompensation of cirrhosis and acute-on-chronic liver failure. J. Hepatol. 62, 437–447 (2015).
Ayres, J. S. Immunometabolism of infections. Nat. Rev. Immunol. 20, 79–80 (2020).
Chowdhury, D. et al. Metallothionein 3 controls the phenotype and metabolic programming of alternatively activated macrophages. Cell Rep. 27, 3873–3886 (2019).
Moreau, R. et al. Blood metabolomics uncovers inflammation-associated mitochondrial dysfunction as a potential mechanism underlying ACLF. J. Hepatol. 72, 688–701 (2020).
Badawy, A. A. B. Kynurenine pathway of tryptophan metabolism: regulatory and functional aspects. Int. J. Tryptophan Res. 10, 1178646917691938 (2017).
Clària, J. et al. Orchestration of tryptophan–kynurenine pathway, acute decompensation, and acute–on–chronic liver failure in cirrhosis. Hepatology 69, 1686–1701 (2019).
Rimola, A. et al. Reticuloendothelial system phagocytic activity in cirrhosis and its relation to bacterial infections and prognosis. Hepatology 4, 53–58 (1984).
Bolognesi, M. et al. Clinical significance of the evaluation of hepatic reticuloendothelial removal capacity in patients with cirrhosis. Hepatology 19, 628–634 (1994).
Laleman, W., Claria, J., Van der Merwe, S., Moreau, R. & Trebicka, J. Systemic inflammation and acute-on-chronic liver failure: too much, not enough. Can. J. Gastroenterol. Hepatol. 2018, 1027152 (2018).
Altorjay, I. et al. Mannose-binding lectin deficiency confers risk for bacterial infections in a large Hungarian cohort of patients with liver cirrhosis. J. Hepatol. 53, 484–491 (2010).
Papp, M. et al. Acute phase proteins in the diagnosis and prediction of cirrhosis associated bacterial infections. Liver Int. 32, 603–611 (2012).
Homann, C. et al. Acquired C3 deficiency in patients with alcoholic cirrhosis predisposes to infection and increased mortality. Gut 40, 544–549 (1997).
Zimmermann, H. W. et al. Functional contribution of elevated circulating and hepatic non-classical CD14+CD16+ monocytes to inflammation and human liver fibrosis. PLoS ONE 5, e11049 (2010).
Nakagawara, A., Inokuchi, K., Ikeda, K., Kumashiro, R. & Tamada, R. Decreased superoxide (O2-)-generating activity of blood monocytes from patients with hepatic cirrhosis. Hepatogastroenterology 31, 201–203 (1984).
Gomez, F., Ruiz, P. & Schreiber, A. D. Impaired function of macrophage Fcγ receptors and bacterial infection in alcoholic cirrhosis. N. Engl. J. Med. 331, 1122–1128 (1994).
Brenig, R. et al. Expression of AXL receptor tyrosine kinase relates to monocyte dysfunction and severity of cirrhosis. Life Sci. Alliance 3, e201900465 (2020).
Berres, M.-L. et al. Longitudinal monocyte Human leukocyte antigen-DR expression is a prognostic marker in critically ill patients with decompensated liver cirrhosis. Liver Int. 29, 536–543 (2009).
Berry, P. A. et al. Severity of the compensatory anti-inflammatory response determined by monocyte HLA-DR expression may assist outcome prediction in cirrhosis. Intensive Care Med. 37, 453–460 (2011).
Xing, T., Li, L., Cao, H. & Huang, J. Altered immune function of monocytes in different stages of patients with acute on chronic liver failure. Clin. Exp. Immunol. 147, 184–188 (2007).
Weichselbaum, L. et al. Epigenetic basis for monocyte dysfunction in patients with severe alcoholic hepatitis. J. Hepatol. 73, 303–314 (2020).
Bernsmeier, C. et al. Patients with acute-on-chronic liver failure have increased numbers of regulatory immune cells expressing the receptor tyrosine kinase MERTK. Gastroenterology 148, 603–615 (2015).
Camenisch, T. D., Koller, B. H., Earp, H. S. & Matsushima, G. K. A novel receptor tyrosine kinase, Mer, inhibits TNF-alpha production and lipopolysaccharide-induced endotoxic shock. J. Immunol. 162, 3498–3503 (1999).
Bernsmeier, C. et al. CD14+CD15−HLA-DR– myeloid-derived suppressor cells impair antimicrobial responses in patients with acute-on-chronic liver failure. Gut 67, 1155–1167 (2018).
Fiuza, C., Salcedo, M., Clemente, G. & Tellado, J. M. In vivo neutrophil dysfunction in cirrhotic patients with advanced liver disease. J. Infect. Dis. 182, 526–533 (2000).
Mookerjee, R. P. et al. Neutrophil dysfunction in alcoholic hepatitis superimposed on cirrhosis is reversible and predicts the outcome. Hepatology 46, 831–840 (2007).
Shawcross, D. L. et al. Ammonia impairs neutrophil phagocytic function in liver disease. Hepatology 48, 1202–1212 (2008).
Bruns, T., Peter, J., Hagel, S., Herrmann, A. & Stallmach, A. The augmented neutrophil respiratory burst in response to Escherichia coli is reduced in liver cirrhosis during infection§. Clin. Exp. Immunol. 164, 346–356 (2011).
Tranah, T. H. et al. Dysfunctional neutrophil effector organelle mobilization and microbicidal protein release in alcohol-related cirrhosis. Am. J. Physiol. Liver Physiol. 313, G203–G211 (2017).
Bukong, T. N. et al. Abnormal neutrophil traps and impaired efferocytosis contribute to liver injury and sepsis severity after binge alcohol use. J. Hepatol. 69, 1145–1154 (2018).
Moreau, R., Périanin, A. & Arroyo, V. Review of defective NADPH oxidase activity and myeloperoxidase release in neutrophils from patients with cirrhosis. Front. Immunol. 10, 1044 (2019).
Artru, F. et al. IL-33/ST2 pathway regulates neutrophil migration and predicts outcome in patients with severe alcoholic hepatitis. J. Hepatol. 72, 1052–1061 (2020).
Taylor, N. J. et al. The severity of circulating neutrophil dysfunction in patients with cirrhosis is associated with 90-day and 1-year mortality. Aliment. Pharmacol. Ther. 40, 705–715 (2014).
Lario, M. et al. Defective thymopoiesis and poor peripheral homeostatic replenishment of T-helper cells cause T-cell lymphopenia in cirrhosis. J. Hepatol. 59, 723–730 (2013).
Riva, A. et al. Mucosa-associated invariant T cells link intestinal immunity with antibacterial immune defects in alcoholic liver disease. Gut 67, 918–930 (2018).
Arruvito, L. et al. Identification and clinical relevance of naturally occurring Human CD8+HLA-DR+ regulatory T cells. J. Immunol. 193, 4469–4476 (2014).
Machicote, A., Belén, S., Baz, P., Billordo, L. A. & Fainboim, L. Human CD8+HLA-DR+ regulatory T cells, similarly to classical CD4+Foxp3+ cells, suppress Immune responses via PD-1/PD-L1 axis. Front. Immunol. 9, 2788 (2018).
Lebossé, F. et al. CD8+ T cells from patients with cirrhosis display a phenotype that may contribute to cirrhosis-associated immune dysfunction. EBioMedicine 49, 258–268 (2019).
Peter, J., Frey, O., Stallmach, A. & Bruns, T. Attenuated antigen-specific T cell responses in cirrhosis are accompanied by elevated serum interleukin-10 levels and down-regulation of HLA-DR on monocytes. BMC Gastroenterol. 13, 37 (2013).
Laso, F. J. et al. Decreased natural killer cytotoxic activity in chronic alcoholism is associated with alcohol liver disease but not active ethanol consumption. Hepatology 25, 1096–1100 (1997).
Doi, H. et al. Dysfunctional B-cell activation in cirrhosis resulting from hepatitis C infection associated with disappearance of CD27-Positive B-cell population. Hepatology 55, 709–719 (2012).
Bandyopadhyay, G. et al. Negative signaling contributes to T-cell anergy in trauma patients. Crit. Care Med. 35, 794–801 (2007).
Monneret, G. et al. The anti-inflammatory response dominates after septic shock: association of low monocyte HLA-DR expression and high interleukin-10 concentration. Immunol. Lett. 95, 193–198 (2004).
Hynninen, M. et al. Predictive value of monocyte histocompatibility leukocyte antigen-DR expression and plasma interleukin-4 and -10 levels in critically ill patients with sepsis. Shock 20, 1–4 (2003).
Oberholzer, A., Oberholzer, C. & Moldawer, L. L. Interleukin-10: a complex role in the pathogenesis of sepsis syndromes and its potential as an anti-inflammatory drug. Crit. Care Med. 30, S58–S63 (2002).
Ward, N. S., Casserly, B. & Ayala, A. The compensatory anti-inflammatory response syndrome (CARS) in critically ill patients. Clin. Chest Med. 29, 617–625 (2008).
Berry, P. A. et al. Admission levels and early changes in serum interleukin-10 are predictive of poor outcome in acute liver failure and decompensated cirrhosis. Liver Int. 30, 733–740 (2010).
Markwick, L. J. L. et al. Blockade of PD1 and TIM3 restores innate and adaptive immunity in patients with acute alcoholic hepatitis. Gastroenterology 148, 590–602.e10 (2015).
Hackstein, C.-P. et al. Gut microbial translocation corrupts myeloid cell function to control bacterial infection during liver cirrhosis. Gut 66, 507–518 (2017).
Jalan, R. et al. Alterations in the functional capacity of albumin in patients with decompensated cirrhosis is associated with increased mortality. Hepatology 50, 555–564 (2009).
Arroyo, V., García-Martinez, R. & Salvatella, X. Human serum albumin, systemic inflammation, and cirrhosis. J. Hepatol. 61, 396–407 (2014).
O’Brien, A. J. et al. Immunosuppression in acutely decompensated cirrhosis is mediated by prostaglandin E2. Nat. Med. 20, 518–523 (2014).
Serezani, C. H. et al. Prostaglandin E2 suppresses bacterial killing in alveolar macrophages by inhibiting NADPH oxidase. Am. J. Respir. Cell Mol. Biol. 37, 562–570 (2007).
Aronoff, D. M., Canetti, C. & Peters-Golden, M. Prostaglandin E2 inhibits alveolar macrophage phagocytosis through an E-prostanoid 2 receptor-mediated increase in intracellular cyclic AMP. J. Immunol. 173, 559–565 (2004).
Schmidl, C. et al. Transcription and enhancer profiling in human monocyte subsets. Blood 123, 90–99 (2014).
Davies, L. C. et al. Peritoneal tissue-resident macrophages are metabolically poised to engage microbes using tissue-niche fuels. Nat. Commun. 8, 2074 (2017).
Villanueva, C. et al. Bacterial infections in patients with compensated cirrhosis and clinically significant portal hypertension: implications on the risk of developing decompensation and on survival. Hepatology 70, 36A–37A (2019).
Arvaniti, V. et al. Infections in patients with cirrhosis increase mortality four-fold and should be used in determining prognosis. Gastroenterology 139, 1246–1256 (2010).
Gustot, T. et al. Impact of infection on the prognosis of critically ill cirrhotic patients: results from a large worldwide study. Liver Int. 34, 1496–1503 (2014).
Fernández, J. et al. Bacterial and fungal infections in acute-on-chronic liver failure: prevalence, characteristics and impact on prognosis. Gut 67, 1870–1880 (2018).
Rao, R. K. Acetaldehyde-induced increase in paracellular permeability in Caco-2 cell monolayer. Alcohol. Clin. Exp. Res. 22, 1724–1730 (1998).
Du Plessis, J. et al. Activated intestinal macrophages in patients with cirrhosis release NO and IL-6 that may disrupt intestinal barrier function. J. Hepatol. 58, 1125–1132 (2013).
Assimakopoulos, S. F. et al. Altered intestinal tight junctions’ expression in patients with liver cirrhosis: a pathogenetic mechanism of intestinal hyperpermeability. Eur. J. Clin. Invest. 42, 439–446 (2012).
Pijls, K. E., Jonkers, D. M. A. E., Elamin, E. E., Masclee, A. A. M. & Koek, G. H. Intestinal epithelial barrier function in liver cirrhosis: an extensive review of the literature. Liver Int. 33, 1457–1469 (2013).
Shah, A. et al. Systematic review and meta-analysis: prevalence of small intestinal bacterial overgrowth in chronic liver disease. Semin. Liver Dis. 37, 388–400 (2017).
Chen, Y. et al. Characterization of fecal microbial communities in patients with liver cirrhosis. Hepatology 54, 562–572 (2011).
Qin, N. et al. Alterations of the human gut microbiome in liver cirrhosis. Nature 513, 59–64 (2014).
Bajaj, J. S. et al. Altered profile of human gut microbiome is associated with cirrhosis and its complications. J. Hepatol. 60, 940–947 (2014).
Solé, C. et al. Alterations in gut microbiome in cirrhosis as assessed by quantitative metagenomics: relationship with acute-on-chronic liver failure and prognosis. Gastroenterology 160, 206–218 (2021).
Bajaj, J. S. et al. Diet affects gut microbiota and modulates hospitalization risk differentially in an international cirrhosis cohort. Hepatology 68, 234–247 (2018).
Garcia-Tsao, G., Lee, F.-Y., Barden, G. E., Cartun, R. & Brian West, A. Bacterial translocation to mesenteric lymph nodes is increased in cirrhotic rats with ascites. Gastroenterology 108, 1835–1841 (1995).
Sorribas, M. et al. FXR modulates the gut-vascular barrier by regulating the entry sites for bacterial translocation in experimental cirrhosis. J. Hepatol. 71, 1126–1140 (2019).
Úbeda, M. et al. Obeticholic acid reduces bacterial translocation and inhibits intestinal inflammation in cirrhotic rats. J. Hepatol. 64, 1049–1057 (2016).
Muñoz, L. et al. Interaction between intestinal dendritic cells and bacteria translocated from the gut in rats with cirrhosis. Hepatology 56, 1861–1869 (2012).
Inamura, T. et al. Alteration of intestinal intraepithelial lymphocytes and increased bacterial translocation in a murine model of cirrhosis. Immunol. Lett. 90, 3–11 (2003).
Tanoue, S., Chang, L.-Y., Li, Y. & Kaplan, D. E. Monocyte-derived dendritic cells from cirrhotic patients retain similar capacity for maturation/activation and antigen presentation as those from healthy subjects. Cell Immunol. 295, 36–45 (2015).
Kaliannan, K. Compromise of α-defensin function in liver cirrhosis facilitates the toxic relationship between gut permeability and endotoxemia. Dig. Dis. Sci. 63, 2492–2494 (2018).
Teltschik, Z. et al. Intestinal bacterial translocation in rats with cirrhosis is related to compromised paneth cell antimicrobial host defense. Hepatology 55, 1154–1163 (2012).
Hassan, M. et al. Paneth cells promote angiogenesis and regulate portal hypertension in response to microbial signals. J. Hepatol. 73, 628–639 (2020).
Genescà, J. et al. Increased tumour necrosis factor alpha production in mesenteric lymph nodes of cirrhotic patients with ascites. Gut 52, 1054–1059 (2003).
Schierwagen, R. et al. Circulating microbiome in blood of different circulatory compartments. Gut 68, 578–580 (2019).
Alvarez-Silva, C. et al. Compartmentalization of immune response and microbial translocation in decompensated cirrhosis. Front. Immunol. 10, 69 (2019).
Bajaj, J. S. et al. Salivary microbiota reflects changes in gut microbiota in cirrhosis with hepatic encephalopathy. Hepatology 62, 1260–1271 (2015).
Bajaj, J. S. et al. Periodontal therapy favorably modulates the oral-gut-hepatic axis in cirrhosis. Am. J. Physiol. Liver Physiol. 315, G824–G837 (2018).
Grønkjær, L. L. et al. Periodontitis in patients with cirrhosis: a cross-sectional study. BMC Oral Health 18, 22 (2018).
Grønkjær, L. L. & Vilstrup, H. Oral health in patients with liver cirrhosis. Eur. J. Gastroenterol. Hepatol. 27, 834–839 (2015).
Kubicka, U. et al. Normal human immune peritoneal cells: Subpopulations and functional characteristics. Scand. J. Immunol. 44, 157–163 (1996).
Ruiz-Alcaraz, A. J. et al. Characterization of human peritoneal monocyte/macrophage subsets in homeostasis: phenotype, GATA6, phagocytic/oxidative activities and cytokines expression. Sci. Rep. 8, 12794 (2018).
Ibidapo-Obe, O. et al. Mucosal-associated invariant T cells redistribute to the peritoneal cavity during spontaneous bacterial peritonitis and contribute to peritoneal inflammation. Cell Mol. Gastroenterol. Hepatol. 9, 661–677 (2020).
Stengel, S. et al. Peritoneal level of CD206 associates with mortality and an inflammatory macrophage phenotype in patients with decompensated cirrhosis and spontaneous bacterial peritonitis. Gastroenterology 158, 1745–1761 (2020).
Irvine, K. M. et al. CRIg-expressing peritoneal macrophages are associated with disease severity in patients with cirrhosis and ascites. JCI Insight 1, e86914 (2016).
Lozano-Ruiz, B. et al. Absent in melanoma 2 triggers a heightened inflammasome response in ascitic fluid macrophages of patients with cirrhosis. J. Hepatol. 62, 64–71 (2015).
Rasaratnam, B., Kaye, D., Jennings, G., Dudley, F. & Chin-Dusting, J. The effect of selective intestinal decontamination on the hyperdynamic circulatory state in cirrhosis. Ann. Intern. Med. 139, 186 (2003).
Chin-Dusting, J. P. F., Rasaratnam, B., Jennings, G. L. R. & Dudley, F. J. Effect of fluoroquinolone on the enhanced nitric oxide-induced peripheral vasodilation seen in cirrhosis. Ann. Intern. Med. 127, 985–988 (1997).
Ginés, P. et al. Norfloxacin prevents spontaneous bacterial peritonitis recurrence in cirrhosis: results of a double-blind, placebo-controlled trial. Hepatology 12, 716–724 (1990).
Moreau, R. et al. Effects of long-term norfloxacin therapy in patients with advanced cirrhosis. Gastroenterology 155, 1816–1827 (2018).
Bajaj, J. S. et al. Modulation of the metabiome by rifaximin in patients with cirrhosis and minimal hepatic encephalopathy. PLoS ONE 8, e60042 (2013).
Fernández, J. et al. Prevalence and risk factors of infections by multiresistant bacteria in cirrhosis: a prospective study. Hepatology 55, 1551–1561 (2012).
Horvath, A. et al. Randomised clinical trial: the effects of a multispecies probiotic vs. placebo on innate immune function, bacterial translocation and gut permeability in patients with cirrhosis. Aliment. Pharmacol. Ther. 44, 926–935 (2016).
Macnaughtan, J. et al. Oral therapy with non-absorbable carbons of controlled porosity (YAQ-001) selectively modulates stool microbiome and its function and this is associated with restoration of immune function and inflammasome activation. J. Hepatol. 62, S240 (2015).
Rice, T. W. et al. A randomized, double-blind, placebo-controlled trial of TAK-242 for the treatment of severe sepsis. Crit. Care Med. 38, 1685–1694 (2010).
Sort, P. et al. Effect of intravenous albumin on renal impairment and mortality in patients with cirrhosis and spontaneous bacterial peritonitis. N. Engl. J. Med. 341, 403–409 (1999).
Caraceni, P. et al. Long-term albumin administration in decompensated cirrhosis (ANSWER): an open-label randomised trial. Lancet 391, 2417–2429 (2018).
Fernández, J. et al. Efficacy of albumin treatment for patients with cirrhosis and infections unrelated to spontaneous bacterial peritonitis. Clin. Gastroenterol. Hepatol. 18, 963–973.e14 (2020).
Lee, K. C. L. et al. Extracorporeal liver assist device to exchange albumin and remove endotoxin in acute liver failure: Results of a pivotal pre-clinical study. J. Hepatol. 63, 634–642 (2015).
Casulleras, M. et al. Albumin internalizes and inhibits endosomal TLR signaling in leukocytes from patients with decompensated cirrhosis. Sci. Transl. Med. 12, eaax5135 (2020).
Dietrich, P. et al. Dysbalance in sympathetic neurotransmitter release and action in cirrhotic rats: impact of exogenous neuropeptide Y. J. Hepatol. 58, 254–261 (2013).
Brinkman, D. J., ten Hove, A. S., Vervoordeldonk, M. J., Luyer, M. D. & de Jonge, W. J. Neuroimmune interactions in the gut and their significance for intestinal immunity. Cells 8, 670 (2019).
Felten, D. L., Felten, S. Y., Carlson, S. L., Olschowka, J. A. & Livnat, S. Noradrenergic and peptidergic innervation of lymphoid tissue. J. Immunol. 135, 755s–765s (1985).
Henriksen, J. H., Ring-Larsen, H., Kanstrup, I. L. & Christensen, N. J. Splanchnic and renal elimination and release of catecholamines in cirrhosis. Evidence of enhanced sympathetic nervous activity in patients with decompensated cirrhosis. Gut 25, 1034–1043 (1984).
Freestone, P. P. et al. Growth stimulation of intestinal commensal Escherichia coli by catecholamines: a possible contributory factor in trauma-induced sepsis. Shock 18, 465–470 (2002).
Chen, C., Brown, D. R., Xie, Y., Green, B. T. & Lyte, M. Catecholamines modulate escherichia coli O157:H7 adherence to murine cecal mucosa. Shock 20, 183–188 (2003).
Piton, G. et al. Catecholamine use is associated with enterocyte damage in critically Ill patients. Shock 43, 437–442 (2015).
Habes, Q. L. M., van Ede, L., Gerretsen, J., Kox, M. & Pickkers, P. Norepinephrine contributes to enterocyte damage in septic shock. Shock 49, 137–143 (2018).
Green, B. T., Lyte, M., Kulkarni-Narla, A. & Brown, D. R. Neuromodulation of enteropathogen internalization in Peyer’s patches from porcine jejunum. J. Neuroimmunol. 141, 74–82 (2003).
Hart, A. & Kamm, M. A. Review article: mechanisms of initiation and perpetuation of gut inflammation by stress. Aliment. Pharmacol. Ther. 16, 2017–2028 (2002).
Straub, R. H., Wiest, R., Strauch, U. G., Harle, P. & Scholmerich, J. The role of the sympathetic nervous system in intestinal inflammation. Gut 55, 1640–1649 (2006).
Worlicek, M. et al. Splanchnic sympathectomy prevents translocation and spreading of E coli but not S aureus in liver cirrhosis. Gut 59, 1127–1134 (2010).
Mehta, G., Mookerjee, R. P., Sharma, V. & Jalan, R. Systemic inflammation is associated with increased intrahepatic resistance and mortality in alcohol-related acute-on-chronic liver failure. Liver Int. 35, 724–734 (2015).
Mookerjee, R. P. et al. Treatment with non-selective beta blockers is associated with reduced severity of systemic inflammation and improved survival of patients with acute-on-chronic liver failure. J. Hepatol. 64, 574–582 (2016).
Forrest, E. H. et al. Baseline neutrophil-to-lymphocyte ratio predicts response to corticosteroids and is associated with infection and renal dysfunction in alcoholic hepatitis. Aliment. Pharmacol. Ther. 50, 442–453 (2019).
Bihari, C. et al. Bone marrow stem cells and their niche components are adversely affected in advanced cirrhosis of the liver. Hepatology 64, 1273–1288 (2016).
Garg, V. et al. Granulocyte colony–stimulating factor mobilizes CD34+ cells and improves survival of patients with acute-on-chronic liver failure. Gastroenterology 142, 505–512 (2012).
Verma, N. et al. Outcomes after multiple courses of granulocyte colony–stimulating factor and growth hormone in decompensated cirrhosis: a randomized trial. Hepatology 68, 1559–1573 (2018).
Engelmann, C. et al. Granulocyte–colony stimulating factor (G–CSF) to treat acute–on–chronic liver failure (Graft Trial): interim analysis of the first randomised European multicentre trial. Hepatology 70, 17 (2019).
Boussif, A. et al. Impaired intracellular signaling, myeloperoxidase release and bactericidal activity of neutrophils from patients with alcoholic cirrhosis. J. Hepatol. 64, 1041–1048 (2016).
Lemmers, A. et al. An inhibitor of interleukin-6 trans-signalling, sgp130, contributes to impaired acute phase response in human chronic liver disease. Clin. Exp. Immunol. 156, 518–527 (2009).
Zimmermann, H. W. et al. Soluble urokinase plasminogen activator receptor is compartmentally regulated in decompensated cirrhosis and indicates immune activation and short-term mortality. J. Intern. Med. 274, 86–100 (2013).
Lehmann, J. M. et al. Circulating CXCL10 in cirrhotic portal hypertension might reflect systemic inflammation and predict ACLF and mortality. Liver Int. 38, 875–884 (2018).
Khanam, A. et al. Blockade of Neutrophil’s chemokine receptors CXCR1/2 abrogate liver damage in acute-on-chronic liver failure. Front. Immunol. 8, 464 (2017).
Xiang, X. et al. Interleukin-22 ameliorates acute-on-chronic liver failure by reprogramming impaired regeneration pathways in mice. J. Hepatol. 72, 736–745 (2020).
Shubham, S. et al. Cellular and functional loss of liver endothelial cells correlates with poor hepatocyte regeneration in acute-on-chronic liver failure. Hepatol. Int. 13, 777–787 (2019).
Rose, C. F. et al. Hepatic encephalopathy: novel insights into classification, pathophysiology and therapy. J. Hepatol. 73, 1526–1547 (2020).
Lee, W.-Y. et al. An intravascular immune response to Borrelia burgdorferi involves Kupffer cells and iNKT cells. Nat. Immunol. 11, 295–302 (2010).
Diehl, L. et al. Tolerogenic maturation of liver sinusoidal endothelial cells promotes B7-homolog 1-dependent CD8+ T cell tolerance. Hepatology 47, 296–305 (2007).
Wang, Y. & Zhang, C. The roles of liver-resident lymphocytes in liver diseases. Front. Immunol. 10, 1582 (2019).
McNamara, H. A. et al. Up-regulation of LFA-1 allows liver-resident memory T cells to patrol and remain in the hepatic sinusoids. Sci. Immunol. 2, 1996 (2017).
Shoukry, N. H. et al. Memory CD8+ T cells are required for protection from persistent hepatitis C virus infection. J. Exp. Med. 197, 1645–1655 (2003).
Pallett, L. J. et al. IL-2high tissue-resident T cells in the human liver: sentinels for hepatotropic infection. J. Exp. Med. 214, 1567–1580 (2017).
Artis, D. & Spits, H. The biology of innate lymphoid cells. Nature 517, 293–301 (2015).
Jeffery, H. C. et al. Biliary epithelium and liver B cells exposed to bacteria activate intrahepatic MAIT cells through MR1. J. Hepatol. 64, 1118–1127 (2016).
Clarembeau, F., Bale, G. & Lanthier, N. Cirrhosis and insulin resistance: current knowledge, pathophysiological mechanisms, complications and potential treatments. Clin. Sci. 134, 2117–2135 (2020).
Bhanji, R. A., Montano–Loza, A. J. & Watt, K. D. Sarcopenia in cirrhosis: looking beyond the skeletal muscle loss to see the systemic disease. Hepatology 70, 2193–2203 (2019).
Angeli, P., Garcia-Tsao, G., Nadim, M. K. & Parikh, C. R. News in pathophysiology, definition and classification of hepatorenal syndrome: a step beyond the International Club of Ascites (ICA) consensus document. J. Hepatol. 71, 811–822 (2019).
Yin, M. et al. Essential role of tumor necrosis factor alpha in alcohol-induced liver injury in mice. Gastroenterology 117, 942–952 (1999).
Acknowledgements
The authors are supported by grants from the Spanish Ministry of Science and Innovation (SAF 2017-86343-R and Instituto de Salud Carlos III PI20/01302). CIBEREHD is funded by the Instituto de Salud Carlos III with grants co-financed by the European Development Regional Fund “A way to achieve Europe” (EDRF).
Author information
Authors and Affiliations
Contributions
A.A. researched data for the article, made a substantial contribution to the discussion of content, wrote the article, and reviewed/edited the manuscript before submission. R.M.-M. made a substantial contribution to the discussion of content, wrote the article, and reviewed/edited the manuscript before submission. S.V.d.M., R.W. and R.J. researched data for the article, made a substantial contributions to the discussion of content, and wrote the article. M.Á.-M. researched data for the article and made a substantial contribution to the discussion of content.
Corresponding author
Ethics declarations
Competing interests
R.J. has research collaborations with Yaqrit and Takeda. R.J. is the inventor of L-ornithine phenylacetate, which has been patented by University College London and licensed to Mallinckrodt Pharma. He is also the founder of Yaqrit Ltd, a spin out company from University College London, Cyberliver Ltd. and Hepyx Ltd. The other authors declare no competing interests.
Additional information
Peer review information
Nature Reviews Gastroenterology & Hepatology thanks Jasmohan Bajaj, Tony Bruns and Frank Tacke for their contribution to the peer review of this work.
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Glossary
- Cirrhosis-associated immune dysfunction
-
(CAID). Dysfunctional immune response associated with cirrhosis and characterized by systemic inflammation and immune paralysis.
- Systemic inflammation
-
Increased expression of surface activation antigens in circulating immune cells and production of pro-inflammatory cytokines.
- Acute-on-chronic liver failure
-
(ACLF). Acute decompensation of cirrhosis associated with organ failure and high short-term mortality.
- Low-grade systemic inflammatory phenotype
-
Increased immune activation and mild to moderate compromise of the immune effector response in patients with compensated cirrhosis or acute decompensation with no organ failure.
- High-grade systemic inflammatory phenotype
-
Extreme activation with a massive release of cytokines along with functional impairment of circulating immune system cells in patients with acute-on-chronic liver failure.
- Gut–liver axis
-
Bidirectional functional connection between the liver and the intestine, particularly its microbiota and immune system.
- Pathogen-associated molecular patterns
-
(PAMPs). Sets of microbial molecular patterns that can be recognized by specific receptors of immune system cells.
- Damage-associated molecular patterns
-
(DAMPs). Intracellular molecules released by injured or dying cells that can be recognized by specific receptors of immune system cells.
- Gut-associated lymphoid tissue
-
(GALT). Lymphoid tissue lining the intestine and composed of Peyer’s patches, intestinal lymphoid follicles, intraepithelial lymphocytes and mesenteric lymph nodes.
- Immunopathology
-
Immune-mediated tissue damage due to excessive immune activation.
Rights and permissions
About this article
Cite this article
Albillos, A., Martin-Mateos, R., Van der Merwe, S. et al. Cirrhosis-associated immune dysfunction. Nat Rev Gastroenterol Hepatol 19, 112–134 (2022). https://doi.org/10.1038/s41575-021-00520-7
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41575-021-00520-7
This article is cited by
-
Elevated CD169 expressing monocyte/macrophage promotes systemic inflammation and disease progression in cirrhosis
Clinical and Experimental Medicine (2024)
-
Prophylaxis and Treatment of Bacterial Infections in Cirrhosis
Current Hepatology Reports (2024)
-
Antimicrobial Resistance in Cirrhosis
Current Hepatology Reports (2024)
-
Risk factors for short-term prognosis of end-stage liver disease complicated by invasive pulmonary aspergillosis
European Journal of Clinical Microbiology & Infectious Diseases (2024)
-
Diagnostic Paracentesis Within 1 Day Is Associated With Reduced Mortality and Length of Hospital Stay in Patients with Cirrhosis and Ascites
Digestive Diseases and Sciences (2024)