Abstract
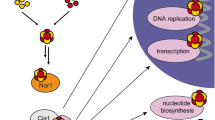
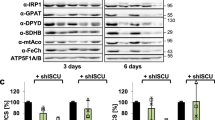
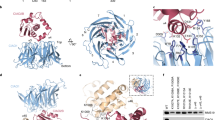
Hundreds of cellular proteins require iron cofactors for activity, and cells express systems for their assembly and distribution. Molecular details of the cytosolic iron pool used for iron cofactors are lacking, but iron chaperones of the poly(rC)-binding protein (PCBP) family play a key role in ferrous ion distribution. Here we show that, in cells and in vitro, PCBP1 coordinates iron via conserved cysteine and glutamate residues and a molecule of noncovalently bound glutathione (GSH). Proteomics analysis of PCBP1-interacting proteins identified BolA2, which functions, in complex with Glrx3, as a cytosolic [2Fe–2S] cluster chaperone. The Fe–GSH-bound form of PCBP1 complexes with cytosolic BolA2 via a bridging Fe ligand. Biochemical analysis of PCBP1 and BolA2, in cells and in vitro, indicates that PCBP1–Fe–GSH–BolA2 serves as an intermediate complex required for the assembly of [2Fe–2S] clusters on BolA2–Glrx3, thereby linking the ferrous iron and Fe–S distribution systems in cells.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
Data availability
The proteomics dataset (Supplementary Table 1) is available at MassIVE Repository (https://massive.ucsd.edu/) with the accession number MSV000083887.
References
Hamza, I. & Dailey, H. A. One ring to rule them all: trafficking of heme and heme synthesis intermediates in the metazoans. Biochim. Biophys. Acta 1823, 1617–1632 (2012).
Ciofi-Baffoni, S., Nasta, V. & Banci, L. Protein networks in the maturation of human iron-sulfur proteins. Metallomics 10, 49–72 (2018).
Lill, R. et al. The role of mitochondria and the CIA machinery in the maturation of cytosolic and nuclear iron-sulfur proteins. Eur. J. Cell Biol. 94, 280–291 (2015).
Philpott, C. C. & Jadhav, S. The ins and outs of iron: escorting iron through the mammalian cytosol. Free Radic. Biol. Med. 133, 112–117 (2019).
Yanatori, I. & Kishi, F. DMT1 and iron transport. Free Radic. Biol. Med. 133, 55–63 (2019).
Chaudhury, A., Chander, P. & Howe, P. H. Heterogeneous nuclear ribonucleoproteins (hnRNPs) in cellular processes: focus on hnRNP E1’s multifunctional regulatory roles. RNA 16, 1449–1462 (2010).
Makeyev, A. V. & Liebhaber, S. A. The poly(C)-binding proteins: a multiplicity of functions and a search for mechanisms. RNA 8, 265–278 (2002).
Ostareck-Lederer, A. & Ostareck, D. H. Control of mRNA translation and stability in haematopoietic cells: the function of hnRNPs K and E1/E2. Biol. Cell 96, 407–411 (2004).
Ostareck-Lederer, A. & Ostareck, D. H. Precision mechanics with multifunctional tools: how hnRNP K and hnRNPs E1/E2 contribute to post-transcriptional control of gene expression in hematopoiesis. Curr. Protein Pept. Sci. 13, 391–400 (2012).
Philpott, C. C., Ryu, M. S., Frey, A. & Patel, S. Cytosolic iron chaperones: proteins delivering iron cofactors in the cytosol of mammalian cells. J. Biol. Chem. 292, 12764–12771 (2017).
Shi, H., Bencze, K. Z., Stemmler, T. L. & Philpott, C. C. A cytosolic iron chaperone that delivers iron to ferritin. Science 320, 1207–1210 (2008).
Epsztejn, S., Kakhlon, O., Glickstein, H., Breuer, W. & Cabantchik, I. Fluorescence analysis of the labile iron pool of mammalian cells. Anal. Biochem. 248, 31–40 (1997).
Leidgens, S. et al. Each member of the poly-r(C)-binding protein 1 (PCBP) family exhibits iron chaperone activity toward ferritin. J. Biol. Chem. 288, 17791–17802 (2013).
Frey, A. G. et al. Iron chaperones PCBP1 and PCBP2 mediate the metallation of the dinuclear iron enzyme deoxyhypusine hydroxylase. Proc. Natl Acad. Sci. USA 111, 8031–8036 (2014).
Nandal, A. et al. Activation of the HIF prolyl hydroxylase by the iron chaperones PCBP1 and PCBP2. Cell Metab. 14, 647–657 (2011).
Ryu, M. S., Zhang, D., Protchenko, O., Shakoury-Elizeh, M. & Philpott, C. C. PCBP1 and NCOA4 regulate erythroid iron storage and heme biosynthesis. J. Clin. Invest. 127, 1786–1797 (2017).
Yanatori, I., Yasui, Y., Tabuchi, M. & Kishi, F. Chaperone protein involved in transmembrane transport of iron. Biochem. J. 462, 25–37 (2014).
Yanatori, I., Richardson, D. R., Imada, K. & Kishi, F. Iron export through the transporter ferroportin 1 is modulated by the iron chaperone PCBP2. J. Biol. Chem. 291, 17303–17318 (2016).
Yanatori, I., Richardson, D. R., Toyokuni, S. & Kishi, F. The iron chaperone poly(rC)-binding protein 2 forms a metabolon with the heme oxygenase 1/cytochrome P450 reductase complex for heme catabolism and iron transfer. J. Biol. Chem. 292, 13205–13229 (2017).
Braymer, J. J. & Lill, R. Iron-sulfur cluster biogenesis and trafficking in mitochondria. J. Biol. Chem. 292, 12754–12763 (2017).
Maio, N. & Rouault, T. A. Iron-sulfur cluster biogenesis in mammalian cells: new insights into the molecular mechanisms of cluster delivery. Biochim. Biophys. Acta 1853, 1493–1512 (2015).
Rouault, T. A. & Maio, N. Biogenesis and functions of mammalian iron-sulfur proteins in the regulation of iron homeostasis and pivotal metabolic pathways. J. Biol. Chem. 292, 12744–12753 (2017).
Srinivasan, V., Pierik, A. J. & Lill, R. Crystal structures of nucleotide-free and glutathione-bound mitochondrial ABC transporter Atm1. Science 343, 1137–1140 (2014).
Haunhorst, P. et al. Crucial function of vertebrate glutaredoxin 3 (PICOT) in iron homeostasis and hemoglobin maturation. Mol. Biol. Cell 24, 1895–1903 (2013).
Li, H., Mapolelo, D. T., Randeniya, S., Johnson, M. K. & Outten, C. E. Human glutaredoxin 3 forms [2Fe–2S]-bridged complexes with human BolA2. Biochemistry 51, 1687–1696 (2012).
Muhlenhoff, U. et al. Cytosolic monothiol glutaredoxins function in intracellular iron sensing and trafficking via their bound iron-sulfur cluster. Cell Metab. 12, 373–385 (2010).
Frey, A. G., Palenchar, D. J., Wildemann, J. D. & Philpott, C. C. A glutaredoxin·BolA complex serves as an iron-sulfur cluster chaperone for the cytosolic cluster assembly machinery. J. Biol. Chem. 291, 22344–22356 (2016).
Li, F., Bullough, K. Z., Vashisht, A. A., Wohlschlegel, J. A. & Philpott, C. C. Poly(rC)-binding protein 2 regulates hippo signaling to control growth in breast epithelial cells. Mol. Cell. Biol 36, 2121–2131 (2016).
Banci, L., Camponeschi, F., Ciofi-Baffoni, S. & Muzzioli, R. Elucidating the molecular function of human BOLA2 in GRX3-dependent anamorsin maturation pathway. J. Am. Chem. Soc. 137, 16133–16143 (2015).
Franco, R., Schoneveld, O. J., Pappa, A. & Panayiotidis, M. I. The central role of glutathione in the pathophysiology of human diseases. Arch. Physiol. Biochem. 113, 234–258 (2007).
Biederbick, A. et al. Role of human mitochondrial Nfs1 in cytosolic iron-sulfur protein biogenesis and iron regulation. Mol. Cell. Biol. 26, 5675–5687 (2006).
Li, H. et al. The yeast iron regulatory proteins Grx3/4 and Fra2 form heterodimeric complexes containing a [2Fe–2S] cluster with cysteinyl and histidyl ligation. Biochemistry 48, 9569–9581 (2009).
Kumanovics, A. et al. Identification of FRA1 and FRA2 as genes involved in regulating the yeast iron regulon in response to decreased mitochondrial iron-sulfur cluster synthesis. J. Biol. Chem. 283, 10276–10286 (2008).
Tang, Y. S. et al. Evidence favoring a positive feedback loop for physiologic auto upregulation of hnRNP-E1 during prolonged folate deficiency in human placental cells. J. Nutr. 147, 482–498 (2017).
Trott, O. & Olson, A. J. AutoDock Vina: improving the speed and accuracy of docking with a new scoring function, efficient optimization, and multithreading. J. Comput. Chem. 31, 455–461 (2010).
Sidiqi, M. et al. Structure and RNA binding of the third KH domain of poly(C)-binding protein 1. Nucleic Acids Res. 33, 1213–1221 (2005).
Patel, S. J. et al. Fine-tuning of substrate affinity leads to alternative roles of Mycobacterium tuberculosis Fe2+-ATPases. J. Biol. Chem. 291, 11529–11539 (2016).
Walkup, G. K. & Imperiali, B. Fluorescent chemosensors for divalent zinc based on zinc finger domains. Enhanced oxidative stability, metal binding affinity, and structural and functional characterization. J. Am. Chem. Soc. 119, 3443–3450 (1997).
Esposito, B. P., Epsztejn, S., Breuer, W. & Cabantchik, Z. I. A review of fluorescence methods for assessing labile iron in cells and biological fluids. Anal. Biochem. 304, 1–18 (2002).
Petrat, F., de Groot, H., Sustmann, R. & Rauen, U. The chelatable iron pool in living cells: a methodically defined quantity. Biol. Chem. 383, 489–502 (2002).
Hider, R. C. & Kong, X. L. Glutathione: a key component of the cytoplasmic labile iron pool. Biometals 24, 1179–1187 (2011).
Kozakov, D. et al. The ClusPro web server for protein-protein docking. Nat. Protoc. 12, 255–278 (2017).
Ba, L. A., Doering, M., Burkholz, T. & Jacob, C. Metal trafficking: from maintaining the metal homeostasis to future drug design. Metallomics 1, 292–311 (2009).
Antony, A. et al. Translational upregulation of folate receptors is mediated by homocysteine via RNA-heterogeneous nuclear ribonucleoprotein E1 interactions. J. Clin. Invest. 113, 285–301 (2004).
Tang, Y. S. et al. Incrimination of heterogeneous nuclear ribonucleoprotein E1 (hnRNP-E1) as a candidate sensor of physiological folate deficiency. J. Biol. Chem. 286, 39100–39115 (2011).
Banci, L. et al. N-terminal domains mediate [2Fe–2S] cluster transfer from glutaredoxin-3 to anamorsin. Nat. Chem. Biol. 11, 772–778 (2015).
Land, T. & Rouault, T. A. Targeting of a human iron-sulfur cluster assembly enzyme, nifs, to different subcellular compartments is regulated through alternative AUG utilization. Mol. Cell 2, 807–815 (1998).
Vashisht, A. A. et al. Control of iron homeostasis by an iron-regulated ubiquitin ligase. Science 326, 718–721 (2009).
Wohlschlegel, J. A. Identification of SUMO-conjugated proteins and their SUMO attachment sites using proteomic mass spectrometry. Methods Mol. Biol. 497, 33–49 (2009).
Acknowledgements
This work was supported by the Intramural Research Program of the National Institutes of Diabetes and Digestive and Kidney Diseases and the Office of Dietary Supplements, Office of the Director, National Institutes of Health. J.A.W. is supported by NIH grant nos. GM089778 and GM112763. We thank C. Outten (University of South Carolina) for plasmids and T. Stemmler (Wayne State University) for plasmids and helpful discussions.
Author information
Authors and Affiliations
Contributions
S.J.P. and A.G.F. conceived and coordinated the study, designed, performed, and analyzed most experiments, prepared figures and wrote the paper. D.J.P. created BolA2-inducible cell lines and S.A. generated KH3 mutant plasmids. K.Z.B., A.V. and J.A.W. performed mass spectrometry experiments. C.C.P. conceived and coordinated the study, prepared the figures and wrote the paper. All authors analyzed results and approved the final version of the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Supplementary Information
Supplementary Tables 1–2 and Supplementary Figures 1–24
Rights and permissions
About this article
Cite this article
Patel, S.J., Frey, A.G., Palenchar, D.J. et al. A PCBP1–BolA2 chaperone complex delivers iron for cytosolic [2Fe–2S] cluster assembly. Nat Chem Biol 15, 872–881 (2019). https://doi.org/10.1038/s41589-019-0330-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41589-019-0330-6
This article is cited by
-
Mechanisms controlling cellular and systemic iron homeostasis
Nature Reviews Molecular Cell Biology (2024)
-
Integrative analysis of TBI data reveals Lgmn as a key player in immune cell-mediated ferroptosis
BMC Genomics (2023)
-
Ferroptotic mechanisms and therapeutic targeting of iron metabolism and lipid peroxidation in the kidney
Nature Reviews Nephrology (2023)
-
Do Marine Polysaccharides Carrageenans Modulate Non-apoptotic Regulated Cell Deaths ? (a Review)
Current Pharmacology Reports (2023)
-
Basic mechanisms and novel potential therapeutic targets for ferroptosis in acute myeloid leukemia
Annals of Hematology (2023)