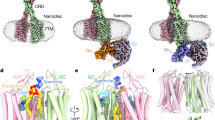
Abstract
The calcium-sensing receptor (CaSR), a cell-surface sensor for Ca2+, is the master regulator of calcium homeostasis in humans and is the target of calcimimetic drugs for the treatment of parathyroid disorders1. CaSR is a family C G-protein-coupled receptor2 that functions as an obligate homodimer, with each protomer composed of a Ca2+-binding extracellular domain and a seven-transmembrane-helix domain (7TM) that activates heterotrimeric G proteins. Here we present cryo-electron microscopy structures of near-full-length human CaSR in inactive or active states bound to Ca2+ and various calcilytic or calcimimetic drug molecules. We show that, upon activation, the CaSR homodimer adopts an asymmetric 7TM configuration that primes one protomer for G-protein coupling. This asymmetry is stabilized by 7TM-targeting calcimimetic drugs adopting distinctly different poses in the two protomers, whereas the binding of a calcilytic drug locks CaSR 7TMs in an inactive symmetric configuration. These results provide a detailed structural framework for CaSR activation and the rational design of therapeutics targeting this receptor.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
Data availability
All data generated or analysed during this study are included in the Article and its Supplementary Information. Cryo-EM maps of active-state CaSR–cinacalcet, active-state CaSR–etelcalcetide–evocalcet, inactive-state CaSR–NPS2143 and CaSR–NPS2143–Ca2+–Trp have been deposited in the Electron Microscopy Data Bank under accession codes EMD-23653, EMD-23654, EMD-23655 and EMD-23652, respectively. The atomic coordinates of active-state CaSR–cinacalcet, active-state CaSR–etelcalcetide–evocalcet, inactive-state CaSR–NPS2143 and CaSR–NPS2143–Ca2+–Trp have been deposited in the Protein Data Bank under the accession codes 7M3F, 7M3G, 7M3J and 7M3E, respectively.
References
Hannan, F. M., Kallay, E., Chang, W., Brandi, M. L. & Thakker, R. V. The calcium-sensing receptor in physiology and in calcitropic and noncalcitropic diseases. Nat. Rev. Endocrinol. 15, 33–51 (2018).
Møller, T. C., Moreno-Delgado, D., Pin, J.-P. & Kniazeff, J. Class C G protein-coupled receptors: reviving old couples with new partners. Biophys. Rep. 3, 57–63 (2017).
Conigrave, A. D., Quinn, S. J. & Brown, E. M. l-amino acid sensing by the extracellular Ca2+-sensing receptor. Proc. Natl Acad. Sci. USA 97, 4814–4819 (2000).
Riccardi, D. & Martin, D. The role of the calcium-sensing receptor in the pathophysiology of secondary hyperparathyroidism. NDT Plus 1 (Suppl 1), i7–i11 (2008).
Nemeth, E. F., Van Wagenen, B. C. & Balandrin, M. F. in Progress in Medicinal Chemistry Vol. 57 (eds Witty, D. R. & Cox, B.) 1–86 (Elsevier, 2018).
Geng, Y. et al. Structural mechanism of ligand activation in human calcium-sensing receptor. eLife 5, e13662 (2016).
Zhang, C. et al. Structural basis for regulation of human calcium-sensing receptor by magnesium ions and an unexpected tryptophan derivative co-agonist. Sci. Adv. 2, e1600241 (2016).
Koehl, A. et al. Structural insights into metabotropic glutamate receptor activation. Nature 566, 79–84 (2019).
Fantini, J. & Barrantes, F. J. How cholesterol interacts with membrane proteins: an exploration of cholesterol-binding sites including CRAC, CARC, and tilted domains. Front. Physiol. 4, 31 (2013).
Isberg, V. et al. Generic GPCR residue numbers—aligning topology maps while minding the gaps. Trends Pharmacol. Sci. 36, 22–31 (2015).
Kifor, O., Diaz, R., Butters, R., Kifor, I. & Brown, E. M. The calcium-sensing receptor is localized in caveolin-rich plasma membrane domains of bovine parathyroid cells. J. Biol. Chem. 273, 21708–21713 (1998).
Timmers, H. J. L. M., Karperien, M., Hamdy, N. A., de Boer, H. & Hermus, A. R. M. M. Normalization of serum calcium by cinacalcet in a patient with hypercalcaemia due to a de novo inactivating mutation of the calcium-sensing receptor. J. Intern. Med. 260, 177–182 (2006).
Kunishima, N. et al. Structural basis of glutamate recognition by a dimeric metabotropic glutamate receptor. Nature 407, 971–977 (2000).
Liu, H. et al. Illuminating the allosteric modulation of the calcium-sensing receptor. Proc. Natl Acad. Sci. USA 117, 21711–21722 (2020).
Bushinsky, D. A. et al. One-year safety and efficacy of intravenous etelcalcetide in patients on hemodialysis with secondary hyperparathyroidism. Nephrol. Dial. Transplant. 35, 1769–1778 (2020).
Alexander, S. T. et al. Critical cysteine residues in both the calcium-sensing receptor and the allosteric activator AMG 416 underlie the mechanism of action. Mol. Pharmacol. 88, 853–865 (2015).
Hannan, F. M. et al. Identification of 70 calcium-sensing receptor mutations in hyper- and hypo-calcaemic patients: evidence for clustering of extracellular domain mutations at calcium-binding sites. Hum. Mol. Genet. 21, 2768–2778 (2012).
Robertson, M. J., van Zundert, G. C. P., Borrelli, K. & Skiniotis, G. GemSpot: a pipeline for robust modeling of ligands into cryo-EM maps. Structure 28, 707–716.e3 (2020).
Leach, K. et al. Towards a structural understanding of allosteric drugs at the human calcium-sensing receptor. Cell Res. 26, 574–592 (2016).
Hlavackova, V. et al. Evidence for a single heptahelical domain being turned on upon activation of a dimeric GPCR. EMBO J. 24, 499–509 (2005).
Jacobsen, S. E., Gether, U. & Bräuner-Osborne, H. Investigating the molecular mechanism of positive and negative allosteric modulators in the calcium-sensing receptor dimer. Sci. Rep. 7, 46355 (2017).
Seven, A. B. et al. G protein activation by a metabotropic glutamate receptor. Nature (in the press).
Huang, S. et al. Interdomain movements in metabotropic glutamate receptor activation. Proc. Natl Acad. Sci. USA 108, 15480–15485 (2011).
Ray, K., Fan, G.-F., Goldsmith, P. K. & Spiegel, A. M. The carboxyl terminus of the human calcium receptor. Requirements for cell-surface expression and signal transduction. J. Biol. Chem. 272, 31355–31361 (1997).
Hu, J. et al. A region in the seven-transmembrane domain of the human Ca2+ receptor critical for response to Ca2+. J. Biol. Chem. 280, 5113–5120 (2005).
Shiohara, M. et al. A novel gain-of-function mutation (F821L) in the transmembrane domain of calcium-sensing receptor is a cause of severe sporadic hypoparathyroidism. Eur. J. Pediatr. 163, 94–98 (2004).
Kobilka, B. K. G protein coupled receptor structure and activation. Biochim. Biophys. Acta 1768, 794–807 (2007).
Wu, H. et al. Structure of a class C GPCR metabotropic glutamate receptor 1 bound to an allosteric modulator. Science 344, 58–64 (2014).
Doré, A. S. et al. Structure of class C GPCR metabotropic glutamate receptor 5 transmembrane domain. Nature 511, 557–562 (2014).
Punjani, A., Rubinstein, J. L., Fleet, D. J. & Brubaker, M. A. cryoSPARC: algorithms for rapid unsupervised cryo-EM structure determination. Nat. Methods 14, 290–296 (2017).
Punjani, A., Zhang, H. & Fleet, D. J. Non-uniform refinement: adaptive regularization improves single-particle cryo-EM reconstruction. Nat. Methods 17, 1214–1221 (2020).
Punjani, A. & Fleet, D. J. 3D variability analysis: resolving continuous flexibility and discrete heterogeneity from single particle cryo-EM. J. Struct. Biol. 213, 107702 (2021).
Pettersen, E. F. et al. UCSF Chimera–a visualization system for exploratory research and analysis. J. Comput. Chem. 25, 1605–1612 (2004).
Goudet, C. et al. Asymmetric functioning of dimeric metabotropic glutamate receptors disclosed by positive allosteric modulators. J. Biol. Chem. 280, 24380–24385 (2005).
Waterhouse, A. et al. SWISS-MODEL: homology modelling of protein structures and complexes. Nucleic Acids Res. 46 (W1), W296–W303 (2018).
Adams, P. D. et al. PHENIX: a comprehensive Python-based system for macromolecular structure solution. Acta Crystallogr. D 66, 213–221 (2010).
Emsley, P. & Cowtan, K. Coot: model-building tools for molecular graphics. Acta Crystallogr. D 60, 2126–2132 (2004).
Chen, V. B. et al. MolProbity: all-atom structure validation for macromolecular crystallography. Acta Crystallogr. D 66, 12–21 (2010).
Barad, B. A. et al. EMRinger: side chain-directed model and map validation for 3D cryo-electron microscopy. Nat. Methods 12, 943–946 (2015).
Olsen, R. H. J. et al. TRUPATH, an open-source biosensor platform for interrogating the GPCR transducerome. Nat. Chem. Biol. 16, 841–849 (2020).
Bond, S. R. & Naus, C. C. RF-Cloning.org: an online tool for the design of restriction-free cloning projects. Nucleic Acids Res. 40, W209–W213 (2012).
Papasergi-Scott, M. M. et al. Structures of metabotropic GABAB receptor. Nature 584, 310–314 (2020).
Isberg, V. et al. GPCRdb: an information system for G protein-coupled receptors. Nucleic Acids Res. 44 (D1), D356–D364 (2016).
Crooks, G. E., Hon, G., Chandonia, J. M. & Brenner, S. E. WebLogo: a sequence logo generator. Genome Res. 14, 1188–1190 (2004).
Acknowledgements
We thank E. Montabana at the Stanford-SLAC cryo-EM facility for support with data collection and B. Kobilka for comments on the manuscript. This work was supported, in part, by R01 NS092695 (G.S. and J.M.M.) and a grant from the Mathers Foundation (G.S.); a Wellcome Trust Investigator Award (grant number 106995/Z/15/Z) (R.V.T); National Institute for Health Research (NIHR) Oxford Biomedical Research Centre Programme (R.V.T.); NIHR Senior Investigator Award (R.V.T.) (grant number NF-SI-0514–10091); T32-GM089626 (J.G.M.); and funding from the Faculty of Health and Medical Sciences (H.B.-O.).
Author information
Authors and Affiliations
Contributions
Y.G. expressed and purified the proteins, prepared cryo-EM samples, collected and processed cryo-EM data, built and refined the structural models, designed the mutagenesis studies and generated the expression constructs. M.J.R. performed ligand docking and assisted in model refinement. S.N.R. performed IP1 assays for profiling of allosteric modulators under the supervision of H.B.-O. A.B.S. assisted in data analysis. C.Z. assisted in cryo-EM data collection. J.G.M. performed the BRET assays and assisted in model refinement. O.P. assisted in cryo-EM data collection. F.M.H. and R.V.T. provided information on disease mutations and provided input in manuscript discussions. J.M.M. performed and analysed cellular signalling experiments. Y.G. and G.S. wrote the manuscript with input from J.G.M., R.V.T., F.M.H., H.B.-O, M.J.R. and J.M.M. G.S. supervised the project.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Peer review information Nature thanks Alexandru Aricescu and the other, anonymous, reviewer(s) for their contribution to the peer review of this work. Peer reviewer reports are available.
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Extended data figures and tables
Extended Data Fig. 1 Functional characterizations of human CaSR and structures of NAM-bound CaSR.
a, size-exclusion chromatography profile of purified CaSR. b, BRET-based assay40 monitoring Gq activation by CaSR upon Ca2+ addition. n = 3 independent experiments, data represent mean ± s.e.m. c-f, Functional responses of CaSR to Ca2+ alone or in combination with PAMs or NAM measured by IP1 accumulation assays. n = 3 independent experiments, data represent mean ± s.e.m. g, Cryo-EM map and model of inactive state CaSR complexed with NAM. h, Cryo-EM map and model of the CaSR-NAM-Ca2+-Trp complex. i, Structure of the inactive-state CaSR ECD region showing an open-closed inactive VFT configuration.
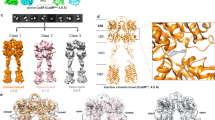
Extended Data Fig. 2 Cryo-EM data processing workflow.
Representative cryo-EM micrographs of different CaSR complexes and flowcharts detailing data processing procedures.
Extended Data Fig. 3 Cryo-EM maps and FSC curves of active-state CaSR complexes.
a–d, Global and local refinement maps with corresponding FSC curves indicating nominal resolutions using the FSC = 0.143 criterion, model-vs-map FSC curves and local resolution maps of CaSR-etelecacetide-evocalcet (a, b) and CaSR-cinacalcet (c, d).
Extended Data Fig. 4 Cryo-EM maps and FSC curves of NAM-bound CaSR complexes.
a–d, Global and local refinement maps with corresponding FSC curves indicating nominal resolutions using the FSC = 0.143 criterion, model-vs-map FSC curves and local resolution maps of inactive-state CaSR (a, b) and CaSR-NAM-Ca2+-Trp (c, d).
Extended Data Fig. 5 Comparisons between CaSR cryo-EM structures and previous family C GPCR structures.
a, ECD from inactive-state CaSR cryo-EM structure with the loop tethering the opposing LB1 colored in red. b, ECD from inactive-state mGlu5 cryo-EM structure8 (PDB: 6N51). c, inactive-state CaSR ECD crystal structure6 (PDE: 5K5T). d, 7TMs from inactive-state CaSR cryo-EM structure (grey) superposed onto inactive CaSR ECD crystal structure based on CRD alignment. e, Comparison of inactive 7TMs orientations between CaSR and mGlu5. f, Active-state CaSR cryo-EM structure aligned with the active CaSR ECD crystal structure6 (grey, PDB: 5K5S) illustrating the difference in CRD orientations. g, Elongated densities observed at the TM6-TM6 interface in cryo-EM maps of active-state CaSR shown with TM6 residues forming the cholesterol-binding CARC motif. h, Sequence alignment logo showing the conservation of CARC motif residues among family C GPCRs (generated using alignment from GPCRdb43 with WebLogo44). i, Comparison of Ca2+ sites in active-state cryo-EM structures with Ca2+ sites in active CaSR ECD crystal structure (PDB: 5K5S)6. j, Alignment of the closed VFT protomer (dark blue) observed in our inactive-state CaSR structure with either the inactive open protomer (left, dark green) or the active closed protomer (right, light green). k, The tube shaped density observed in closed inactive CaSR VFT with L-Trp docked in. l, Crystal structure of open-closed mGlu1 VFTs13 (PDB: 1EWV).
Extended Data Fig. 6 PAM and NAM models and TM6-TM6 interfaces in cryo-EM densities.
a, Etelcalcetide model in cryo-EM density. b, Active-state CaSR TM6-TM6 interface model in cryo-EM density (shown here is CaSR-cinacalcet. The TM6-TM6 interface of CaSR-evocalcet-etelcalcetide is highly similar). c, Cinacalcet models in cryo-EM densities. d, Evocalcet models in cryo-EM densities. e, NAM model in cryo-EM density for the CaSR-NAM-Ca2+-Trp complex. f, CaSR-NAM-Ca2+-Trp complex TM6-TM6 interface model in cryo-EM density. g, Inactive-state NAM binding pocket. h, Inactive-state NAM model in cryo-EM density.
Extended Data Fig. 7 Binding modes of cinacalcet and evocalcet.
a, Interaction network in the CRD-ECL2-ECL3 region of cinacalcet-bound active-state CaSR. b, Chemical structures of evocalcet and cinacalcet. c, Structure of active-state CaSR 7TMs complexed with evocalcet. Similar to cinacalcet (Fig. 3a), evocalcet adopts an extended conformation in 7TMA (green) and a bent conformation in 7TMB (blue), making distinct interactions with the two protomers.
Extended Data Fig. 8 Cell surface expression levels and calcium response curves of various CaSR constructs.
a, Ca2+ response curves (left and middle panels) of the wild-type or Q6813.33A mutant CaSR expressed at similar levels (plasma membrane expression levels shown on the right panel) in HEK293 cells in the absence or presence of the PAM cinacalcet or NAM NPS-2143 as monitored by IP1 accumulation assays. Data represent mean ± s.e.m. from six independent experiments each performed in duplicate. b, Top three panels depict results from IP1 accumulation assays monitoring the Ca2+ responses of the CaSR heterodimer without or with the PAM-binding deficient mutant E837A in both protomers. Middle and bottom left panels show results from cell surface ELISA assays verifying the different C1 and C2 construct combinations yield similar expression levels of CaSR heterodimer at the plasma membrane. Bottom right panel shows the transfection with only one protomer containing either a C1 or C2 tail results in no IP1 accumulation signals in response to stimulation with Ca2+. Functional IP1 data represent mean ± s.e.m. from 5 independent experiments (top row) or 3 independent experiments (3rd row) each performed in duplicate, whereas cell surface ELISA data represent mean ± s.e.m. from 3 independent experiments performed in triplicate. c, Structural illustrations of how the introduced mutations would occlude extended or bent PAM conformations.
Extended Data Fig. 9 Ordered C terminus in CaSR and structural comparison between CaSR and mGlu2.
a, The ordered C terminus from the 7TM with a bent PAM (7TMB) shown in cryo-EM densities with unsharpened maps. b, Asymmetric 7TMs in the active-state mGlu2 alone structure (accompanying manuscript). TM6 are shown as solid cartoon with representative residues shown as sticks to highlight the asymmetry. c, Superposition of the G protein-coupling 7TM (7TMGC) from mGlu2-Gi complex structure (accompanying manuscript) onto the active-state CaSR 7TM with a straight PAM (7TMA) showing that the G protein would fit well on the membrane plane and the tilt of CaSR 7TMB leads to the sequestration of its C terminus in the membrane. The comparison between mGlu 7TMGC and CaSR 7TMA illustrates that the receptor likely would couple to G proteins through downward extensions of both ICL2 and C terminus.
Extended Data Fig. 10 Schematic of CaSR activation mechanism.
In the inactive state, CaSR is relatively flexible and the 7TMs are separated facing each other at the TM5-TM6 plane. The VFTs adopt inactive open-open/closed conformations. The open-closed conformation can be stabilized by aromatic amino acids (AAs) or their derivatives, thus priming the receptor for activation. NAM binds at both 7TMs with the same conformation and locks the TM6 toggle switch in an inactive conformation. Under high Ca2+ and high Trp conditions, the ECD adopts a closed-closed active conformation, while the presence of the NAM prevents the 7TMs from adopting the active asymmetric configuration. Upon activation by high Ca2+ concentration, the VFTs adopt an active closed-closed conformation, which is stabilized by L-Trp bound at the cleft of each VFT and the ECD PAM etelcalcetide further stabilizes the interface between LB2 of the closed-closed VFTs. Closure of the VFTs leads to rearrangement of the CRDs, bringing the 7TMs together to form an asymmetric TM6-TM6 interface. The asymmetric configuration is stabilized by 7TM PAMs adopting distinct poses. The 7TM with a bent PAM is more tilted than the opposing 7TM with its C terminus sequestered in the membrane, and likely unable to couple to G protein.
Supplementary information
Video 1
: Inactive-state CaSR 3D variability analysis component 1. Particles of inactive-state CaSR were subjected to 3D variability analysis. This video illustrates the motions corresponding to the first principal component revealed by the analysis.
Video 2
: Inactive-state CaSR 3D variability analysis component 2. Particles of inactive-state CaSR were subjected to 3D variability analysis. This video illustrates the motions corresponding to the second principal component revealed by the analysis.
Video 3
: Inactive-state CaSR 3D variability analysis component 3. Particles of inactive-state CaSR were subjected to 3D variability analysis. This video illustrates the motions corresponding to the third principal component revealed by the analysis.
Video 4
: Active-state CaSR 3D variability analysis component 1. Particles of active-state CaSR complexed with etelcalcetide and evocalcet were subjected to 3D variability analysis. This video illustrates the motions corresponding to the first principal component revealed by the analysis.
Video 5
: Active-state CaSR 3D variability analysis component 2. Particles of active-state CaSR complexed with etelcalcetide and evocalcet were subjected to 3D variability analysis. This video illustrates the motions corresponding to the second principal component revealed by the analysis.
Video 6
: Active-state CaSR 3D variability analysis component 3. Particles of active-state CaSR complexed with etelcalcetide and evocalcet were subjected to 3D variability analysis. This video illustrates the motions corresponding to the third principal component revealed by the analysis.
Rights and permissions
About this article
Cite this article
Gao, Y., Robertson, M.J., Rahman, S.N. et al. Asymmetric activation of the calcium-sensing receptor homodimer. Nature 595, 455–459 (2021). https://doi.org/10.1038/s41586-021-03691-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41586-021-03691-0
This article is cited by
-
G protein-coupled receptors (GPCRs): advances in structures, mechanisms, and drug discovery
Signal Transduction and Targeted Therapy (2024)
-
Constitutive activation mechanism of a class C GPCR
Nature Structural & Molecular Biology (2024)
-
Allosteric modulation and G-protein selectivity of the Ca2+-sensing receptor
Nature (2024)
-
Promiscuous G-protein activation by the calcium-sensing receptor
Nature (2024)
-
Recent advances in chemical protein synthesis: method developments and biological applications
Science China Chemistry (2024)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.