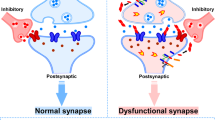
Abstract
Alzheimer disease (AD) is characterized by progressive cognitive decline in older individuals accompanied by the presence of two pathological protein aggregates — amyloid-β and phosphorylated tau — in the brain. The disease results in brain atrophy caused by neuronal loss and synapse degeneration. Synaptic loss strongly correlates with cognitive decline in both humans and animal models of AD. Indeed, evidence suggests that soluble forms of amyloid-β and tau can cause synaptotoxicity and spread through neural circuits. These pathological changes are accompanied by an altered phenotype in the glial cells of the brain — one hypothesis is that glia excessively ingest synapses and modulate the trans-synaptic spread of pathology. To date, effective therapies for the treatment or prevention of AD are lacking, but understanding how synaptic degeneration occurs will be essential for the development of new interventions. Here, we highlight the mechanisms through which synapses degenerate in the AD brain, and discuss key questions that still need to be answered. We also cover the ways in which our understanding of the mechanisms of synaptic degeneration is leading to new therapeutic approaches for AD.
Key points
-
Synaptic degeneration is a prominent feature of Alzheimer disease (AD) both in humans and in preclinical models of the disease.
-
Evidence indicates that synaptic degeneration is the best neuropathological correlate of cognitive decline in AD; however, effective treatments to slow down or stop synaptic loss are lacking.
-
Amyloid-β (Aβ) and tau are the most well-studied contributors to synaptic degeneration in AD and, although most anti-Aβ therapies have so far failed in clinical trials, targeting these proteins earlier in the disease process might ameliorate neurodegeneration.
-
Microglia and astrocytes can drive synaptic degeneration in animal models of ageing and AD via ingestion of tagged synapses, contributing to cognitive decline.
-
Many clinical trials are now focusing on the interactions between immune responses and neurons in AD, as opposed to focusing only on the reduction of Aβ and tau levels.
-
New synaptic biomarkers are being developed with the aim of aiding the earlier diagnosis of AD and distinguishing between people who will stay cognitively healthy as they age and people who will develop AD.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
WHO. Global action plan on the public health response to dementia 2017–2025 (WHO, 2017).
Spires-Jones, T. L. Alzheimer’s research – breakthrough or breakdown? Brain Commun. 3, fcab217 (2021).
Biogen. Lecanemab confirmatory phase 3 CLARITY AD study met primary endpoubt, showing highly statistically significant reduction of clinical decline in large global clinical study of 1,795 participants with early Alzheimer’s disease. Biogen https://investors.biogen.com/news-releases/news-release-details/lecanemab-confirmatory-phase-3-clarity-ad-study-met-primary (2022).
DeKosky, S. T. & Scheff, S. W. Synapse loss in frontal cortex biopsies in Alzheimer’s disease: correlation with cognitive severity. Ann. Neurol. 27, 457–464 (1990).
Terry, R. D. et al. Physical basis of cognitive alterations in Alzheimer’s disease: synapse loss is the major correlate of cognitive impairment. Ann. Neurol. 30, 572–580 (1991).
Mecca, A. P. et al. Synaptic density and cognitive performance in Alzheimer’s disease: a PET imaging study with [11C]UCB-J. Alzheimers Dement. https://doi.org/10.1002/alz.12582 (2022).
Kopeikina, K. J., Hyman, B. T. & Spires-Jones, T. L. Soluble forms of tau are toxic in Alzheimer’s disease. Transl Neurosci. 3, 223–233 (2012).
Spires-Jones, T. L. & Hyman, B. T. The intersection of amyloid beta and tau at synapses in Alzheimer’s disease. Neuron 82, 756–771 (2014).
Polydoro, M. et al. Soluble pathological tau in the entorhinal cortex leads to presynaptic deficits in an early Alzheimer’s disease model. Acta Neuropathol. 127, 257–270 (2014).
Fá, M. et al. Extracellular tau oligomers produce an immediate impairment of LTP and memory. Sci. Rep. 6, 19393 (2016).
Shankar, G. M. et al. Amyloid-β protein dimers isolated directly from Alzheimer’s brains impair synaptic plasticity and memory. Nat. Med. 14, 837–842 (2008).
Hong, W. et al. Diffusible, highly bioactive oligomers represent a critical minority of soluble Aβ in Alzheimer’s disease brain. Acta Neuropathol. 136, 19–40 (2018).
Klein, W. L. Synaptotoxic amyloid-β oligomers: a molecular basis for the cause, diagnosis, and treatment of Alzheimer’s disease? J. Alzheimers Dis. 33, S49–S65 (2013).
Perez-Nievas, B. G. et al. Dissecting phenotypic traits linked to human resilience to Alzheimer’s pathology. Brain 136, 2510–2526 (2013).
Mc Donald, J. M. et al. The presence of sodium dodecyl sulphate-stable Aβ dimers is strongly associated with Alzheimer-type dementia. Brain 133, 1328–1341 (2010).
de Calignon, A. et al. Propagation of tau pathology in a model of early Alzheimer’s disease. Neuron 73, 685–697 (2012).
Pickett, E. K. et al. Spread of tau down neural circuits precedes synapse and neuronal loss in the rTgTauEC mouse model of early Alzheimer’s disease. Synapse 71, e21965 (2017).
Jucker, M. & Walker, L. C. Self-propagation of pathogenic protein aggregates in neurodegenerative diseases. Nature 501, 45–51 (2013).
d’Errico, P. & Meyer-Luehmann, M. Mechanisms of pathogenic tau and Aβ protein spreading in Alzheimer’s disease. Front. Aging Neurosci. 12, 265 (2020).
Takeda, S. et al. Neuronal uptake and propagation of a rare phosphorylated high-molecular-weight tau derived from Alzheimer’s disease brain. Nat. Commun. 6, 8490 (2015).
Pooler, A. M., Phillips, E. C., Lau, D. H. W., Noble, W. & Hanger, D. P. Physiological release of endogenous tau is stimulated by neuronal activity. EMBO Rep. 14, 389–394 (2013).
Yamada, K. et al. Neuronal activity regulates extracellular tau in vivo. J. Exp. Med. 211, 387–393 (2014).
Cirrito, J. R. et al. Endocytosis is required for synaptic activity-dependent release of amyloid-β in vivo. Neuron 58, 42–51 (2008).
Harris, J. A. et al. Human P301L-mutant tau expression in mouse entorhinal-hippocampal network causes tau aggregation and presynaptic pathology but no cognitive deficits. PLoS ONE 7, e45881 (2012).
Liu, L. et al. Trans-synaptic spread of tau pathology in vivo. PLoS ONE 7, e31302 (2012).
Wegmann, S. et al. Experimental evidence for the age dependence of tau protein spread in the brain. Sci. Adv. 5, eaaw6404 (2019).
Henstridge, C. M., Hyman, B. T. & Spires-Jones, T. L. Beyond the neuron-cellular interactions early in Alzheimer disease pathogenesis. Nat. Rev. Neurosci. 20, 94–108 (2019).
De Strooper, B. & Karran, E. The cellular phase of Alzheimer’s disease. Cell 164, 603–615 (2016).
Keren-Shaul, H. et al. A unique microglia type associated with restricting development of Alzheimer’s disease. Cell 169, 1276–1290 (2017).
Habib, N. et al. Disease-associated astrocytes in Alzheimer’s disease and aging. Nat. Neurosci. 23, 701–706 (2020).
Jonsson, T. et al. Variant of TREM2 associated with the risk of Alzheimer’s disease. N. Engl. J. Med. 368, 107–116 (2013).
Guerreiro, R. et al. TREM2 variants in Alzheimer’s disease. N. Engl. J. Med. 368, 117–127 (2013).
Strittmatter, W. J. & Roses, A. D. Apolipoprotein E and Alzheimer’s disease. Annu. Rev. Neurosci. 19, 53–77 (1996).
Krasemann, S. et al. The TREM2-APOE pathway drives the transcriptional phenotype of dysfunctional microglia in neurodegenerative diseases. Immunity 47, 566–581 (2017).
Kent, S. A., Spires-Jones, T. L. & Durrant, C. S. The physiological roles of tau and Aβ: implications for Alzheimer’s disease pathology and therapeutics. Acta Neuropathol. 140, 417–447 (2020).
Hardy, J. A. & Higgins, G. A. Alzheimer’s disease: the amyloid cascade hypothesis. Science 256, 184–185 (1992).
Hardy, J. The amyloid hypothesis for Alzheimer’s disease: a critical reappraisal. J. Neurochem. 110, 1129–1134 (2009).
O’Brien, R. J. & Wong, P. C. Amyloid precursor protein processing and Alzheimer’s disease. Annu. Rev. Neurosci. 34, 185–204 (2011).
Cole, S. L. & Vassar, R. The Alzheimer’s disease β-secretase enzyme, BACE1. Mol. Neurodegener. 2, 22 (2007).
Richter, M. C. et al. Distinct in vivo roles of secreted APP ectodomain variants APPsα and APPsβ in regulation of spine density, synaptic plasticity, and cognition. EMBO J. 37, e98335 (2018).
Kuhn, P.-H. et al. ADAM10 is the physiologically relevant, constitutive α-secretase of the amyloid precursor protein in primary neurons. EMBO J. 29, 3020–3032 (2010).
Jackson, R. J. et al. Clusterin accumulates in synapses in Alzheimer’s disease and is increased in apolipoprotein E4 carriers. Brain Commun. 1, fcz003 (2019).
Koffie, R. M. et al. Apolipoprotein E4 effects in Alzheimer’s disease are mediated by synaptotoxic oligomeric amyloid-β. Brain 135, 2155–2168 (2012).
Pickett, E. K. et al. Amyloid beta and tau cooperate to cause reversible behavioral and transcriptional deficits in a model of Alzheimer’s disease. Cell Rep. 29, 3592–3604.e5 (2019).
Koffie, R. M. et al. Oligomeric amyloid associates with postsynaptic densities and correlates with excitatory synapse loss near senile plaques. Proc. Natl Acad. Sci. USA 106, 4012–4017 (2009).
Fein, J. A. et al. Co-localization of amyloid beta and tau pathology in Alzheimer’s disease synaptosomes. Am. J. Pathol. 172, 1683–1692 (2008).
Tai, H.-C. et al. The synaptic accumulation of hyperphosphorylated tau oligomers in Alzheimer disease is associated with dysfunction of the ubiquitin-proteasome system. Am. J. Pathol. 181, 1426–1435 (2012).
Paspalas, C. D. et al. The aged rhesus macaque manifests Braak stage III/IV Alzheimer’s-like pathology. Alzheimers Dement. 14, 680–691 (2018).
Hoover, B. R. et al. Tau mislocalization to dendritic spines mediates synaptic dysfunction independently of neurodegeneration. Neuron 68, 1067–1081 (2010).
Kopeikina, K. J. et al. Synaptic alterations in the rTg4510 mouse model of tauopathy. J. Comp. Neurol. 521, 1334–1353 (2013).
Zhou, L. et al. Tau association with synaptic vesicles causes presynaptic dysfunction. Nat. Commun. 8, 15295 (2017).
Rozkalne, A., Spires-Jones, T. L., Stern, E. A. & Hyman, B. T. A single dose of passive immunotherapy has extended benefits on synapses and neurites in an Alzheimer’s disease mouse model. Brain Res. 1280, 178–185 (2009).
Spires-Jones, T. L. et al. Passive immunotherapy rapidly increases structural plasticity in a mouse model of Alzheimer disease. Neurobiol. Dis. 33, 213–220 (2009).
Sydow, A. et al. Tau-induced defects in synaptic plasticity, learning, and memory are reversible in transgenic mice after switching off the toxic tau mutant. J. Neurosci. 31, 2511–2525 (2011).
Roberson, E. D. et al. Amyloid-β/Fyn-induced synaptic, network, and cognitive impairments depend on tau levels in multiple mouse models of Alzheimer’s disease. J. Neurosci. 31, 700–711 (2011).
Roberson, E. D. et al. Reducing endogenous tau ameliorates amyloid-induced deficits in an Alzheimer’s disease mouse model. Science 316, 750–754 (2007).
Shankar, G. M. et al. Natural oligomers of the Alzheimer amyloid-β protein induce reversible synapse loss by modulating an NMDA-type glutamate receptor-dependent signaling pathway. J. Neurosci. 27, 2866–2875 (2007).
Townsend, M., Shankar, G. M., Mehta, T., Walsh, D. M. & Selkoe, D. J. Effects of secreted oligomers of amyloid β-protein on hippocampal synaptic plasticity: a potent role for trimers. J. Physiol. 572, 477–492 (2006).
Walsh, D. M. et al. Naturally secreted oligomers of amyloid β protein potently inhibit hippocampal long-term potentiation in vivo. Nature 416, 535–539 (2002).
Cleary, J. P. et al. Natural oligomers of the amyloid-β protein specifically disrupt cognitive function. Nat. Neurosci. 8, 79–84 (2005).
Walsh, D. M. et al. The role of cell-derived oligomers of Abeta in Alzheimer’s disease and avenues for therapeutic intervention. Biochem. Soc. Trans. 33, 1087–1090 (2005).
Beckman, D. et al. Oligomeric Aβ in the monkey brain impacts synaptic integrity and induces accelerated cortical aging. Proc. Natl Acad. Sci. USA 116, 26239–26246 (2019).
Acquarone, E. et al. Synaptic and memory dysfunction induced by tau oligomers is rescued by up-regulation of the nitric oxide cascade. Mol. Neurodegener. 14, 26 (2019).
Kaniyappan, S., Chandupatla, R. R., Mandelkow, E.-M. & Mandelkow, E. Extracellular low-n oligomers of tau cause selective synaptotoxicity without affecting cell viability. Alzheimers Dement. 13, 1270–1291 (2017).
Decker, J. M. et al. Pro-aggregant Tau impairs mossy fiber plasticity due to structural changes and Ca(++) dysregulation. Acta Neuropathol. Commun. 3, 23 (2015).
Moreno, H. et al. Tau pathology-mediated presynaptic dysfunction. Neuroscience 325, 30–38 (2016).
Lacor, P. N. et al. Synaptic targeting by Alzheimer’s-related amyloid β oligomers. J. Neurosci. 24, 10191–10200 (2004).
Laurén, J., Gimbel, D. A., Nygaard, H. B., Gilbert, J. W. & Strittmatter, S. M. Cellular prion protein mediates impairment of synaptic plasticity by amyloid-β oligomers. Nature 457, 1128–1132 (2009).
Renner, M. et al. Deleterious effects of amyloid β oligomers acting as an extracellular scaffold for mGluR5. Neuron 66, 739–754 (2010).
Barry, A. E. et al. Alzheimer’s disease brain-derived amyloid-β-mediated inhibition of LTP in vivo is prevented by immunotargeting cellular prion protein. J. Neurosci. 31, 7259–7263 (2011).
Hu, N.-W. et al. mGlu5 receptors and cellular prion protein mediate amyloid-β-facilitated synaptic long-term depression in vivo. Nat. Commun. 5, 3374 (2014).
Zhang, D. et al. Targeting glutamatergic and cellular prion protein mechanisms of amyloid β-mediated persistent synaptic plasticity disruption: longitudinal studies. Neuropharmacology 121, 231–246 (2017).
Um, J. W. et al. Metabotropic glutamate receptor 5 is a coreceptor for Alzheimer Aβ oligomer bound to cellular prion protein. Neuron 79, 887–902 (2013).
Larson, M. et al. The complex PrP(c)-Fyn couples human oligomeric Aβ with pathological tau changes in Alzheimer’s disease. J. Neurosci. 32, 16857–16871 (2012).
Salazar, S. V. et al. Conditional deletion of Prnp rescues behavioral and synaptic deficits after disease onset in transgenic Alzheimer’s disease. J. Neurosci. 37, 9207–9221 (2017).
Kaufman, A. C. et al. Fyn inhibition rescues established memory and synapse loss in Alzheimer mice. Ann. Neurol. 77, 953–971 (2015).
van Dyck, C. H. et al. Effect of AZD0530 on cerebral metabolic decline in Alzheimer disease: a randomized clinical trial. JAMA Neurol. 76, 1219–1229 (2019).
Folch, J. et al. Memantine for the treatment of dementia: a review on its current and future applications. J. Alzheimers Dis. 62, 1223–1240 (2018).
Gunn, A. P. et al. Amyloid-β peptide Aβ3pE-42 induces lipid peroxidation, membrane permeabilization, and calcium influx in neurons. J. Biol. Chem. 291, 6134–6145 (2016).
Wei, W. et al. Amyloid beta from axons and dendrites reduces local spine number and plasticity. Nat. Neurosci. 13, 190–196 (2010).
Fani, G. et al. Aβ oligomers dysregulate calcium homeostasis by mechanosensitive activation of AMPA and NMDA receptors. ACS Chem. Neurosci. 12, 766–781 (2021).
Gomes, G. M. et al. Inhibition of the polyamine system counteracts β-amyloid peptide-induced memory impairment in mice: involvement of extrasynaptic NMDA receptors. PLoS ONE 9, e99184 (2014).
Dieterich, D. C. et al. Caldendrin–Jacob: a protein liaison that couples NMDA receptor signalling to the nucleus. PLoS Biol. 6, e34 (2008).
Rönicke, R. et al. Early neuronal dysfunction by amyloid β oligomers depends on activation of NR2B-containing NMDA receptors. Neurobiol. Aging 32, 2219–2228 (2011).
Yin, Y. et al. Tau accumulation induces synaptic impairment and memory deficit by calcineurin-mediated inactivation of nuclear CaMKIV/CREB signaling. Proc. Natl Acad. Sci. USA 113, E3773–E3781 (2016).
McClendon, M. J., Hernandez, S., Smyth, K. A. & Lerner, A. J. Memantine and acetylcholinesterase inhibitor treatment in cases of CDR 0.5 or questionable impairment. J. Alzheimers Dis. 16, 577–583 (2009).
Wang, H.-F. et al. Efficacy and safety of cholinesterase inhibitors and memantine in cognitive impairment in Parkinson’s disease, Parkinson’s disease dementia, and dementia with Lewy bodies: systematic review with meta-analysis and trial sequential analysis. J. Neurol. Neurosurg. Psychiatry 86, 135–143 (2015).
Cissé, M. et al. Reversing EphB2 depletion rescues cognitive functions in Alzheimer model. Nature 469, 47–52 (2011).
Ohnishi, T. et al. Na,K-ATPase α3 is a death target of Alzheimer patient amyloid-β assembly. Proc. Natl Acad. Sci. USA 112, E4465–E4474 (2015).
Magdesian, M. H. et al. Amyloid-β binds to the extracellular cysteine-rich domain of Frizzled and inhibits Wnt/β-catenin signaling. J. Biol. Chem. 283, 9359–9368 (2008).
Zhao, W.-Q. et al. Amyloid beta oligomers induce impairment of neuronal insulin receptors. FASEB J. 22, 246–260 (2008).
Costantini, C. et al. Characterization of the signaling pathway downstream p75 neurotrophin receptor involved in β-amyloid peptide-dependent cell death. J. Mol. Neurosci. 25, 141–156 (2005).
Yamamoto, N. et al. A ganglioside-induced toxic soluble Aβ assembly. Its enhanced formation from Aβ bearing the Arctic mutation. J. Biol. Chem. 282, 2646–2655 (2007).
Riad, A. et al. The sigma-2 receptor/TMEM97, PGRMC1, and LDL receptor complex are responsible for the cellular uptake of Aβ42 and its protein aggregates. Mol. Neurobiol. 57, 3803–3813 (2020).
Izzo, N. J. et al. Alzheimer’s therapeutics targeting amyloid beta 1-42 oligomers II: sigma-2/PGRMC1 receptors mediate Abeta 42 oligomer binding and synaptotoxicity. PLoS ONE 9, e111899 (2014).
Marcello, E. et al. Endocytosis of synaptic ADAM10 in neuronal plasticity and Alzheimer’s disease. J. Clin. Invest. 123, 2523–2538 (2013).
Musardo, S. et al. The development of ADAM10 endocytosis inhibitors for the treatment of Alzheimer’s disease. Mol. Ther. 30, 2474–2490 (2022).
Bold, C. S. et al. APPsα rescues tau-induced synaptic pathology. J. Neurosci. 42, 5782–5802 (2022).
Gómez-Ramos, A., Díaz-Hernández, M., Rubio, A., Miras-Portugal, M. T. & Avila, J. Extracellular tau promotes intracellular calcium increase through M1 and M3 muscarinic receptors in neuronal cells. Mol. Cell Neurosci. 37, 673–681 (2008).
McInnes, J. et al. Synaptogyrin-3 mediates presynaptic dysfunction induced by tau. Neuron 97, 823–835 (2018).
Largo-Barrientos, P. et al. Lowering synaptogyrin-3 expression rescues Tau-induced memory defects and synaptic loss in the presence of microglial activation. Neuron 109, 767–777 (2021).
Liu, C., Song, X., Nisbet, R. & Götz, J. Co-immunoprecipitation with tau isoform-specific antibodies reveals distinct protein interactions and highlights a putative role for 2N tau in disease. J. Biol. Chem. 291, 8173–8188 (2016).
Lasagna-Reeves, C. A. et al. Tau oligomers impair memory and induce synaptic and mitochondrial dysfunction in wild-type mice. Mol. Neurodegener. 6, 39 (2011).
Balaji, V., Kaniyappan, S., Mandelkow, E., Wang, Y. & Mandelkow, E.-M. Pathological missorting of endogenous MAPT/Tau in neurons caused by failure of protein degradation systems. Autophagy 14, 2139–2154 (2018).
Zhao, X. et al. Caspase-2 cleavage of tau reversibly impairs memory. Nat. Med. 22, 1268–1276 (2016).
Ittner, L. M. et al. Dendritic function of tau mediates amyloid-β toxicity in Alzheimer’s disease mouse models. Cell 142, 387–397 (2010).
Tang, S. J. et al. Fyn kinase inhibition reduces protein aggregation, increases synapse density and improves memory in transgenic and traumatic tauopathy. Acta Neuropathol. Commun. 8, 96 (2020).
Zempel, H. et al. Amyloid-β oligomers induce synaptic damage via Tau-dependent microtubule severing by TTLL6 and spastin. EMBO J. 32, 2920–2937 (2013).
Busche, M. A. et al. Critical role of soluble amyloid-β for early hippocampal hyperactivity in a mouse model of Alzheimer’s disease. Proc. Natl Acad. Sci. USA 109, 8740–8745 (2012).
Kuchibhotla, K. V. et al. Aβ plaques lead to aberrant regulation of calcium homeostasis in vivo resulting in structural and functional disruption of neuronal networks. Neuron 59, 214–225 (2008).
Arbel-Ornath, M. et al. Soluble oligomeric amyloid-β induces calcium dyshomeostasis that precedes synapse loss in the living mouse brain. Mol. Neurodegener. 12, 27 (2017).
Jang, H. et al. β-Barrel topology of Alzheimer’s β-amyloid ion channels. J. Mol. Biol. 404, 917–934 (2010).
Kokubo, H. et al. Amyloid beta annular protofibrils in cell processes and synapses accumulate with aging and Alzheimer-associated genetic modification. Int. J. Alzheimers Dis. 2009, 689285 (2009).
Lal, R., Lin, H. & Quist, A. P. Amyloid beta ion channel: 3D structure and relevance to amyloid channel paradigm. Biochim. Biophys. Acta Biomembr. 1768, 1966–1975 (2007).
Kayed, R. et al. Annular protofibrils are a structurally and functionally distinct type of amyloid oligomer. J. Biol. Chem. 284, 4230–4237 (2009).
Halpain, S., Hipolito, A. & Saffer, L. Regulation of F-actin stability in dendritic spines by glutamate receptors and calcineurin. J. Neurosci. 18, 9835–9844 (1998).
Wu, H.-Y. et al. Amyloid β induces the morphological neurodegenerative triad of spine loss, dendritic simplification, and neuritic dystrophies through calcineurin activation. J. Neurosci. 30, 2636–2649 (2010).
Rozkalne, A., Hyman, B. T. & Spires-Jones, T. L. Calcineurin inhibition with FK506 ameliorates dendritic spine density deficits in plaque-bearing Alzheimer model mice. Neurobiol. Dis. 41, 650–654 (2011).
Hesse, R. et al. Comparative profiling of the synaptic proteome from Alzheimer’s disease patients with focus on the APOE genotype. Acta Neuropathol. Commun. 7, 214 (2019).
Biasetti, L. et al. Elevated amyloid beta disrupts the nanoscale organization and function of synaptic vesicle pools in hippocampal neurons. Cereb. Cortex https://doi.org/10.1093/cercor/bhac134 (2022).
Usenovic, M. et al. Internalized tau oligomers cause neurodegeneration by inducing accumulation of pathogenic tau in human neurons derived from induced pluripotent stem cells. J. Neurosci. 35, 14234–14250 (2015).
Kopeikina, K. J. et al. Tau causes synapse loss without disrupting calcium homeostasis in the rTg4510 model of tauopathy. PLoS ONE 8, e80834 (2013).
Kuchibhotla, K. V. et al. Neurofibrillary tangle-bearing neurons are functionally integrated in cortical circuits in vivo. Proc. Natl Acad. Sci. USA 111, 510–514 (2014).
Mattson, M. P. Calcium and neurodegeneration. Aging Cell 6, 337–350 (2007).
Arnsten, A. F. T., Datta, D., Del Tredici, K. & Braak, H. Hypothesis: tau pathology is an initiating factor in sporadic Alzheimer’s disease. Alzheimers Dement. 17, 115–124 (2021).
Datta, D. et al. Age-related calcium dysregulation linked with tau pathology and impaired cognition in non-human primates. Alzheimers Dement. 17, 920–932 (2021).
Mattson, M. P., Gary, D. S., Chan, S. L. & Duan, W. Perturbed endoplasmic reticulum function, synaptic apoptosis and the pathogenesis of Alzheimer’s disease. Biochem. Soc. Symp. 67, 151–162 (2001).
Adamec, E., Mohan, P., Vonsattel, J. P. & Nixon, R. A. Calpain activation in neurodegenerative diseases: confocal immunofluorescence study with antibodies specifically recognizing the active form of calpain 2. Acta Neuropathol. 104, 92–104 (2002).
Jin, M. et al. Soluble amyloid beta-protein dimers isolated from Alzheimer cortex directly induce Tau hyperphosphorylation and neuritic degeneration. Proc. Natl Acad. Sci. USA 108, 5819–5824 (2011).
Busche, M. A. et al. Tau impairs neural circuits, dominating amyloid-β effects, in Alzheimer models in vivo. Nat. Neurosci. 22, 57–64 (2019).
Marinković, P. et al. In vivo imaging reveals reduced activity of neuronal circuits in a mouse tauopathy model. Brain 142, 1051–1062 (2019).
Wu, Q. et al. Increased neuronal activity in motor cortex reveals prominent calcium dyshomeostasis in tauopathy mice. Neurobiol. Dis. 147, 105165 (2021).
Cenini, G. & Voos, W. Mitochondria as potential targets in Alzheimer disease therapy: an update. Front. Pharmacol. 10, 902 (2019).
Hauptmann, S. et al. Mitochondrial dysfunction: an early event in Alzheimer pathology accumulates with age in AD transgenic mice. Neurobiol. Aging 30, 1574–1586 (2009).
Bell, S. M. et al. Mitochondrial dysfunction in Alzheimer’s disease: a biomarker of the future? Biomedicines 9, 63 (2021).
Xie, H. et al. Mitochondrial alterations near amyloid plaques in an Alzheimer’s disease mouse model. J. Neurosci. 33, 17042–17051 (2013).
Pickett, E. K. et al. Region-specific depletion of synaptic mitochondria in the brains of patients with Alzheimer’s disease. Acta Neuropathol. 136, 747–757 (2018).
Hansson Petersen, C. A. et al. The amyloid β-peptide is imported into mitochondria via the TOM import machinery and localized to mitochondrial cristae. Proc. Natl Acad. Sci. USA 105, 13145–13150 (2008).
Hernandez-Zimbron, L. F. et al. Amyloid-β peptide binds to cytochrome c oxidase subunit 1. PLoS ONE 7, e42344 (2012).
Lustbader, J. W. et al. ABAD directly links Aβ to mitochondrial toxicity in Alzheimer’s disease. Science 304, 448–452 (2004).
Wang, X. et al. Amyloid-β overproduction causes abnormal mitochondrial dynamics via differential modulation of mitochondrial fission/fusion proteins. Proc. Natl Acad. Sci. USA 105, 19318–19323 (2008).
Kopeikina, K. J. et al. Tau accumulation causes mitochondrial distribution deficits in neurons in a mouse model of tauopathy and in human Alzheimer’s disease brain. Am. J. Pathol. 179, 2071–2082 (2011).
Manczak, M. & Reddy, P. H. Abnormal interaction between the mitochondrial fission protein Drp1 and hyperphosphorylated tau in Alzheimer’s disease neurons: implications for mitochondrial dysfunction and neuronal damage. Hum. Mol. Genet. 21, 2538–2547 (2012).
D’Amelio, M. et al. Caspase-3 triggers early synaptic dysfunction in a mouse model of Alzheimer’s disease. Nat. Neurosci. 14, 69–76 (2011).
Park, G. et al. Caspase activation and caspase-mediated cleavage of APP is associated with amyloid β-protein-induced synapse loss in Alzheimer’s disease. Cell Rep. 31, 107839 (2020).
Louneva, N. et al. Caspase-3 is enriched in postsynaptic densities and increased in Alzheimer’s disease. Am. J. Pathol. 173, 1488–1495 (2008).
Baumgartner, H. K. et al. Calcium elevation in mitochondria is the main Ca2+ requirement for mitochondrial permeability transition pore (mPTP) opening. J. Biol. Chem. 284, 20796–20803 (2009).
D’Amelio, M., Cavallucci, V. & Cecconi, F. Neuronal caspase-3 signaling: not only cell death. Cell Death Differ. 17, 1104–1114 (2010).
Pérez, M. J., Vergara-Pulgar, K., Jara, C., Cabezas-Opazo, F. & Quintanilla, R. A. Caspase-cleaved tau impairs mitochondrial dynamics in Alzheimer’s disease. Mol. Neurobiol. 55, 1004–1018 (2018).
Bertholet, A. M. et al. OPA1 loss of function affects in vitro neuronal maturation. Brain 136, 1518–1533 (2013).
Bero, A. W. et al. Neuronal activity regulates the regional vulnerability to amyloid-β deposition. Nat. Neurosci. 14, 750–756 (2011).
Jack, C. R. et al. Tracking pathophysiological processes in Alzheimer’s disease: an updated hypothetical model of dynamic biomarkers. Lancet Neurol. 12, 207–216 (2013).
Tzioras, M., Davies, C., Newman, A., Jackson, R. & Spires-Jones, T. Invited review: APOE at the interface of inflammation, neurodegeneration and pathological protein spread in Alzheimer’s disease. Neuropathol. Appl. Neurobiol. 45, 327–346 (2019).
Greicius, M. D., Srivastava, G., Reiss, A. L. & Menon, V. Default-mode network activity distinguishes Alzheimer’s disease from healthy aging: evidence from functional MRI. Proc. Natl Acad. Sci. USA 101, 4637–4642 (2004).
Hafkemeijer, A., van der Grond, J. & Rombouts, S. A. R. B. Imaging the default mode network in aging and dementia. Biochim. Biophys. Acta Mol. Basis Dis. 1822, 431–441 (2012).
Badhwar, A. et al. Resting-state network dysfunction in Alzheimer’s disease: a systematic review and meta-analysis. Alzheimers Dement. 8, 73–85 (2017).
Mevel, K., Chételat, G., Eustache, F. & Desgranges, B. The default mode network in healthy aging and Alzheimer’s disease. Int. J. Alzheimers Dis. 2011, e535816 (2011).
Sperling, R. A. et al. Amyloid deposition is associated with impaired default network function in older persons without dementia. Neuron 63, 178–188 (2009).
Palmqvist, S. et al. Earliest accumulation of β-amyloid occurs within the default-mode network and concurrently affects brain connectivity. Nat. Commun. 8, 1214 (2017).
Aloisi, F. Immune function of microglia. Glia 36, 165–179 (2001).
Diaz-Aparicio, I. et al. Microglia actively remodel adult hippocampal neurogenesis through the phagocytosis secretome. J. Neurosci. 40, 1453–1482 (2020).
Abbott, N. J., Rönnbäck, L. & Hansson, E. Astrocyte–endothelial interactions at the blood–brain barrier. Nat. Rev. Neurosci. 7, 41–53 (2006).
Banker, G. A. Trophic interactions between astroglial cells and hippocampal neurons in culture. Science 209, 809–810 (1980).
Li, X. et al. MEK is a key regulator of gliogenesis in the developing brain. Neuron 75, 1035–1050 (2012).
Schafer, D. P. et al. Microglia sculpt postnatal neural circuits in an activity and complement-dependent manner. Neuron 74, 691–705 (2012).
Paolicelli, R. C. et al. Synaptic pruning by microglia is necessary for normal brain development. Science 333, 1456–1458 (2011).
Oosterhof, N. et al. Homozygous mutations in CSF1R cause a pediatric-onset leukoencephalopathy and can result in congenital absence of microglia. Am. J. Hum. Genet. 104, 936–947 (2019).
Wu, T. et al. Complement C3 is activated in human AD brain and is required for neurodegeneration in mouse models of amyloidosis and tauopathy. Cell Rep. 28, 2111–2123 (2019).
Chung, W.-S. et al. Astrocytes mediate synapse elimination through MEGF10 and MERTK pathways. Nature 504, 394–400 (2013).
Scott-Hewitt, N. et al. Local externalization of phosphatidylserine mediates developmental synaptic pruning by microglia. EMBO J. 39, e105380 (2020).
Vainchtein, I. D. et al. Astrocyte-derived interleukin-33 promotes microglial synapse engulfment and neural circuit development. Science 359, 1269–1273 (2018).
Spurrier, J. et al. Reversal of synapse loss in Alzheimer mouse models by targeting mGluR5 to prevent synaptic tagging by C1Q. Sci. Transl Med. 14, eabi8593 (2022).
Hong, S. et al. Complement and microglia mediate early synapse loss in Alzheimer mouse models. Science 352, 712–716 (2016).
Bie, B., Wu, J., Foss, J. F. & Naguib, M. Activation of mGluR1 mediates C1q-dependent microglial phagocytosis of glutamatergic synapses in Alzheimer’s rodent models. Mol. Neurobiol. 56, 5568–5585 (2019).
Shi, Q. et al. Complement C3 deficiency protects against neurodegeneration in aged plaque-rich APP/PS1 mice. Sci. Transl Med. 9, eaaf6295 (2017).
Brelstaff, J., Tolkovsky, A. M., Ghetti, B., Goedert, M. & Spillantini, M. G. Living neurons with tau filaments aberrantly expose phosphatidylserine and are phagocytosed by microglia. Cell Rep. 24, 1939–1948 (2018).
Dejanovic, B. et al. Changes in the synaptic proteome in tauopathy and rescue of tau-induced synapse loss by C1q antibodies. Neuron 100, 1322–1336 (2018).
Benetatos, J. et al. PTEN activation contributes to neuronal and synaptic engulfment by microglia in tauopathy. Acta Neuropathol. 140, 7–24 (2020).
Olmos-Alonso, A. et al. Pharmacological targeting of CSF1R inhibits microglial proliferation and prevents the progression of Alzheimer’s-like pathology. Brain 139, 891–907 (2016).
Spangenberg, E. E. et al. Eliminating microglia in Alzheimer’s mice prevents neuronal loss without modulating amyloid-β pathology. Brain 139, 1265–1281 (2016).
Mancuso, R. et al. CSF1R inhibitor JNJ-40346527 attenuates microglial proliferation and neurodegeneration in P301S mice. Brain 142, 3243–3264 (2019).
Oberheim, N. A., Wang, X., Goldman, S. & Nedergaard, M. Astrocytic complexity distinguishes the human brain. Trends Neurosci. 29, 547–553 (2006).
Wang, W.-Y., Tan, M.-S., Yu, J.-T. & Tan, L. Role of pro-inflammatory cytokines released from microglia in Alzheimer’s disease. Ann. Transl Med. 3, 136 (2015).
Combs, C. K., Karlo, J. C., Kao, S. C. & Landreth, G. E. β-Amyloid stimulation of microglia and monocytes results in TNFα-dependent expression of inducible nitric oxide synthase and neuronal apoptosis. J. Neurosci. 21, 1179–1188 (2001).
Azevedo, E. P. et al. Activated microglia mediate synapse loss and short-term memory deficits in a mouse model of transthyretin-related oculoleptomeningeal amyloidosis. Cell Death Dis. 4, e789 (2013).
Sheppard, O., Coleman, M. P. & Durrant, C. S. Lipopolysaccharide-induced neuroinflammation induces presynaptic disruption through a direct action on brain tissue involving microglia-derived interleukin 1 beta. J. Neuroinflamm. 16, 106 (2019).
Zhu, Y. et al. APOE genotype alters glial activation and loss of synaptic markers in mice. Glia 60, 559–569 (2012).
El Hajj, H. et al. Ultrastructural evidence of microglial heterogeneity in Alzheimer’s disease amyloid pathology. J. Neuroinflamm. 16, 87 (2019).
Gomez-Arboledas, A. et al. Phagocytic clearance of presynaptic dystrophies by reactive astrocytes in Alzheimer’s disease. Glia 66, 637–653 (2018).
Sanchez-Mico, M. V. et al. Amyloid-β impairs the phagocytosis of dystrophic synapses by astrocytes in Alzheimer’s disease. Glia 69, 997–1011 (2021).
Franco-Bocanegra, D. K. et al. Microglial morphology in Alzheimer’s disease and after Aβ immunotherapy. Sci. Rep. 11, 15955 (2021).
Hannestad, J. et al. Safety and tolerability of GRF6019 infusions in severe Alzheimer’s disease: a phase II double-blind placebo-controlled trial. J. Alzheimers Dis. 81, 1649–1662 (2021).
Izzo, N. J. et al. Preclinical and clinical biomarker studies of CT1812: a novel approach to Alzheimer’s disease modification. Alzheimers Dement. 17, 1365–1382 (2021).
US National Library of Medicine. ClinicalTrials.gov https://clinicaltrials.gov/ct2/show/NCT03817684 (2019).
Farlow, M. R. et al. A randomized, double-blind, placebo-controlled, phase II study assessing safety, tolerability, and efficacy of bryostatin in the treatment of moderately severe to severe Alzheimer’s disease. J. Alzheimers Dis. 67, 555–570 (2019).
Ismail, R. et al. The effect of 40-Hz light therapy on amyloid load in patients with prodromal and clinical Alzheimer’s disease. Int. J. Alzheimers Dis. 2018, 6852303 (2018).
Pleen, J. & Townley, R. Alzheimer’s disease clinical trial update 2019–2021. J. Neurol. 269, 1038–1051 (2022).
US National Library of Medicine. ClinicalTrials.gov https://clinicaltrials.gov/ct2/show/NCT04805983 (2022).
Lansita, J. A. et al. Nonclinical development of ANX005: a humanized anti-C1q antibody for treatment of autoimmune and neurodegenerative diseases. Int. J. Toxicol. 36, 449–462 (2017).
US National Library of Medicine. ClinicalTrials.gov https://clinicaltrials.gov/ct2/show/NCT04701164 (2021).
US National Library of Medicine. ClinicalTrials.gov https://clinicaltrials.gov/ct2/show/NCT04514367 (2022).
US National Library of Medicine. ClinicalTrials.gov https://clinicaltrials.gov/ct2/show/NCT04121208 (2020).
Cannarile, M. A. et al. Colony-stimulating factor 1 receptor (CSF1R) inhibitors in cancer therapy. J. Immunother. Cancer 5, 53 (2017).
Livingston, G. et al. Dementia prevention, intervention, and care: 2020 report of the Lancet Commission. Lancet 396, 413–446 (2020).
Casaletto, K. B. et al. Microglial correlates of late life physical activity: relationship with synaptic and cognitive aging in older adults. J. Neurosci. 42, 288–298 (2022).
Spires-Jones, T. L. & Ritchie, C. W. A brain boost to fight Alzheimer’s disease. Science 361, 975–976 (2018).
Choi, S. H. et al. Combined adult neurogenesis and BDNF mimic exercise effects on cognition in an Alzheimer’s mouse model. Science 361, eaan8821 (2018).
Yiannopoulou, K. G., Anastasiou, A. I., Zachariou, V. & Pelidou, S.-H. Reasons for failed trials of disease-modifying treatments for Alzheimer disease and their contribution in recent research. Biomedicines 7, E97 (2019).
Colom-Cadena, M. et al. The clinical promise of biomarkers of synapse damage or loss in Alzheimer’s disease. Alzheimers Res. Ther. 12, 21 (2020).
Utz, J. et al. Cerebrospinal fluid of patients with Alzheimer’s disease contains increased percentages of synaptophysin-bearing microvesicles. Front. Aging Neurosci. 13, 683115 (2021).
Xiao, M.-F. et al. NPTX2 and cognitive dysfunction in Alzheimer’s disease. Elife 6, e23798 (2017).
Brinkmalm, G. et al. A parallel reaction monitoring mass spectrometric method for analysis of potential CSF biomarkers for Alzheimer’s disease. Proteom. Clin. Appl. 12, 1700131 (2018).
Kvartsberg, H. et al. The intact postsynaptic protein neurogranin is reduced in brain tissue from patients with familial and sporadic Alzheimer’s disease. Acta Neuropathol. 137, 89–102 (2019).
Portelius, E. et al. Cerebrospinal fluid neurogranin: relation to cognition and neurodegeneration in Alzheimer’s disease. Brain 138, 3373–3385 (2015).
Tarawneh, R. et al. Diagnostic and prognostic utility of the synaptic marker neurogranin in Alzheimer disease. JAMA Neurol. 73, 561–571 (2016).
Lleó, A. et al. Changes in synaptic proteins precede neurodegeneration markers in preclinical Alzheimer’s disease cerebrospinal fluid. Mol. Cell Proteom. 18, 546–560 (2019).
Suárez-Calvet, M. et al. sTREM2 cerebrospinal fluid levels are a potential biomarker for microglia activity in early-stage Alzheimer’s disease and associate with neuronal injury markers. EMBO Mol. Med. 8, 466–476 (2016).
Piccio, L. et al. Cerebrospinal fluid soluble TREM2 is higher in Alzheimer disease and associated with mutation status. Acta Neuropathol. 131, 925–933 (2016).
Heslegrave, A. et al. Increased cerebrospinal fluid soluble TREM2 concentration in Alzheimer’s disease. Mol. Neurodegener. 11, 3 (2016).
Fukuyama, R., Izumoto, T. & Fushiki, S. The cerebrospinal fluid level of glial fibrillary acidic protein is increased in cerebrospinal fluid from Alzheimer’s disease patients and correlates with severity of dementia. Eur. Neurol. 46, 35–38 (2001).
Benedet, A. L. et al. Differences between plasma and cerebrospinal fluid glial fibrillary acidic protein levels across the Alzheimer disease continuum. JAMA Neurol. 78, 1471–1483 (2021).
Teitsdottir, U. D. et al. Association of glial and neuronal degeneration markers with Alzheimer’s disease cerebrospinal fluid profile and cognitive functions. Alzheimers Res. Ther. 12, 92 (2020).
Olsson, B. et al. Microglial markers are elevated in the prodromal phase of Alzheimer’s disease and vascular dementia. J. Alzheimers Dis. 33, 45–53 (2013).
Craig-Schapiro, R. et al. YKL-40: a novel prognostic fluid biomarker for preclinical Alzheimer’s disease. Biol. Psychiatry 68, 903–912 (2010).
Corrêa, J. D., Starling, D., Teixeira, A. L., Caramelli, P. & Silva, T. A. Chemokines in CSF of Alzheimer’s disease patients. Arq. Neuropsiquiatr. 69, 455–459 (2011).
Mattsson, N. et al. Cerebrospinal fluid microglial markers in Alzheimer’s disease: elevated chitotriosidase activity but lack of diagnostic utility. Neuromol. Med. 13, 151–159 (2011).
Watabe-Rudolph, M. et al. Chitinase enzyme activity in CSF is a powerful biomarker of Alzheimer disease. Neurology 78, 569–577 (2012).
Humpel, C. & Hochstrasser, T. Cerebrospinal fluid and blood biomarkers in Alzheimer’s disease. World J. Psychiatry 1, 8–18 (2011).
Palmqvist, S. et al. Cerebrospinal fluid and plasma biomarker trajectories with increasing amyloid deposition in Alzheimer’s disease. EMBO Mol. Med. 11, e11170 (2019).
Stevenson, A. J. et al. Characterisation of an inflammation-related epigenetic score and its association with cognitive ability. Clin. Epigenetics 12, 113 (2020).
Finnema, S. J. et al. Imaging synaptic density in the living human brain. Sci. Transl Med. 8, 348ra96 (2016).
Mecca, A. P. et al. In vivo measurement of widespread synaptic loss in Alzheimer’s disease with SV2A PET. Alzheimers Dement. 16, 974–982 (2020).
Chen, M.-K. et al. Assessing synaptic density in Alzheimer disease with synaptic vesicle glycoprotein 2A positron emission tomographic imaging. JAMA Neurol. 75, 1215–1224 (2018).
Mecca, A. P. et al. Association of entorhinal cortical tau deposition and hippocampal synaptic density in older individuals with normal cognition and early Alzheimer’s disease. Neurobiol. Aging 111, 44–53 (2022).
Scheff, S. W., Price, D. A., Schmitt, F. A., DeKosky, S. T. & Mufson, E. J. Synaptic alterations in CA1 in mild Alzheimer disease and mild cognitive impairment. Neurology 68, 1501–1508 (2007).
Beach, T. G., Walker, R. & McGeer, E. G. Patterns of gliosis in Alzheimer’s disease and aging cerebrum. Glia 2, 420–436 (1989).
US National Library of Medicine. ClinicalTrials.gov https://clinicaltrials.gov/ct2/show/NCT02386306 (2016).
US National Library of Medicine. ClinicalTrials.gov https://clinicaltrials.gov/ct2/show/NCT04015076 (2020).
Hu, W. et al. Development of a novel therapeutic suppressor of brain proinflammatory cytokine up-regulation that attenuates synaptic dysfunction and behavioral deficits. Bioorg. Med. Chem. Lett. 17, 414–418 (2007).
US National Library of Medicine. ClinicalTrials.gov https://clinicaltrials.gov/ct2/show/NCT04120233 (2021).
US National Library of Medicine. ClinicalTrials.gov https://clinicaltrials.gov/ct2/show/NCT04008355 (2022).
Decourt, B. et al. MCLENA-1: a phase II clinical trial for the assessment of safety, tolerability, and efficacy of lenalidomide in patients with mild cognitive impairment due to Alzheimer’s disease. Open. Access. J. Clin. Trials 12, 1–13 (2020).
Marschallinger, J. et al. Structural and functional rejuvenation of the aged brain by an approved anti-asthmatic drug. Nat. Commun. 6, 8466 (2015).
Xiong, L. Y. et al. Leukotriene receptor antagonist use and cognitive decline in normal cognition, mild cognitive impairment, and Alzheimer’s dementia. Alzheimers Res. Ther. 13, 147 (2021).
US National Library of Medicine. ClinicalTrials.gov https://clinicaltrials.gov/ct2/show/NCT03991988 (2021).
Munoz, L. et al. A novel p38α MAPK inhibitor suppresses brain proinflammatory cytokine up-regulation and attenuates synaptic dysfunction and behavioral deficits in an Alzheimer’s disease mouse model. J. Neuroinflamm. 4, 21 (2007).
Scheltens, P. et al. An exploratory clinical study of p38α kinase inhibition in Alzheimer’s disease. Ann. Clin. Transl Neurol. 5, 464–473 (2018).
Prins, N. D. et al. A phase 2 double-blind placebo-controlled 24-week treatment clinical study of the p38 alpha kinase inhibitor neflamapimod in mild Alzheimer’s disease. Alzheimers Res. Ther. 13, 106 (2021).
Ricciarelli, R. & Fedele, E. Phosphodiesterase 4D: an enzyme to remember. Br. J. Pharmacol. 172, 4785–4789 (2015).
Cui, S.-Y. et al. Protection from amyloid β peptide-induced memory, biochemical, and morphological deficits by a phosphodiesterase-4D allosteric inhibitor. J. Pharmacol. Exp. Ther. 371, 250–259 (2019).
Brazier, D., Perry, R., Keane, J., Barrett, K. & Elmaleh, D. R. Pharmacokinetics of cromolyn and ibuprofen in healthy elderly volunteers. Clin. Drug Investig. 37, 1025–1034 (2017).
Xiao, S. et al. A 36-week multicenter, randomized, double-blind, placebo-controlled, parallel-group, phase 3 clinical trial of sodium oligomannate for mild-to-moderate Alzheimer’s dementia. Alzheimers Res. Ther. 13, 62 (2021).
Wang, T. et al. A phase II randomized trial of sodium oligomannate in Alzheimer’s dementia. Alzheimers Res. Ther. 12, 110 (2020).
US National Library of Medicine. ClinicalTrials.gov https://clinicaltrials.gov/ct2/show/NCT02097056 (2016).
US National Library of Medicine. ClinicalTrials.gov https://clinicaltrials.gov/ct2/show/NCT01054976 (2012).
US National Library of Medicine. ClinicalTrials.gov https://clinicaltrials.gov/ct2/show/NCT00622713 (2011).
Davis, K. L. et al. A double-blind, placebo-controlled multicenter study of tacrine for Alzheimer’s disease. N. Engl. J. Med. 327, 1253–1259 (1992).
US National Library of Medicine. ClinicalTrials.gov https://clinicaltrials.gov/ct2/show/NCT00469456 (2009).
US National Library of Medicine. ClinicalTrials.gov https://clinicaltrials.gov/ct2/show/NCT02750306 (2019).
US National Library of Medicine. ClinicalTrials.gov https://clinicaltrials.gov/ct2/show/NCT03822208 (2021).
Tao, C.-C. et al. Galectin-3 promotes Aβ oligomerization and Aβ toxicity in a mouse model of Alzheimer’s disease. Cell Death Differ. 27, 192–209 (2020).
Boza-Serrano, A. et al. Galectin-3, a novel endogenous TREM2 ligand, detrimentally regulates inflammatory response in Alzheimer’s disease. Acta Neuropathol. 138, 251–273 (2019).
US National Library of Medicine. ClinicalTrials.gov https://clinicaltrials.gov/ct2/show/NCT05074498 (2022).
Smith, E. S. et al. SEMA4D compromises blood-brain barrier, activates microglia, and inhibits remyelination in neurodegenerative disease. Neurobiol. Dis. 73, 254–268 (2015).
US National Library of Medicine. ClinicalTrials.gov https://clinicaltrials.gov/ct2/show/NCT04381468 (2022).
US National Library of Medicine. ClinicalTrials.gov https://clinicaltrials.gov/ct2/show/NCT04592874 (2022).
Butchart, J. et al. Etanercept in Alzheimer disease: a randomized, placebo-controlled, double-blind, phase 2 trial. Neurology 84, 2161–2168 (2015).
US National Library of Medicine. ClinicalTrials.gov https://clinicaltrials.gov/ct2/show/NCT00818662 (2021).
Macpherson, L. J. et al. Dynamic labelling of neural connections in multiple colours by trans-synaptic fluorescence complementation. Nat. Commun. 6, 10024 (2015).
Sama, D. M. et al. Inhibition of soluble tumor necrosis factor ameliorates synaptic alterations and Ca2+ dysregulation in aged rats. PLoS ONE 7, e38170 (2012).
US National Library of Medicine. ClinicalTrials.gov https://clinicaltrials.gov/ct2/show/NCT03943264 (2022).
Kiyota, T. et al. Granulocyte-macrophage colony-stimulating factor neuroprotective activities in Alzheimer’s disease mice. J. Neuroimmunol. 319, 80–92 (2018).
US National Library of Medicine ClinicalTrials.gov https://clinicaltrials.gov/ct2/show/NCT04902703 (2022).
Hongpaisan, J., Sun, M.-K. & Alkon, D. L. PKC ε activation prevents synaptic loss, Aβ elevation, and cognitive deficits in Alzheimer’s disease transgenic mice. J. Neurosci. 31, 630–643 (2011).
McClean, P. L., Parthsarathy, V., Faivre, E. & Hölscher, C. The diabetes drug liraglutide prevents degenerative processes in a mouse model of Alzheimer’s disease. J. Neurosci. 31, 6587–6594 (2011).
Robbins, M., Clayton, E. & Kaminski Schierle, G. S. Synaptic tau: a pathological or physiological phenomenon? Acta Neuropathol. Commun. 9, 149 (2021).
Carlyle, B. C. et al. cAMP-PKA phosphorylation of tau confers risk for degeneration in aging association cortex. Proc. Natl Acad. Sci. USA 111, 5036–5041 (2014).
Li, C. & Götz, J. Somatodendritic accumulation of Tau in Alzheimer’s disease is promoted by Fyn‐mediated local protein translation. EMBO J. 36, 3120–3138 (2017).
Alonso, A. et al. Hyperphosphorylation induces self-assembly of τ into tangles of paired helical filaments/straight filaments. Proc. Natl Acad. Sci. USA 98, 6923–6928 (2001).
Ballatore, C., Lee, V. M.-Y. & Trojanowski, J. Q. Tau-mediated neurodegeneration in Alzheimer’s disease and related disorders. Nat. Rev. Neurosci. 8, 663–672 (2007).
Rocher, A. B. et al. Structural and functional changes in tau mutant mice neurons are not linked to the presence of NFTs. Exp. Neurol. 223, 385–393 (2010).
Crimins, J. L., Rocher, A. B. & Luebke, J. I. Electrophysiological changes precede morphological changes to frontal cortical pyramidal neurons in the rTg4510 mouse model of progressive tauopathy. Acta Neuropathol. 124, 777–795 (2012).
Yoshiyama, Y. et al. Synapse loss and microglial activation precede tangles in a P301S tauopathy mouse model. Neuron 53, 337–351 (2007).
Ghag, G. et al. Soluble tau aggregates, not large fibrils, are the toxic species that display seeding and cross‐seeding behavior. Protein Sci. 27, 1901–1909 (2018).
Jiang, L., Zhao, J., Cheng, J.-X. & Wolozin, B. Tau oligomers and fibrils exhibit differential patterns of seeding and association with RNA binding proteins. Front. Neurol. 11, 579434 (2020).
Menkes-Caspi, N. et al. Pathological tau disrupts ongoing network activity. Neuron 85, 959–966 (2015).
Meyer-Luehmann, M. et al. Exogenous induction of cerebral β-amyloidogenesis is governed by agent and host. Science 313, 1781–1784 (2006).
Clavaguera, F. et al. Transmission and spreading of tauopathy in transgenic mouse brain. Nat. Cell Biol. 11, 909–913 (2009).
Falcon, B. et al. Conformation determines the seeding potencies of native and recombinant tau aggregates. J. Biol. Chem. 290, 1049–1065 (2015).
Furman, J. L. et al. Widespread tau seeding activity at early Braak stages. Acta Neuropathol. 133, 91–100 (2017).
Mirbaha, H., Holmes, B. B., Sanders, D. W., Bieschke, J. & Diamond, M. I. Tau trimers are the minimal propagation unit spontaneously internalized to seed intracellular aggregation. J. Biol. Chem. 290, 14893–14903 (2015).
DeVos, S. L. et al. Synaptic tau seeding precedes tau pathology in human Alzheimer’s disease brain. Front. Neurosci. 12, 267 (2018).
d’Errico, P. et al. Microglia contribute to the propagation of Aβ into unaffected brain tissue. Nat. Neurosci. 25, 20–25 (2022).
Hopp, S. C. et al. The role of microglia in processing and spreading of bioactive tau seeds in Alzheimer’s disease. J. Neuroinflamm. 15, 269 (2018).
Asai, H. et al. Depletion of microglia and inhibition of exosome synthesis halt tau propagation. Nat. Neurosci. 18, 1584–1593 (2015).
Clayton, K. et al. Plaque associated microglia hyper-secrete extracellular vesicles and accelerate tau propagation in a humanized APP mouse model. Mol. Neurodegener. 16, 18 (2021).
Busche, M. A. & Hyman, B. T. Synergy between amyloid-β and tau in Alzheimer’s disease. Nat. Neurosci. 23, 1183–1193 (2020).
Pooler, A. M. et al. Amyloid accelerates tau propagation and toxicity in a model of early Alzheimer’s disease. Acta Neuropathol. Commun. 3, 14 (2015).
Adams, J. N., Maass, A., Harrison, T. M., Baker, S. L. & Jagust, W. J. Cortical tau deposition follows patterns of entorhinal functional connectivity in aging. eLife 8, e49132 (2019).
Wan, Y.-W. et al. Meta-analysis of the Alzheimer’s disease human brain transcriptome and functional dissection in mouse models. Cell Rep. 32, 107908 (2020).
Chen, W.-T. et al. Spatial transcriptomics and in situ sequencing to study Alzheimer’s disease. Cell 182, 976–991 (2020).
Zhou, Y. et al. Human and mouse single-nucleus transcriptomics reveal TREM2-dependent and TREM2-independent cellular responses in Alzheimer’s disease. Nat. Med. 26, 131–142 (2020).
Mehta, D., Jackson, R., Paul, G., Shi, J. & Sabbagh, M. Why do trials for Alzheimer’s disease drugs keep failing? A discontinued drug perspective for 2010–2015. Expert. Opin. Investig. Drugs 26, 735–739 (2017).
Makin, S. The amyloid hypothesis on trial. Nature 559, S4–S7 (2018).
Lazic, S. E. The problem of pseudoreplication in neuroscientific studies: is it affecting your analysis? BMC Neurosci. 11, 5 (2010).
Brown, A. W., Kaiser, K. A. & Allison, D. B. Issues with data and analyses: errors, underlying themes, and potential solutions. Proc. Natl Acad. Sci. USA 115, 2563–2570 (2018).
du Sert, N. P. et al. Reporting animal research: explanation and elaboration for the ARRIVE guidelines 2.0. PLoS Biol. 18, e3000411 (2020).
Deaton, A. & Cartwright, N. Understanding and misunderstanding randomized controlled trials. Soc. Sci. Med. 210, 2–21 (2018).
Author information
Authors and Affiliations
Contributions
The authors contributed equally to all aspects of the article.
Corresponding author
Ethics declarations
Competing interests
T.L.S.-J. is on the Scientific Advisory Board of Cognition Therapeutics and receives collaborative grant funding from two industry partners. None of these had any influence over the current paper. The other authors declare no competing interests.
Peer review
Peer review information
Nature Reviews Neurology thanks Monica di Luca, Erik Roberson and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Related links
AD-SOLES: https://github.com/kaitlynhair/AD-SOLES
British Neuroscience Association: https://www.bnacredibility.org.uk
Glossary
- APP/PS1 transgenic mice
-
Mice that express the human amyloid precursor protein (APP) gene with the Swedish mutation and presenilin gene with exon 9 deletion; both are genetic causes of early-onset AD.
- BV2 microglia
-
A mouse immortalized cell line developed to model microglia in vitro.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Tzioras, M., McGeachan, R.I., Durrant, C.S. et al. Synaptic degeneration in Alzheimer disease. Nat Rev Neurol 19, 19–38 (2023). https://doi.org/10.1038/s41582-022-00749-z
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41582-022-00749-z
This article is cited by
-
Profiling of long non-coding RNAs in hippocampal–entorhinal system subfields: impact of RN7SL1 on neuroimmune response modulation in Alzheimer’s disease
Journal of Neuroinflammation (2024)
-
Delivering synaptic protein mRNAs via extracellular vesicles ameliorates cognitive impairment in a mouse model of Alzheimer’s disease
BMC Medicine (2024)
-
Protein translation: biological processes and therapeutic strategies for human diseases
Signal Transduction and Targeted Therapy (2024)
-
Bilirubin impairs neuritogenesis and synaptogenesis in NSPCs by downregulating NMDAR-CREB-BDNF signaling
In Vitro Cellular & Developmental Biology - Animal (2024)
-
Vivaria housing conditions expose sex differences in brain oxidation, microglial activation, and immune system states in aged hAPOE4 mice
Experimental Brain Research (2024)