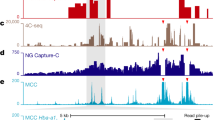
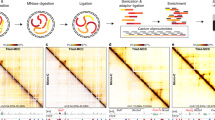
Abstract
Although enhancers are central regulators of mammalian gene expression, the mechanisms underlying enhancer–promoter (E-P) interactions remain unclear. Chromosome conformation capture (3C) methods effectively capture large-scale three-dimensional (3D) genome structure but struggle to achieve the depth necessary to resolve fine-scale E-P interactions. Here, we develop Region Capture Micro-C (RCMC) by combining micrococcal nuclease (MNase)-based 3C with a tiling region-capture approach and generate the deepest 3D genome maps reported with only modest sequencing. By applying RCMC in mouse embryonic stem cells and reaching the genome-wide equivalent of ~317 billion unique contacts, RCMC reveals previously unresolvable patterns of highly nested and focal 3D interactions, which we term microcompartments. Microcompartments frequently connect enhancers and promoters, and although loss of loop extrusion and inhibition of transcription disrupts some microcompartments, most are largely unaffected. We therefore propose that many E-P interactions form through a compartmentalization mechanism, which may partially explain why acute cohesin depletion only modestly affects global gene expression.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
Data availability
The data generated in this study can be found at NCBI Gene Expression Omnibus under accession number GSE207225. Source data are provided with this paper.
Code availability
All custom code and scripts used for data analyses in this paper are available on GitHub at https://github.com/ahansenlab/RCMC_analysis_code and on Zenodo at https://zenodo.org/record/7641852. Source data are provided with this paper.
References
Dekker, J. & Mirny, L. The 3D genome as moderator of chromosomal communication. Cell 164, 1110–1121 (2016).
Oudelaar, A. M. et al. The relationship between genome structure and function. Nat. Rev. Genet. 22, 154–168 (2021).
Lieberman-Aiden, E. et al. Comprehensive mapping of long-range interactions reveals folding principles of the human genome. Science. 326, 289–293 (2009).
Nuebler, J. et al. Chromatin organization by an interplay of loop extrusion and compartmental segregation. Proc. Natl Acad. Sci. USA 115, E6697–E6706 (2018).
Dixon, J. R. et al. Topological domains in mammalian genomes identified by analysis of chromatin interactions. Nature 485, 376–380 (2012).
Nora, E. P. et al. Spatial partitioning of the regulatory landscape of the X-inactivation centre. Nature 485, 381–385 (2012).
Rao, S. S. P. et al. A 3D map of the human genome at kilobase resolution reveals principles of chromatin looping. Cell 159, 1665–1680 (2014).
Goel, V. Y. et al. The macro and micro of chromosome conformation capture. WIREs Dev. Biol. 10, e395 (2020).
Sanborn, A. L. et al. Chromatin extrusion explains key features of loop and domain formation in wild-type and engineered genomes. Proc. Natl Acad. Sci. USA 112, 6456–6465 (2015).
Fudenberg, G. et al. Formation of chromosomal domains by loop extrusion. Cell Rep. 15, 2038–2049 (2016).
Krietenstein, N. et al. Ultrastructural details of mammalian chromosome architecture. Mol. Cell 78, 554–565 (2020).
Hsieh, T.-H. S. et al. Resolving the 3D landscape of transcription-linked mammalian chromatin folding. Mol. Cell 78, 539–553 (2020).
Hsieh, T.-H. S. et al. Enhancer–promoter interactions and transcription are largely maintained upon acute loss of CTCF, cohesin, WAPL or YY1. Nat. Genet. 54, 1919–1932 (2022).
Harris, H. et al. Chromatin alternates between A and B compartments at kilobase scale for subgenic organization. Nat. Commun. (in the press).
Hansen, A. S. et al. Distinct classes of chromatin loops revealed by deletion of an RNA-binding region in CTCF. Mol. Cell 76, 395–411 (2019).
Hua, P. et al. Defining genome architecture at base-pair resolution. Nature 595, 125–129 (2021).
Aljahani, A. et al. Analysis of sub-kilobase chromatin topology reveals nano-scale regulatory interactions with variable dependence on cohesin and CTCF. Nat. Commun. 13, 2139 (2022).
Gasperini, M. et al. A Genome-wide framework for mapping gene regulation via cellular genetic screens. Cell 176, 377–390 (2019).
Barshad, G. et al. RNA polymerase II and PARP1 shape enhancer-promoter contacts. Preprint at https://www.biorxiv.org/content/10.1101/2022.07.07.499190v1 (2022).
Zhang, S. et al. Enhancer–promoter contact formation requires RNAPII and antagonizes loop extrusion. Nat. Genet. https://doi.org/10.1038/s41588-023-01364-4 (2023).
Schwarzer, W. et al. Two independent modes of chromatin organization revealed by cohesin removal. Nature 551, 51–56 (2017).
Nora, E. P. et al. Targeted degradation of CTCF decouples local insulation of chromosome domains from genomic compartmentalization. Cell 169, 930–944 (2017).
Gassler, J. et al. A mechanism of cohesin-dependent loop extrusion organizes zygotic genome architecture. EMBO J. 36, 3600–3618 (2017).
Wutz, G. et al. Topologically associating domains and chromatin loops depend on cohesin and are regulated by CTCF, WAPL, and PDS5 proteins. EMBO J. 36, 3573–3599 (2017).
Rao, S. S. P. et al. Cohesin loss eliminates all loop domains. Cell 171, 305–320 (2017).
Haarhuis, J. H. I. et al. The cohesin release factor WAPL restricts chromatin loop extension. Cell 169, 693–707 (2017).
El Khattabi, L. et al. A pliable mediator acts as a functional rather than an architectural bridge between promoters and enhancers. Cell 178, 1145–1158 (2019).
Oudelaar, A. M. et al. Dynamics of the 4D genome during in vivo lineage specification and differentiation. Nat. Commun. 11, 2722 (2020).
Jäger, R. et al. Capture Hi-C identifies the chromatin interactome of colorectal cancer risk loci. Nat. Commun. 6, 6178 (2015).
Imakaev, M. et al. Iterative correction of Hi-C data reveals hallmarks of chromosome organization. Nat. Methods 9, 999–1003 (2012).
Bonev, B. et al. Multiscale 3D genome rewiring during mouse neural development. Cell 171, 557–572 (2017).
Zhou, H. Y. et al. A Sox2 distal enhancer cluster regulates embryonic stem cell differentiation potential. Genes Dev. 28, 2699–2711 (2014).
Li, Y. et al. CRISPR reveals a distal super-enhancer required for Sox2 expression in mouse embryonic stem cells. PLoS One 9, e114485 (2014).
Chakraborty, S. et al. Enhancer–promoter interactions can bypass CTCF-mediated boundaries and contribute to phenotypic robustness. Nat. Genet. 55, 280–290 (2023).
Akgol Oksuz, B. et al. Systematic evaluation of chromosome conformation capture assays. Nat. Methods 18, 1046–1055 (2021).
Abdennur, N. et al. Cooltools: enabling high-resolution Hi-C analysis in Python. Preprint at https://www.biorxiv.org/content/10.1101/2022.10.31.514564v1 (2022).
Roayaei Ardakany, A. et al. Mustache: multi-scale detection of chromatin loops from Hi-C and Micro-C maps using scale-space representation. Genome Biol. 21, 256 (2020).
Gabriele, M. et al. Dynamics of CTCF- and cohesin-mediated chromatin looping revealed by live-cell imaging. Science. 376, 496–501 (2022).
Wang, Z. et al. Prediction of histone post-translational modification patterns based on nascent transcription data. Nat. Genet. 54, 295–305 (2022).
Rosencrance, C. D. et al. Chromatin hyperacetylation impacts chromosome folding by forming a nuclear subcompartment. Mol. Cell 78, 112–126 (2020).
You, Q. et al. Direct DNA crosslinking with CAP-C uncovers transcription-dependent chromatin organization at high resolution. Nat. Biotechnol. 39, 225–235 (2021).
Rippe, K. et al. Functional organization of RNA polymerase II in nuclear subcompartments. Curr. Opin. Cell Biol. 74, 88–96 (2022).
Leibler, L. Theory of microphase separation in block copolymers. Macromolecules 13, 1602–1617 (1980).
Meier, D. J. Theory of block copolymers. I. Domain formation in A-B block copolymers. J. Polym. Sci. Part C. Polym. Symp. 26, 81–98 (1969).
Fujishiro, S. et al. Generation of dynamic three-dimensional genome structure through phase separation of chromatin. Proc. Natl Acad. Sci. USA 119, e2109838119 (2022).
Thiecke, M. J. et al. Cohesin-dependent and -independent mechanisms mediate chromosomal contacts between promoters and enhancers. Cell Rep. 32, 107929 (2020).
Kane, L. et al. Cohesin is required for long-range enhancer action at the Shh locus. Nat. Struct. Mol. Biol. 29, 891–897 (2022).
Calderon, L. et al. Cohesin-dependence of neuronal gene expression relates to chromatin loop length. Elife 11, e76539 (2022).
Rinzema, N. J. et al. Building regulatory landscapes reveals that an enhancer can recruit cohesin to create contact domains, engage CTCF sites and activate distant genes. Nat. Struct. Mol. Biol. 29, 563–574 (2022).
Cuartero, S. et al. Control of inducible gene expression links cohesin to hematopoietic progenitor self-renewal and differentiation. Nat. Immunol. 19, 932–941 (2018).
Navarro Gonzalez, J. et al. The UCSC Genome Browser database: 2021 update. Nucleic Acids Res. 49, D1046–D1057 (2021).
Pettitt, S. J. et al. Agouti C57BL/6N embryonic stem cells for mouse genetic resources. Nat. Methods 6, 493–495 (2009).
Hansen, A. S. et al. CTCF and cohesin regulate chromatin loop stability with distinct dynamics. Elife 6, e25776 (2017).
Kerpedjiev, P. et al. HiGlass: web-based visual exploration and analysis of genome interaction maps. Genome Biol. 19, 125 (2018).
Xu, W. et al. CoolBox: a flexible toolkit for visual analysis of genomics data. BMC Bioinformatics 22, 489 (2021).
Zhao, H. et al. CrossMap: a versatile tool for coordinate conversion between genome assemblies. Bioinformatics 30, 1006–1007 (2014).
Robinson, J. T. et al. Integrative genomics viewer. Nat. Biotechnol. 29, 24–26 (2011).
Yang, T. et al. HiCRep: assessing the reproducibility of Hi-C data using a stratum-adjusted correlation coefficient. Genome Res. 27, 1939–1949 (2017).
Venev, S. et al. open2c/cooltools: v0.4.1 (v0.4.1). Zenodo https://doi.org/10.5281/zenodo.5214125 (2021).
Abdennur, N. et al. Cooler: scalable storage for Hi-C data and other genomically labeled arrays. Bioinformatics 36, 311–316 (2020).
Robinson, J. T. et al. Juicebox.js provides a cloud-based visualization system for Hi-C data. Cell Syst. 6, 256–258 (2018).
Acknowledgements
We thank T. H. S. Hsieh, the co-inventor of Micro-C, for holding a Micro-C workshop to teach us the protocol. We also thank T. H. S. Hsieh, C. Cattoglio and L. Mirny for extensive and insightful discussions throughout this project. We thank L. Mirny and G. Fudenberg for insightful discussions on ICE normalization. We thank E. Dimitrova and A. Hughes for their guidance on performing RNA Pol II ChIP-seq. We thank J. Dekker, S. Grosse-Holz, E. Navarette, S. Abraham, and the Hansen laboratory for helpful discussions throughout this project. We thank E. Nora, M. Huntley, A. Boettiger, T. H. S. Hsieh, D. Narducci, S. Nemsick, A. Jha, M. Gabriele, J. H. Yang, C. Eyler, M. Aryee, S. Johnstone, C. Cerda-Smith and V. Sankaran for critical feedback on the paper. We thank M. Oudelaar for providing TMCC numbers and feedback on the TMCC vs RCMC comparison and the paper. We thank J. Rowley for sharing the Hi-C data generated in Harris et al.14. We thank the MIT Koch Institute’s Robert A. Swanson (1969) Biotechnology Center for technical support, specifically the Integrated Genomics and Bioinformatics Core and MIT BioMicro Center, and this work was supported in part by the Koch Institute Support (core) Grant P30-CA14051 from the National Cancer Institute. We also thank the Walk-Up Sequencing services of the Broad Institute of MIT and Harvard. This work was supported by National Institutes of Health grants DP2GM140938 (A.S.H.), R33CA257878 (A.S.H.) and UM1HG011536 (A.S.H.), National Science Foundation grant 2036037 (A.S.H.), a Solomon Buchsbaum Research Support Committee award (A.S.H.) and the Koch Institute Frontier Research Fund (A.S.H.). V.Y.G. is supported by a graduate research fellowship from the Ludwig Center at MIT’s Koch Institute. M.K.H. is supported by a National Institutes of Health F32GM140548 fellowship and a nonstipendiary EMBO fellowship.
Author information
Authors and Affiliations
Contributions
V.Y.G. and A.S.H. designed the project. V.Y.G. developed and optimized the RCMC protocol with input from M.K.H. and A.S.H. V.Y.G. performed cell culture and RCMC, analyzed sequencing data, compared RCMC to previous studies, and performed loop and compartment calling, identification of individual microcompartments and validation of ICE balancing. M.K.H. performed and analyzed western blotting and Pol II ChIP-seq experiments and analyzed reproducibility of RCMC datasets and features of microcompartment interactions. V.Y.G. and M.K.H. performed pileup and microcompartment interaction strength analysis. A.S.H. supervised the project. All authors contributed to drafting and editing the paper and figures.
Corresponding author
Ethics declarations
Competing interests
V.Y.G., M.K.H. and A.S.H. are considering RCMC for a patent application.
Peer review
Peer review information
Nature Genetics thanks the anonymous reviewers for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Extended data
Extended Data Fig. 1 RCMC efficiently and reproducibly captures ligated dinucleosomal fragments, giving rise to deep contact maps.
(a) Representative MNase titration DNA gel indicating the ideal level of digestion by MNase, based on the ratio of fragment sizes, for the RCMC protocol. (b) Representative size-selection gel for the RCMC protocol showing the dinucleosomal band that is extracted to obtain ligated fragments. (c) Overview of the capture probe design workflow for RCMC. 80-mer probes tiling the region of interest are designed, removing those which overlap highly repetitive regions. (d) Summary of the capture efficiency for each of the five regions for which probes were designed. The locations and sizes of the regions, the number of ligated fragments which mapped at single loci at both ends in total and in the region, and the capture efficiencies are given. Because different capture probe sets were used for Biological Replicates 1 (two separate sets of capture probes) and 2 (simultaneous capture for all five loci), numbers are separately provided for each Biological Replicate. (e) Contact maps comparing raw, unbalanced data (upper panel, lower triangle), ICE-balanced30 to all aligned reads (lower panel, lower triangle) and ICE-balanced to reads in captured loci only (both panels, upper triangle). Balancing only to data entirely within captured loci was necessary to remove artifacts due to capture bias. (f) Contact maps comparing the entire Fbn2 TAD in RCMC and in Hi-C31 and Micro-C12. Gene annotations and ChIP-seq signal tracks are shown below the contact maps. (g) Measurement of reproducibility between WT replicates across all five capture loci, with reproducibility scores determined using HiCRep58 at 10 kb resolution, clustered according to similarity. Three technical RCMC replicates (denoted by ‘TR#’) comprise Biological Replicate 1, while ‘BR2’ denotes Biological Replicate 2. TR3_WT is noted in blue text at the Sox2 and Nanog loci because very little TR3_WT pre-Capture library remained for input to Sox2 & Nanog capture after the initial Ppm1g, Klf1 and Fbn2 capture experiment; accordingly, relative to all other replicates, TR3_WT has much lower sequencing depth (0.5–2.4% the number of unique contacts) at the Sox2 & Nanog loci.
Extended Data Fig. 2 Benchmarking of RCMC against other 3C methods.
(a) Contact probability curves comparing RCMC against the highest resolution Tiled-Micro-Capture-C (TMCC)17, Micro-C12, and Hi-C31 mESC datasets across contact distances. (b) Benchmarking comparison of RCMC’s ability to fill out high-resolution contact matrices against TMCC17, Micro-C12, and Hi-C31. Region-averaged calculations are shown for all methods, and calculations for individual captured regions are also shown for RCMC and TMCC. The x axis shows the contact distance in bp, and the y axis shows the fraction of all bins at a given contact distance within the captured locus that contain at least one read at 100 bp resolution. (c) Summary of read counts across RCMC, TMCC17, Micro-C12, and Hi-C31. The number of mapped sequencing reads, the fraction of unique reads, and the fraction of structurally informative (defined as cis contacts > =1 kb) unique reads are given for each method. Two versions of quantification are provided for TMCC. In black are numbers processed using the same bioinformatic pipeline as for RCMC. Capture region-specific quantifications (defined here as all reads with at least one of two read mates mapped to the locus) are also provided for all RCMC loci and the Sox2 and Nanog TMCC loci; the Oct4 and Prdm14 TMCC loci are not considered in this manuscript. In blue are numbers kindly provided by Dr. A Marieke Oudelaar, obtained using the custom TMCC-specific bioinformatic pipeline from Aljahani et al.17. Values with asterisks denote quantifications of all unique contact pairs mapped to captured loci (not filtered to be > = 1 kb in size). (d) Contact map comparisons of RCMC data generated in this manuscript, starting from the full dataset (topmost) and successively downsampled by orders of two down to 1/128th of the data (bottommost), shown for the Klf1 locus at 500 bp resolution. (e) As in (b), benchmarking comparison of successively downsampled RCMC’s ability to fill out high-resolution contact matrices against Micro-C12 at the Klf1 locus. (f) Contact map comparisons of 1/64th and 1/128th downsampled RCMC (left) against the highest-resolution available mESC Micro-C12 (right; Hsieh 2020) dataset, shown for the Klf1 locus at 500 bp resolution.
Extended Data Fig. 3 RCMC generates deeper contact maps than other 3C methods across all 5 captured loci.
Contact map comparisons of RCMC against the highest-resolution available mESC Hi-C31 (top; Bonev et al. 2017) and Micro-C12 (bottom; Hsieh et al. 2020) datasets at the Klf1, Ppm1g, Sox2, Nanog, and Fbn2 loci. Full captured regions are shown for each locus at resolutions ranging from 1–5 kb, as well as Klf1 and Ppm1g zoom-ins at 800 and 1000 bp, respectively. Gene annotations and ATAC, ChIP-seq, and RNA-seq tracks (Supplementary Table 1) are shown below the contact maps, while the contact intensity scales are shown next to the maps.
Extended Data Fig. 4 RCMC maps the Sox2 locus more deeply and efficiently than sister methods, uncovering previously unseen interactions.
(a) Contact map comparisons of RCMC against Hi-C31 (top) and Micro-C12 (bottom) at the Sox2 locus at 1.6 kb resolution. Arrows mark contacts between Sox2, the SCR, and Fxr1 not mapped by Hi-C and Micro-C. (b) Contact map comparisons of RCMC against Tiled-Micro-Capture-C17 (TMCC) across the whole TMCC-Captured locus (left, 1600 bp resolution) and in the Sox2 and SCR regulatory cluster (right, 500 bp resolution). Full datasets are visualized in the top contact maps, and TMCC has been downsampled to match the total number of RCMC sequencing reads in view in the bottom contact maps.
Extended Data Fig. 5 RCMC identifies microcompartments, which are not visible in other methods and not reliably called by existing algorithms.
(a, b) Contact maps comparison of RCMC (top) against Hi-C31 (bottom, a) and Micro-C12 (bottom, b) at the Klf1 locus at 500 and 250 bp resolutions and at the Ppm1g locus at 1000 and 250 bp resolutions. (c) Contact maps of the Klf1 and Ppm1g loci at 1 kb resolution with loop calls by Mustache37 overlaid on the bottom half of the map and compartment calls by cooltools59,60 shown below the map. (d) Contact maps of the entire Klf1 (3.2 kb resolution) and Ppm1g (5 kb resolution) captured loci with manually called loops (see Methods) overlaid on the bottom halves of the maps.
Extended Data Fig. 6 Microcompartments are not artifacts resulting from incomplete ICE balancing nor chromatin accessibility.
(a) Comparison of ICE balancing across methods and captured loci. Distributions of the sums of ICE-balanced contact matrix rows at 250 bp resolution are shown at the Klf1, Ppm1g, Fbn2, and Sox2 loci for RCMC, Micro-C12, and Hi-C31, as well as for the subset of RCMC rows containing microcompartment anchors. A sharp unimodal peak is consistent with ICE’s baseline assumption that all contact matrix rows and columns must sum to the same value. (b) Metaplots (above) and heatmaps (below) depicting ATAC signal at microcompartment anchors (left, separated by whether anchors coincide with an ATAC peak) and at all ATAC peaks in the Klf1 and Ppm1g capture loci (right, separated by whether peaks coincide with a microcompartment anchor). Signals are plotted in a 2 kb window centered on the anchor (left) or the ATAC peak (right). (c) RCMC contact maps at the Klf1 (left, 250 bp resolution) and Ppm1g (right, 1.6 kb resolution) loci indicating ATAC peaks that do not form microcompartments (left, magenta arrows) and a microcompartment anchor that does not coincide with an ATAC peak (right, cyan arrow). Black arrows (right) indicate microcompartmental loops involving the ATAC-negative microcompartment anchor. (d) Venn diagram breakdown of the overlap between all manually annotated microcompartment anchors and all ATAC peaks across the Klf1 and Ppm1g capture loci. Of 132 annotated microcompartment anchors, 12 do not coincide with ATAC peaks (cyan) while 120 do (purple, *). Of 353 called ATAC peaks, 187 do not form microcompartment anchors (magenta) while 166 do (purple, **). The apparent discrepancy of 120 microcompartment anchors being anchored by 166 ATAC peaks is due to two close ATAC peaks occasionally anchoring a single microcompartment.
Extended Data Fig. 8 Cohesin depletion disrupts CTCF/Cohesin loops, but generally not most microcompartmental loops.
(a) Contact maps comparing a DMSO control (above) and RAD21-depleted samples (below) are shown for the Klf1, Ppm1g, Sox2, Nanog, and Fbn2 loci at resolutions spanning 800 bp – 5 kb in F1M RAD21-mAID-BFP-V5 mESCs13,38. Arrows mark contacts lost upon RAD21 depletion. ChIP-seq data from Hsieh et al.,13 is shown below the maps before and after the IAA treatment (500 μM, 3 hours). Two versions of the Fbn2 locus are shown, with the left using logarithmic contact frequency scaling and the right using linear scaling. Loss of the Fbn2 loop38 is most clearly seen on linear scale. (b) Contact probability curves comparing RAD21-depleted RCMC samples against a DMSO control (top) and RAD21-depleted Micro-C samples against a DMSO control (bottom). Arrows indicate the contact frequency ‘bump’ lost upon RAD21 depletion.
Extended Data Fig. 9 Inhibition of transcription does not significantly alter genome organization in captured loci.
Contact maps comparing control data against 45 min (top) and 4 hr (bottom) transcriptional inhibition data (from 1 μM triptolide treatments) are shown for the Klf1, Ppm1g, Sox2, Nanog, and Fbn2 loci at resolutions spanning 800 bp – 5 kb in mESC WT cells. RNA Pol II ChIP-seq data is shown below the maps for each treatment condition.
Extended Data Fig. 10 Microcompartment-like structures are also visible in ultra-deep Hi-C data.
(a–d) Contact maps of ultra-deep Hi-C data in human lymphoblastoid cells Harris et al.,14 showing loci with structures sharing many microcompartmental features. Maps were generated using Juicebox’s web interface61 kindly provided by Dr. Jordan Rowley. Maps are shown at 1 kb resolution, with GM12878 gene annotations, CTCF (ENCFF364OXN) and H3K27ac (ENCFF180LKW) ChIP-seq, and RNA-seq (ENCFF604VIC) signal tracks shown below the contact maps.
Supplementary information
Supplementary Information
Supplementary Methods, Supplementary Figure 1, Supplementary Protocol
Supplementary Table 1
A list of all publicly available datasets used in the paper and the figures in which they appear.
Source data
Source Data Fig. 4
Unprocessed western blots.
Source Data Extended Data Fig./Table 1
Unprocessed gels.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Goel, V.Y., Huseyin, M.K. & Hansen, A.S. Region Capture Micro-C reveals coalescence of enhancers and promoters into nested microcompartments. Nat Genet 55, 1048–1056 (2023). https://doi.org/10.1038/s41588-023-01391-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41588-023-01391-1
This article is cited by
-
Enhancer selectivity in space and time: from enhancer–promoter interactions to promoter activation
Nature Reviews Molecular Cell Biology (2024)
-
Computational methods for analysing multiscale 3D genome organization
Nature Reviews Genetics (2024)
-
KLF5 regulates actin remodeling to enhance the metastasis of nasopharyngeal carcinoma
Oncogene (2024)
-
Mapping nucleosome-resolution chromatin organization and enhancer-promoter loops in plants using Micro-C-XL
Nature Communications (2024)
-
The impact of DNA methylation on CTCF-mediated 3D genome organization
Nature Structural & Molecular Biology (2024)