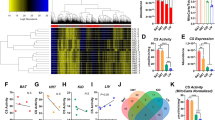
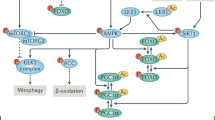
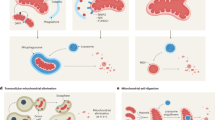
Abstract
Mitochondrial membrane potential (ΔΨm) is a universal selective indicator of mitochondrial function and is known to play a central role in many human pathologies, such as diabetes mellitus, cancer and Alzheimer’s and Parkinson’s diseases. Here, we report the design, synthesis and several applications of mitochondria-activatable luciferin (MAL), a bioluminescent probe sensitive to ΔΨm, and partially to plasma membrane potential (ΔΨp), for non-invasive, longitudinal monitoring of ΔΨm in vitro and in vivo. We applied this new technology to evaluate the aging-related change of ΔΨm in mice and showed that nicotinamide riboside (NR) reverts aging-related mitochondrial depolarization, revealing another important aspect of the mechanism of action of this potent biomolecule. In addition, we demonstrated application of the MAL probe for studies of brown adipose tissue (BAT) activation and non-invasive in vivo assessment of ΔΨm in animal cancer models, opening exciting opportunities for understanding the underlying mechanisms and for discovery of effective treatments for many human pathologies.

This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
Data availability
The data that support the findings of this study are available from the corresponding author upon request. CCDC nos. 1940412, 1940411, 1940410 and 1940409 for compounds 7, 9, 13 and 16, contain the supplementary crystallographic data for this paper. These data can be obtained, free of charge, from The Cambridge Crystallographic Data Centre via www.ccdc.cam.ac.uk/structures.
References
Herst, P. M., Rowe, M. R., Carson, G. M. & Berridge, M. V. Functional mitochondria in health and disease. Front. Endocrinol. (Lausanne) 8, 296 (2017).
Duchen, M. R. Mitochondria in health and disease: perspectives on a new mitochondrial biology. Mol. Aspects Med. 25, 365–451 (2004).
Wang, Y., Xu, E., Musich, P. R. & Lin, F. Mitochondrial dysfunction in neurodegenerative diseases and the potential countermeasure. CNS Neurosci. Ther. 25, 816–824 (2019).
Zorova, L. D. et al. Mitochondrial membrane potential. Anal. Biochem. 552, 50–59 (2018).
Momcilovic, M. et al. In vivo imaging of mitochondrial membrane potential in non-small-cell lung cancer. Nature 575, 380–384 (2019).
Lee, J. H. et al. The role of adipose tissue mitochondria: regulation of mitochondrial function for the treatment of metabolic diseases. Int. J. Mol. Sci. 20, 4924 (2019).
Vyas, S., Zaganjor, E. & Haigis, M. C. Mitochondria and cancer. Cell 166, 555–566 (2016).
Weinberg, S. E. & Chandel, N. S. Targeting mitochondria metabolism for cancer therapy. Nat. Chem. Biol. 11, 9–15 (2015).
Perry, S. W., Norman, J. P., Barbieri, J., Brown, E. B. & Gelbard, H. A. Mitochondrial membrane potential probes and the proton gradient: a practical usage guide. Biotechniques 50, 98–115 (2011).
Rottenberg, H. & Wu, S. Quantitative assay by flow cytometry of the mitochondrial membrane potential in intact cells. Biochim. Biophys. Acta 1404, 393–404 (1998).
Gerencser, A. A. et al. Quantitative measurement of mitochondrial membrane potential in cultured cells: calcium-induced de- and hyperpolarization of neuronal mitochondria. J. Physiol. 590, 2845–2871 (2012).
Madar, I. et al. Characterization of uptake of the new PET imaging compound F-18-fluorobenzyl triphenyl phosphonium in dog myocardium. J. Nucl. Med. 47, 1359–1366 (2006).
Kim, D. Y. et al. Evaluation of a mitochondrial voltage sensor, (18F-fluoropentyl)triphenylphosphonium cation, in a rat myocardial infarction model. J. Nucl. Med. 53, 1779–1785 (2012).
Logan, A. et al. Assessing the mitochondrial membrane potential in cells and in vivo using targeted click chemistry and mass spectrometry. Cell Metab. 23, 379–385 (2016).
Gaskova, D., DeCorby, A. & Lemire, B. D. DiS-C-3(3) monitoring of in vivo mitochondrial membrane potential in C. elegans. Biochem. Biophys. Res. Commun. 354, 814–819 (2007).
Sasagawa, S. et al. In vivo detection of mitochondrial dysfunction induced by clinical drugs and disease-associated genes using a novel dye ZMJ214 in zebrafish. ACS Chem. Biol. 11, 381–388 (2016).
Massoud, T. F. & Gambhir, S. S. Molecular imaging in living subjects: seeing fundamental biological processes in a new light. Gene Dev. 17, 545–580 (2003).
Sweeney, T. J. et al. Visualizing the kinetics of tumor-cell clearance in living animals. Proc. Natl Acad. Sci. USA 96, 12044–12049 (1999).
Mezzanotte, L., van ‘t Root, M., Karatas, H., Goun, E. A. & Lowik, C. In vivo molecular bioluminescence imaging: new tools and applications. Trends Biotechnol. 35, 640–652 (2017).
Rathbun, C. M. & Prescher, J. A. Bioluminescent probes for imaging biology beyond the culture dish. Biochemistry 56, 5178–5184 (2017).
Su, T. A., Bruemmer, K. J. & Chang, C. J. Caged luciferins for bioluminescent activity-based sensing. Curr. Opin. Biotechnol. 60, 198–204 (2019).
Maric, T. et al. Bioluminescent-based imaging and quantification of glucose uptake in vivo. Nat. Methods 16, 526–532 (2019).
Zagozdzon, A. M. et al. Generation of a new bioluminescent model for visualisation of mammary tumour development in transgenic mice. BMC Cancer 12, 209 (2012).
Cao, Y. A. et al. Shifting foci of hematopoiesis during reconstitution from single stem cells. Proc. Natl Acad. Sci. USA 101, 221–226 (2004).
Manni, I., de Latouliere, L., Gurtner, A. & Piaggio, G. Transgenic animal models to visualize cancer-related cellular processes by bioluminescence imaging. Front. Pharmacol. 10, 235 (2019).
Saxon, E. & Bertozzi, C. R. Cell surface engineering by a modified Staudinger reaction. Science 287, 2007–2010 (2000).
Myers, E. L. & Raines, R. T. A phosphine-mediated conversion of azides into diazo compounds. Angew. Chem. Int. Ed. 48, 2359–2363 (2009).
Sundhoro, M., Jeon, S., Park, J., Ramstrom, O. & Yan, M. Perfluoroaryl azide Staudinger reaction: a fast and bioorthogonal reaction. Angew. Chem. Int. Ed. 56, 12117–12121 (2017).
Cohen, A. S., Dubikovskaya, E. A., Rush, J. S. & Bertozzi, C. R. Real-time bioluminescence imaging of glycans on live cells. J. Am. Chem. Soc. 132, 8563–8565 (2010).
Murphy, M. P. Targeting lipophilic cations to mitochondria. Biochim. Biophys. Acta 1777, 1028–1031 (2008).
Prime, T. A. et al. A mitochondria-targeted S-nitrosothiol modulates respiration, nitrosates thiols, and protects against ischemia-reperfusion injury. Proc. Natl Acad. Sci. USA 106, 10764–10769 (2009).
Jameson, V. J. A. et al. Synthesis of triphenylphosphonium vitamin E derivatives as mitochondria-targeted antioxidants. Tetrahedron 71, 8444–8453 (2015).
Ross, M. F. et al. Lipophilic triphenylphosphonium cations as tools in mitochondrial bioenergetics and free radical biology. Biochemistry (Mosc.) 70, 222–230 (2005).
Woodroofe, C. C. et al. N-alkylated 6′-aminoluciferins are bioluminescent substrates for Ultra-Glo and QuantiLum luciferase: new potential scaffolds for bioluminescent assays. Biochemistry 47, 10383–10393 (2008).
Zhang, J. H., Chung, T. D. Y. & Oldenburg, K. R. A simple statistical parameter for use in evaluation and validation of high throughput screening assays. J. Biomol. Screen. 4, 67–73 (1999).
Nicholls, D. G. Simultaneous monitoring of ionophore- and inhibitor-mediated plasma and mitochondrial membrane potential changes in cultured neurons. J. Biol. Chem. 281, 14864–14874 (2006).
Erez, Y. et al. Comparative study of the photoprotolytic reactions of D-luciferin and oxyluciferin. J. Phys. Chem. A 116, 7452–7461 (2012).
Wyatt, C. N. & Buckler, K. J. The effect of mitochondrial inhibitors on membrane currents in isolated neonatal rat carotid body type I cells. J. Physiol. 556, 175–191 (2004).
Lou, P. H. et al. Mitochondrial uncouplers with an extraordinary dynamic range. Biochem. J. 407, 129–140 (2007).
Manago, A. et al. Early effects of the antineoplastic agent salinomycin on mitochondrial function. Cell Death Dis. 6, e1930 (2015).
Martens, C. R. et al. Chronic nicotinamide riboside supplementation is well-tolerated and elevates NAD+ in healthy middle-aged and older adults. Nat. Commun. 9, 1286 (2018).
Mouchiroud, L. et al. The NAD+/Sirtuin pathway modulates longevity through activation of mitochondrial UPR and FOXO signaling. Cell 154, 430–441 (2013).
Vannini, N. et al. The NAD-booster nicotinamide riboside potently stimulates hematopoiesis through increased mitochondrial clearance. Cell Stem Cell 24, 405–418 (2019).
Lowell, B. B. & Spiegelman, B. M. Towards a molecular understanding of adaptive thermogenesis. Nature 404, 652–660 (2000).
Cypess, A. M. et al. Activation of human brown adipose tissue by a β3-adrenergic receptor agonist. Cell Metab. 21, 33–38 (2015).
Virtanen, K. A. Activation of human brown adipose tissue (BAT): focus on nutrition and eating. Handb. Exp. Pharmacol 251, 349–357 (2018).
Yang, Y. et al. Mitochondria and mitochondrial ROS in cancer: novel targets for anticancer therapy. J. Cell. Physiol. 231, 2570–2581 (2016).
Schondorf, D. C. et al. The NAD+ precursor nicotinamide riboside rescues mitochondrial defects and neuronal loss in iPSC and fly models of Parkinson’s disease. Cell Rep. 23, 2976–2988 (2018).
Szabadkai, G. & Duchen, M. R. Mitochondria mediated cell death in diabetes. Apoptosis 14, 1405–1423 (2009).
Kleinert, M. et al. Animal models of obesity and diabetes mellitus. Nat. Rev. Endocrinol. 14, 140–162 (2018).
Zha, L. et al. The histone demethylase UTX promotes brown adipocyte thermogenic program via coordinated regulation of H3K27 demethylation and acetylation. J. Biol. Chem. 290, 25151–25163 (2015).
Erlich-Hadad, T. et al. TAT-MTS-MCM fusion proteins reduce MMA levels and improve mitochondrial activity and liver function in MCM-deficient cells. J. Cell. Mol. Med. 22, 1601–1613 (2018).
Livak, K. J. & Schmittgen, T. D. Analysis of relative gene expression data using real-time quantitative PCR and the 2−ΔΔCT method. Methods 25, 402–408 (2001).
Acknowledgements
We thank the EPFL NMR facility staff and especially P. Mieville for help with acquiring 2D spectra, the EPFL XRD facility staff for help with crystal structure determination, and the EPFL MS facility for help with HRMS measurements. We thank O. Naveiras and A. Oggier (ISREC, EPFL), who kindly provided NR-enriched and control diets. We also thank G. Karateev for help and advice regarding the synthesis of TPP-CL2. We appreciate the help of R. Combe and M. Kulagin, members of the CPG facility at EPFL, in measuring the oxygen consumption by mice. We thank the Swiss National Foundation (grant no. 31003A_150134) for funding this work.
Author information
Authors and Affiliations
Contributions
E.A.G. conceptualized the study and acquired the funding. E.A.G. and A.A.B. designed the experiments. A.A.B., R.S. and N.S. synthesized the compounds. A.A.B. performed the experiments and analyzed the data. A.A.B., A.H. and U.D.M. designed and performed cellular OCR measurements. T.M. measured the clearance of TPP-CL2 in vivo, and designed and drew the graphical abstract. G.B. assisted in measurement of the nigericin effect in cells with TMRM. E.A.G. and A.A.B. wrote the manuscript. All authors edited the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Supplementary Information
Supplementary Figs. 1–28, Tables 1–3 and notes 1–3.
Supplementary Video 1
Working principle of MAL probe.
Rights and permissions
About this article
Cite this article
Bazhin, A.A., Sinisi, R., De Marchi, U. et al. A bioluminescent probe for longitudinal monitoring of mitochondrial membrane potential. Nat Chem Biol 16, 1385–1393 (2020). https://doi.org/10.1038/s41589-020-0602-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41589-020-0602-1
This article is cited by
-
Intracellular MUC20 variant 2 maintains mitochondrial calcium homeostasis and enhances drug resistance in gastric cancer
Gastric Cancer (2022)
-
Mitochondrial copper depletion suppresses triple-negative breast cancer in mice
Nature Biotechnology (2021)