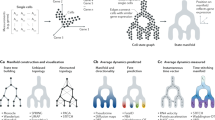
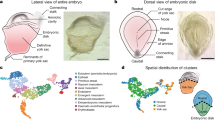
Abstract
During mammalian embryonic development, a single fertilized egg cell will proliferate and differentiate into all the cell lineages and cell types that eventually form the adult organism. Cell lineage diversification involves repeated cell fate choices that ultimately occur at the level of the individual cell rather than at the cell-population level. Improvements in single-cell technologies are transforming our understanding of mammalian development, not only by overcoming the limitations presented by the extremely low cell numbers of early embryos but also by enabling the study of cell fate specification in greater detail. In this Review, we first discuss recent advances in single-cell transcriptomics and imaging and provide a brief outline of current bioinformatics methods available to analyse the resulting data. We then discuss how these techniques have contributed to our understanding of pre-implantation and early post-implantation development and of in vitro pluripotency. Finally, we overview the current challenges facing single-cell research and highlight the latest advances and potential future avenues.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$189.00 per year
only $15.75 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Spitzer, M. H. & Nolan, G. P. Mass cytometry: single cells, many features. Cell 165, 780–791 (2016).
Herzenberg, L. A. et al. The history and future of the fluorescence activated cell sorter and flow cytometry: a view from Stanford. Clin. Chem. 48, 1819–1827 (2002).
Brady, G., Barbara, M. & Iscove, N. Representative in vitro cDNA amplification from individual hemopoietic cells and colonies. Methods Mol. Cell. Biol. 2, 17–23 (1990).
Higuchi, R., Dollinger, G., Walsh, P. S. & Griffith, R. Simultaneous Amplification and detection of specific DNA sequences. Nat. Biotechnol. 10, 413–417 (1992).
Higuchi, R., Fockler, C., Dollinger, G. & Watson, R. Kinetic PCR analysis: real-time monitoring of DNA amplification reactions. Nat. Biotechnol. 11, 1026–1030 (1993).
Guo, G. et al. Resolution of cell fate decisions revealed by single-cell gene expression analysis from zygote to blastocyst. Dev. Cell 18, 675–685 (2010).
Hu, M. et al. Multilineage gene expression precedes commitment in the hemopoietic system. Genes Dev. 11, 774–785 (1997).
Miyamoto, T. et al. Myeloid or lymphoid promiscuity as a critical step in hematopoietic lineage commitment. Dev. Cell 3, 137–147 (2002).
Haghverdi, L., Büttner, M., Wolf, F. A., Buettner, F. & Theis, F. J. Diffusion pseudotime robustly reconstructs lineage branching. Nat. Methods 13, 845–848 (2016).
Moignard, V. et al. Decoding the regulatory network of early blood development from single-cell gene expression measurements. Nat. Biotechnol. 33, 269–276 (2015).
Kurimoto, K. et al. An improved single-cell cDNA amplification method for efficient high-density oligonucleotide microarray analysis. Nucleic Acids Res. 34, e42–e42 (2006).
Tang, F. et al. mRNA-Seq whole-transcriptome analysis of a single cell. Nat. Methods 6, 377–382 (2009).
Hashimshony, T., Wagner, F., Sher, N. & Yanai, I. CEL-Seq: single-cell RNA-Seq by multiplexed linear amplification. Cell Rep. 2, 666–673 (2012).
Hashimshony, T. et al. CEL-Seq2: sensitive highly-multiplexed single-cell RNA-Seq. Genome Biol. 17, 77 (2016).
Picelli, S. et al. Smart-seq2 for sensitive full-length transcriptome profiling in single cells. Nat. Methods 10, 1096–1098 (2013). This report presents the Smart-seq2 protocol for scRNA-seq.
Ramsköld, D. et al. Full-length mRNA-Seq from single-cell levels of RNA and individual circulating tumor cells. Nat. Biotechnol. 30, 777–782 (2012).
Klein, A. M. et al. Droplet barcoding for single-cell transcriptomics applied to embryonic stem cells. Cell 161, 1187–1201 (2015).
Macosko, E. Z. et al. Highly parallel genome-wide expression profiling of individual cells using nanoliter droplets. Cell 161, 1202–1214 (2015). This article presents Drop-seq, a droplet-based protocol that performs scRNA-seq.
Ibarra-Soria, X. et al. Defining murine organogenesis at single cell resolution reveals a role for the leukotriene pathway in regulating blood progenitor formation. Nat. Cell Biol. 20, 127–134 (2018).
Islam, S. et al. Quantitative single-cell RNA-seq with unique molecular identifiers. Nat. Methods 11, 163–166 (2014).
Brennecke, P. et al. Accounting for technical noise in single-cell RNA-seq experiments. Nat. Methods 10, 1093–1095 (2013).
Anders, S. & Huber, W. Differential expression analysis for sequence count data. Genome Biol. 11, R106 (2010).
Jiang, L. et al. Synthetic spike-in standards for RNA-seq experiments. Genome Res. 21, 1543–1551 (2011).
Kolodziejczyk, A. A. et al. Single cell RNA-sequencing of pluripotent states unlocks modular transcriptional variation. Cell Stem Cell 17, 471–485 (2015).
Qiu, X. et al. Single-cell mRNA quantification and differential analysis with Census. Nat. Methods 14, 309–315 (2017).
Maaten, L. J. P. van der & Hinton, G. E. Visualizing high-dimensional data using t-SNE. J. Machine Learn. Res. 9, 2579–2605 (2008).
Coifman, R. R. et al. Geometric diffusions as a tool for harmonic analysis and structure definition of data: diffusion maps. Proc. Natl Acad. Sci. USA 102, 7426–7431 (2005).
Haghverdi, L., Buettner, F. & Theis, F. J. Diffusion maps for high-dimensional single-cell analysis of differentiation data. Bioinformatics 31, 2989–2998 (2015).
Scialdone, A. et al. Resolving early mesoderm diversification through single-cell expression profiling. Nature 535, 289–293 (2016). This study characterizes the E6.5 epiblast, the Flk1+ mesodermal progenitor population and the differentiation path towards blood during gastrulation using the Smart-seq2 protocol.
Brunskill, E. W. et al. A gene expression atlas of early craniofacial development. Dev. Biol. 391, 133–146 (2014).
DeLaughter, D. M. et al. Single-cell resolution of temporal gene expression during heart development. Dev. Cell 39, 480–490 (2016).
Petropoulos, S. et al. Single-cell RNA-Seq reveals lineage and X chromosome dynamics in human preimplantation embryos. Cell 165, 1012–1026 (2016).
Setty, M. et al. Wishbone identifies bifurcating developmental trajectories from single-cell data. Nat. Biotechnol. 34, 637–645 (2016).
Trapnell, C. et al. The dynamics and regulators of cell fate decisions are revealed by pseudotemporal ordering of single cells. Nat. Biotechnol. 32, 381–386 (2014).
Bendall, S. C. et al. Single-cell trajectory detection uncovers progression and regulatory coordination in human B cell development. Cell 157, 714–725 (2014).
Lubeck, E. & Cai, L. Single-cell systems biology by super-resolution imaging and combinatorial labeling. Nat. Methods 9, 743–748 (2012).
Lubeck, E., Coskun, A. F., Zhiyentayev, T., Ahmad, M. & Cai, L. Single-cell in situ RNA profiling by sequential hybridization. Nat. Methods 11, 360–361 (2014).
Raj, A., van den Bogaard, P., Rifkin, S. A., van Oudenaarden, A. & Tyagi, S. Imaging individual mRNA molecules using multiple singly labeled probes. Nat. Methods 5, 877–879 (2008).
Singer, Z. S. et al. Dynamic heterogeneity and DNA methylation in embryonic stem cells. Mol. Cell 55, 319–331 (2014). This study describes the expression dynamics of Nanog in mESCs, which includes transcription bursts.
Frieda, K. L. et al. Synthetic recording and in situ readout of lineage information in single cells. Nature 541, 107–111 (2017).
Lee, J. H. et al. Fluorescent in situ sequencing (FISSEQ) of RNA for gene expression profiling in intact cells and tissues. Nat. Protoc 10, 442–458 (2015).
Haim, L., Zipor, G., Aronov, S. & Gerst, J. E. A genomic integration method to visualize localization of endogenous mRNAs in living yeast. Nat. Methods 4, 409–412 (2007).
Hocine, S., Raymond, P., Zenklusen, D., Chao, J. A. & Singer, R. H. Single-molecule analysis of gene expression using two-color RNA labeling in live yeast. Nat. Methods 10, 119–121 (2013).
Graf, T. & Enver, T. Forcing cells to change lineages. Nature 462, 587–594 (2009).
Hoppe, P. S. et al. Early myeloid lineage choice is not initiated by random PU.1 to GATA1 protein ratios. Nature 535, 299–302 (2016).
Strumpf, D. et al. Cdx2 is required for correct cell fate specification and differentiation of trophectoderm in the mouse blastocyst. Development 132, 2093–2102 (2005).
Palmieri, S. L., Peter, W., Hess, H. & Schöler, H. R. Oct-4 Transcription factor is differentially expressed in the mouse embryo during establishment of the first two extraembryonic cell lineages involved in implantation. Dev. Biol. 166, 259–267 (1994).
Nichols, J. et al. Formation of pluripotent stem cells in the mammalian embryo depends on the POU transcription factor Oct4. Cell 95, 379–391 (1998).
Chambers, I. et al. Functional expression cloning of Nanog, a pluripotency sustaining factor in embryonic stem cells. Cell 113, 643–655 (2003).
Mitsui, K. et al. The homeoprotein Nanog is required for maintenance of pluripotency in mouse epiblast and ES cells. Cell 113, 631–642 (2003).
Schrode, N., Saiz, N., Di Talia, S. & Hadjantonakis, A.-K. GATA6 levels modulate primitive endoderm cell fate choice and timing in the mouse blastocyst. Dev. Cell 29, 454–467 (2014).
Dietrich, J.-E. & Hiiragi, T. Stochastic patterning in the mouse pre-implantation embryo. Development 134, 4219–4231 (2007).
Goolam, M. et al. Heterogeneity in Oct4 and Sox2 targets biases cell fate in 4-cell mouse embryos. Cell 165, 61–74 (2016).
Alarcón, V. B. & Marikawa, Y. Deviation of the blastocyst axis from the first cleavage plane does not affect the quality of mouse postimplantation development. Biol. Reprod. 69, 1208–1212 (2003).
Hiiragi, T. & Solter, D. First cleavage plane of the mouse egg is not predetermined but defined by the topology of the two apposing pronuclei. Nature 430, 360–364 (2004).
Motosugi, N., Bauer, T., Polanski, Z., Solter, D. & Hiiragi, T. Polarity of the mouse embryo is established at blastocyst and is not prepatterned. Genes Dev. 19, 1081–1092 (2005).
Anani, S., Bhat, S., Honma-Yamanaka, N., Krawchuk, D. & Yamanaka, Y. Initiation of Hippo signaling is linked to polarity rather than to cell position in the pre-implantation mouse embryo. Development 141, 2813–2824 (2014).
Bischoff, M., Parfitt, D.-E. & Zernicka-Goetz, M. Formation of the embryonic-abembryonic axis of the mouse blastocyst: relationships between orientation of early cleavage divisions and pattern of symmetric/asymmetric divisions. Development 135, 953–962 (2008).
Johnson, M. H. & Ziomek, C. A. The foundation of two distinct cell lineages within the mouse morula. Cell 24, 71–80 (1981).
Korotkevich, E. et al. The apical domain is required and sufficient for the first lineage segregation in the mouse embryo. Dev. Cell 40, 235–247.e7 (2017).
Piotrowska, K. & Zernicka-Goetz, M. Role for sperm in spatial patterning of the early mouse embryo. Nature 409, 517–521 (2001).
Plachta, N., Bollenbach, T., Pease, S., Fraser, S. E. & Pantazis, P. Oct4 kinetics predict cell lineage patterning in the early mammalian embryo. Nat. Cell Biol. 13, 117–123 (2011).
Tabansky, I. et al. Developmental bias in cleavage-stage mouse blastomeres. Curr. Biol. 23, 21–31 (2013).
Torres-Padilla, M.-E., Parfitt, D.-E., Kouzarides, T. & Zernicka-Goetz, M. Histone arginine methylation regulates pluripotency in the early mouse embryo. Nature 445, 214–218 (2007).
Zernicka-Goetz, M. Development: do mouse embryos play dice? Curr. Biol. 23, R15–R17 (2013).
Biase, F., Cao, X. & Zhong, S. Cell fate inclination within 2-cell and 4-cell mouse embryos revealed by single-cell RNA sequencing. Genome Res. 24, 1787–1796 (2014).
Shi, J. et al. Dynamic transcriptional symmetry-breaking in pre-implantation mammalian embryo development revealed by single-cell RNA-seq. Development 142, 3468–3477 (2015).
Flach, G., Johnson, M. H., Braude, P. R., Taylor, R. A. & Bolton, V. N. The transition from maternal to embryonic control in the 2-cell mouse embryo. EMBO J. 1, 681–686 (1982).
Blakeley, P. et al. Defining the three cell lineages of the human blastocyst by single-cell RNA-seq. Development 142, 3151–3165 (2015).
Braude, P., Bolton, V. & Moore, S. Human gene expression first occurs between the four- and eight-cell stages of preimplantation development. Nature 332, 459–461 (1988).
Niakan, K. K. & Eggan, K. Analysis of human embryos from zygote to blastocyst reveals distinct gene expression patterns relative to the mouse. Dev. Biol. 375, 54–64 (2013).
Xue, Z. et al. Genetic programs in human and mouse early embryos revealed by single-cell RNA sequencing. Nature 500, 593–597 (2013).
Maître, J.-L. et al. Asymmetric division of contractile domains couples cell positioning and fate specification. Nature 536, 344 (2016).
Maître, J.-L. Mechanics of blastocyst morphogenesis. Biol. Cell 109, 323–338 (2017).
Chan, C. J., Heisenberg, C.-P. & Hiiragi, T. Coordination of morphogenesis and cell-fate specification in development. Curr. Biol. 27, R1024–R1035 (2017).
Chazaud, C., Yamanaka, Y., Pawson, T. & Rossant, J. Early lineage segregation between epiblast and primitive endoderm in mouse blastocysts through the Grb2-MAPK pathway. Dev. Cell 10, 615–624 (2006).
Morris, S. A. et al. Origin and formation of the first two distinct cell types of the inner cell mass in the mouse embryo. Proc. Natl Acad. Sci. USA 107, 6364–6369 (2010).
Morris, S. A., Graham, S. J. L., Jedrusik, A. & Zernicka-Goetz, M. The differential response to Fgf signalling in cells internalized at different times influences lineage segregation in preimplantation mouse embryos. Open Biol. 3, 130104 (2013).
Yamanaka, Y., Lanner, F. & Rossant, J. FGF signal-dependent segregation of primitive endoderm and epiblast in the mouse blastocyst. Development 137, 715–724 (2010).
Ohnishi, Y. et al. Cell-to-cell expression variability followed by signal reinforcement progressively segregates early mouse lineages. Nat. Cell Biol. 16, 27–37 (2014). Using single-cell transcriptomics, this study identifies Fgf4 as one of the first genes to be differentially expressed within the ICM. Evaluation of Fgf4 mutant embryos with scRNA-seq shows that Fgf4 −/− cells are arrested before the decision between epiblast and primitive endoderm occurs.
Xenopoulos, P., Kang, M., Puliafito, A., Di Talia, S. & Hadjantonakis, A.-K. Heterogeneities in Nanog expression drive stable commitment to pluripotency in the mouse blastocyst. Cell Rep. 10, 1508–1520 (2015).
Frankenberg, S. et al. Primitive endoderm differentiates via a three-step mechanism involving Nanog and RTK signaling. Dev. Cell 21, 1005–1013 (2011).
Molotkov, A., Mazot, P., Brewer, J. R., Cinalli, R. M. & Soriano, P. Distinct requirements for FGFR1 and FGFR2 in primitive endoderm development and exit from pluripotency. Dev. Cell 41, 511–526.e4 (2017).
Kang, M., Garg, V. & Hadjantonakis, A.-K. Lineage establishment and progression within the inner cell mass of the mouse blastocyst requires FGFR1 and FGFR2. Dev. Cell 41, 496–510.e5 (2017).
Arnold, S. J. & Robertson, E. J. Making a commitment: cell lineage allocation and axis patterning in the early mouse embryo. Nat. Rev. Mol. Cell Biol. 10, 91–103 (2009).
Sutherland, A. E. Tissue morphodynamics shaping the early mouse embryo. Semin. Cell Dev. Biol. 55, 89–98 (2016).
Tam, P. P. L. & Behringer, R. R. Mouse gastrulation: the formation of a mammalian body plan. Mech. Dev. 68, 3–25 (1997).
Tam, P. P., Parameswaran, M., Kinder, S. J. & Weinberger, R. P. The allocation of epiblast cells to the embryonic heart and other mesodermal lineages: the role of ingression and tissue movement during gastrulation. Development 124, 1631–1642 (1997).
Wen, J. et al. Single-cell analysis reveals lineage segregation in early post-implantation mouse embryos. J. Biol. Chem. 292, 9840–9854 (2017). Using single-cell transcriptional profiling, this study reveals the existence of a population of mesendodermal cells as early as E5.5, potentially one of the earliest populations after the exit from epiblast.
Rodaway, A. & Patient, R. Mesendoderm: an ancient germ layer? Cell 105, 169–172 (2001).
Tada, S. et al. Characterization of mesendoderm: a diverging point of the definitive endoderm and mesoderm in embryonic stem cell differentiation culture. Development 132, 4363–4374 (2005).
Ema, M. et al. Combinatorial effects of Flk1 and Tal1 on vascular and hematopoietic development in the mouse. Genes Dev. 17, 380–393 (2003).
Motoike, T., Markham, D. W., Rossant, J. & Sato, T. N. Evidence for novel fate of Flk1 + progenitor: contribution to muscle lineage. Genes 35, 153–159 (2003).
Yamashita, J. et al. Flk1-positive cells derived from embryonic stem cells serve as vascular progenitors. Nature 408, 92–96 (2000).
Ferdous, A. et al. Nkx2–5 transactivates the Ets-related protein 71 gene and specifies an endothelial/endocardial fate in the developing embryo. Proc. Natl Acad. Sci. USA 106, 814–819 (2009).
Rasmussen, T. L. et al. ER71 directs mesodermal fate decisions during embryogenesis. Development 138, 4801–4812 (2011).
Gong, W. et al. Dpath software reveals hierarchical haemato-endothelial lineages of Etv2 progenitors based on single-cell transcriptome analysis. Nat. Commun. 8, 14362 (2017).
Saga, Y. et al. MesP1 is expressed in the heart precursor cells and required for the formation of a single heart tube. Development 126, 3437–3447 (1999).
Devine, W. P., Wythe, J. D., George, M., Koshiba-Takeuchi, K. & Bruneau, B. G. Early patterning and specification of cardiac progenitors in gastrulating mesoderm. eLife 3, e03848 (2014).
Lescroart, F. et al. Early lineage restriction in temporally distinct populations of Mesp1 progenitors during mammalian heart development. Nat. Cell Biol. 16, 829–840 (2014).
Evans, M. J. & Kaufman, M. H. Establishment in culture of pluripotential cells from mouse embryos. Nature 292, 154–156 (1981).
Weinberger, L., Ayyash, M., Novershtern, N. & Hanna, J. H. Dynamic stem cell states: naive to primed pluripotency in rodents and humans. Nat. Rev. Mol. Cell Biol. 17, 155–169 (2016).
Guo, G. et al. Serum-based culture conditions provoke gene expression variability in mouse embryonic stem cells as revealed by single-cell analysis. Cell Rep. 14, 956–965 (2016).
Martinez Arias, A. & Brickman, J. M. Gene expression heterogeneities in embryonic stem cell populations: origin and function. Curr. Opin. Cell Biol. 23, 650–656 (2011).
Filipczyk, A. et al. Network plasticity of pluripotency transcription factors in embryonic stem cells. Nat. Cell Biol. 17, 1235–1246 (2015).
Cannon, D., Corrigan, A. M., Miermont, A., McDonel, P. & Chubb, J. R. Multiple cell and population-level interactions with mouse embryonic stem cell heterogeneity. Development 142, 2840–2849 (2015).
Kalmar, T. et al. Regulated fluctuations in Nanog expression mediate cell fate decisions in embryonic stem cells. PLoS Biol. 7, e1000149 (2009).
Macfarlan, T. S. et al. Embryonic stem cell potency fluctuates with endogenous retrovirus activity. Nature 487, 57–63 (2012). Using single-cell imaging, this study identifies 2C-like cells, a rare subpopulation within mESCs that resembles the in vivo 2-cell stage.
Zalzman, M. et al. Zscan4 regulates telomere elongation and genomic stability in ES cells. Nature 464, 858–863 (2010).
Eckersley-Maslin, M. A. et al. MERVL/Zscan4 network activation results in transient genome-wide DNA demethylation of mESCs. Cell Rep. 17, 179–192 (2016). This study shows similarities between the in vivo 2-cell state and 2 C-like cells at the transcriptional level. Furthermore, 2 C-like cells have open chromatin and hypomethylated DNA, both characteristics of the in vivo 2-cell stage.
Takahashi, K. & Yamanaka, S. Induction of pluripotent stem cells from mouse embryonic and adult fibroblast cultures by defined factors. Cell 126, 663–676 (2006).
Takahashi, K. & Yamanaka, S. A decade of transcription factor-mediated reprogramming to pluripotency. Nat. Rev. Mol. Cell Biol. 17, 183–193 (2016).
Lujan, E. et al. Early reprogramming regulators identified by prospective isolation and mass cytometry. Nature 521, 352–356 (2015).
O’Malley, J. et al. High-resolution analysis with novel cell-surface markers identifies routes to iPS cells. Nature 499, 88–91 (2013).
Buganim, Y. et al. Single-cell expression analyses during cellular reprogramming reveal an early stochastic and a late hierarchic phase. Cell 150, 1209–1222 (2012).
Chung, K.-M. et al. Single cell analysis reveals the stochastic phase of reprogramming to pluripotency is an ordered probabilistic process. PLoS ONE 9, e95304 (2014).
Zunder, E. R., Lujan, E., Goltsev, Y., Wernig, M. & Nolan, G. P. A. Continuous molecular roadmap to iPSC reprogramming through progression analysis of single-cell mass cytometry. Cell Stem Cell 16, 323–337 (2015).
Kim, D. H. et al. Single-cell transcriptome analysis reveals dynamic changes in lncRNA expression during reprogramming. Cell Stem Cell 16, 88–101 (2015).
Polo, J. M. et al. A molecular roadmap of reprogramming somatic cells into iPS cells. Cell 151, 1617–1632 (2012). Transcriptional analyses define the reprogramming towards iPSC as a two-step process, where DNA methylation changes occur late in reprogramming.
Smith, Z. D., Nachman, I., Regev, A. & Meissner, A. Dynamic single-cell imaging of direct reprogramming reveals an early specifying event. Nat. Biotechnol. 28, 521–526 (2010).
Apostolou, E. & Hochedlinger, K. Chromatin dynamics during cellular reprogramming. Nature 502, 462–471 (2013).
Pasque, V. et al. X chromosome reactivation dynamics reveal stages of reprogramming to pluripotency. Cell 159, 1681–1697 (2014).
Achim, K. et al. High-throughput spatial mapping of single-cell RNA-seq data to tissue of origin. Nat. Biotechnol. 33, 503–509 (2015).
Satija, R., Farrell, J. A., Gennert, D., Schier, A. F. & Regev, A. Spatial reconstruction of single-cell gene expression data. Nat. Biotechnol. 33, 495–502 (2015).
Espina, V. et al. Laser-capture microdissection. Nat. Protoc. 1, 586–603 (2006).
Junker, J. P. et al. Genome-wide RNA tomography in the zebrafish embryo. Cell 159, 662–675 (2014).
Peng, G. et al. Spatial transcriptome for the molecular annotation of lineage fates and cell identity in mid-gastrula mouse embryo. Dev. Cell 36, 681–697 (2016). This study presents a spatial transcriptome map of the E7.0 epiblast. This was achieved using laser capture microdissection and profiling pools of 20 cells by scRNA-seq while keeping track of their original locations.
Clark, S. J. et al. scNMT-seq enables joint profiling of chromatin accessibility DNA methylation and transcription in single cells. Nat. Commun. 9, 781 (2018). This study reports a combined method to obtain the transcriptome, chromatin accessibility and DNA methylation states of individual cells.
Macaulay, I. C. et al. G&T-seq: parallel sequencing of single-cell genomes and transcriptomes. Nat. Methods 12, 519–522 (2015).
Byrne, A. et al. Nanopore long-read RNAseq reveals widespread transcriptional variation among the surface receptors of individual B cells. Nat. Commun. 8, 16027 (2017).
McKenna, A. et al. Whole-organism lineage tracing by combinatorial and cumulative genome editing. Science 353, aaf7907 (2016). This study presents a proof-of-principle experiment of lineage tracking at single-cell resolution. Using a genomic barcode harbouring unique CRISPR–Cas9 target sites in a fertilized zebrafish egg gives rise to the accumulation of thousands of uniquely edited barcodes in the offspring cells of the adult fish, enabling the authors to infer lineage relationships between all adult cells.
Acknowledgements
B.P.-S. is funded by the Wellcome Trust 4-Year PhD programme in Stem Cell Biology and Medicine and the University of Cambridge, UK. C.G. is funded by the Swedish Research Council. Research in the Göttgens laboratory is supported by programme grants from the Wellcome Trust, CRUK and Bloodwise and by a Wellcome Strategic Award to study cell fate decisions during gastrulation (105031/D/14/Z). The authors also gratefully acknowledge core support funding from the Wellcome Trust to the Wellcome–Medical Research Council Cambridge Stem Cell Institute.
Author information
Authors and Affiliations
Contributions
B.P.-S. researched data for the article and wrote the article. C.G. contributed substantially to the discussion of the content. B.P.-S., C.G. and B.G. reviewed and edited the manuscript before submission.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Glossary
- Fluorescence-activated cell sorting
-
Flow cytometry method to analyse and sort single cells based on the expression of cell-surface markers.
- Microfluidics systems
-
Automated technologies based on the use of microminiaturized devices for mixing and manipulating low fluid volumes aiming to achieve multiplexing and high-throughput yields.
- Blastocyst
-
Embryonic stage composed of inner cell mass cells, a fluid-filled cavity called blastocoel and outer trophectoderm cells.
- Epiblast
-
Group of cells derived from the inner cell mass that will give rise to the embryo proper.
- Primitive endoderm
-
Group of cells derived from the inner cell mass that contribute to extra-embryonic tissues such as the yolk sac.
- Unique molecular identifiers
-
Short sequences that uniquely tag individual RNA molecules.
- Dimensionality-reduction approaches
-
Methods used in high-dimensional datasets, where each gene represents a dimension, to reduce the number of dimensions and elicit the visualization of the data set structure in a two-dimensional or three-dimensional plot.
- Gastrulation
-
Embryonic process, following implantation, where epiblast cells become specified into the three germ layers (ectoderm, mesoderm and endoderm).
- Inner cell mass
-
(ICM). Group of cells located inside the blastocyst that will give rise to the primitive endoderm and the epiblast.
- Trophectoderm
-
Group of cells located on the outer part of the blastocyst that will become the supportive extra-embryonic tissues, such as the placenta.
- Blastomeres
-
The cells resulting from the first divisions of the fertilized egg.
- Maternal-to-zygotic transition
-
Process occurring shortly after fertilization, where maternal RNA and proteins are degraded and the zygotic genome is activated and produces RNA and proteins.
- Bimodal distribution
-
In gene expression analyses, a gene being either (a) highly expressed or (b) not or lowly expressed, with small numbers of cells displaying intermediate levels. Cells can thus be divided into two subpopulations based on the expression levels of that particular gene.
- Salt-and-pepper
-
Group of inner cell mass cells with heterogeneous expression of epiblast and primitive endoderm markers, where cells expressing more epiblast markers are intermingled with cells expressing more primitive endoderm markers.
- Morula
-
Early embryonic stage where the embryo is composed of a symmetric ball of morphologically similar cells.
- Primitive streak
-
Morphological structure at the posterior side of the embryo formed by the accumulation of cells. It is where epiblast cells will ingress to become mesoderm or endoderm.
- Mesendodermal progenitor
-
A cell that can give rise to either mesoderm or endoderm.
- Yolk sac
-
Extra-embryonic tissue that originates from the primitive endoderm.
- Boolean algorithm
-
Qualitative algorithm based on the Boolean (binary) logic, where only two values are accepted. In gene regulatory networks, one value will be active and the other one inactive.
- Unimodal distribution
-
In gene expression analyses, unimodal distribution refers to a gene being mostly expressed at intermediate levels.
- X-chromosome reactivation
-
Process where the inactivated X chromosome in mammalian female cells becomes reactivated.
Rights and permissions
About this article
Cite this article
Pijuan-Sala, B., Guibentif, C. & Göttgens, B. Single-cell transcriptional profiling: a window into embryonic cell-type specification. Nat Rev Mol Cell Biol 19, 399–412 (2018). https://doi.org/10.1038/s41580-018-0002-5
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41580-018-0002-5
This article is cited by
-
Single-cell RNA sequencing to identify cellular heterogeneity and targets in cardiovascular diseases: from bench to bedside
Basic Research in Cardiology (2023)
-
Engineering a niche supporting hematopoietic stem cell development using integrated single-cell transcriptomics
Nature Communications (2022)
-
iMAP: integration of multiple single-cell datasets by adversarial paired transfer networks
Genome Biology (2021)
-
Transposable elements shape the evolution of mammalian development
Nature Reviews Genetics (2021)
-
Single-cell mapping of DNA G-quadruplex structures in human cancer cells
Scientific Reports (2021)