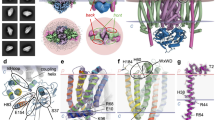
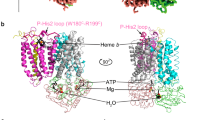
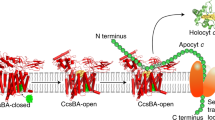
Abstract
The covalent attachment of one or multiple heme cofactors to cytochrome c protein chains enables cytochrome c proteins to be used in electron transfer and redox catalysis in extracytoplasmic environments. A dedicated heme maturation machinery, whose core component is a heme lyase, scans nascent peptides after Sec-dependent translocation for CXnCH-binding motifs. Here we report the three-dimensional (3D) structure of the heme lyase CcmF, a 643-amino acid integral membrane protein, from Thermus thermophilus. CcmF contains a heme b cofactor at the bottom of a large cavity that opens toward the extracellular side to receive heme groups from the heme chaperone CcmE for cytochrome maturation. A surface groove on CcmF may guide the extended apoprotein to heme attachment at or near a loop containing the functionally essential WXWD motif, which is situated above the putative cofactor binding pocket. The structure suggests heme delivery from within the membrane, redefining the role of the chaperone CcmE.

This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
Data availability
The structural model and structure factors for TtCcmF have been deposited in the Protein Data Bank at www.pdb.org under the accession number 6ZMQ.
References
Anson, M. L. & Mirsky, A. E. The heme compounds in nature and biological oxidations. Science 68, 647–648 (1928).
Pauling, L. & Coryell, C. D. The magnetic properties and structure of hemoglobin, oxyhemoglobin and carbonmonoxyhemoglobin. Proc. Natl Acad. Sci. USA 22, 210–216 (1936).
Fita, I. & Rossmann, M. G. The active center of catalase. J. Mol. Biol. 185, 21–37 (1985).
Einsle, O. et al. Structure of cytochrome c nitrite reductase. Nature 400, 476–480 (1999).
Hermann, B., Kern, M., La Pietra, L., Simon, J. & Einsle, O. The octaheme MccA is a heme c-copper sulfite reductase. Nature 520, 706–709 (2015).
Schlichting, I. et al. The catalytic pathway of cytochrome P450cam at atomic resolution. Science 287, 1615–1622 (2000).
Margoliash, E., Barlow, G. H. & Byers, V. Differential binding properties of cytochrome c: possible relevance for mitochondrial ion transport. Nature 228, 723–726 (1970).
Poulos, T. L. Heme enzyme structure and function. Chem. Rev. 114, 3919–3962 (2014).
Weichsel, A., Andersen, J. F., Roberts, S. A. & Montfort, W. R. Nitric oxide binding to nitrophorin 4 induces complete distal pocket burial. Nat. Struct. Biol. 7, 551–554 (2000).
Perutz, M. F., Kendrew, J. C. & Watson, H. C. Structure and function of hemeoglobin: II. Some relations between polypeptide chain configuration and amino acid sequence. J. Mol. Biol. 13, 669–678 (1965).
Barker, P. D. & Ferguson, S. J. Still a puzzle: why is heme covalently attached in c-type cytochromes? Structure 7, R281–R290 (1999).
Moore, G. R. & Pettigrew, G. W. Cytochromes c. Evolutionary, Structural and Physicochemical Aspects. (Springer, 1990).
Keilin, D. On cytochrome, a respiratory pigment, common to animals, yeast, and higher plants. Proc. R. Soc. Lond. B 98, 312–339 (1925).
Thöny-Meyer, L. Biogenesis of respiratory cytochromes in bacteria. Microbiol. Mol. Biol. Rev. 61, 337–376 (1997).
Kranz, R., Lill, R., Goldman, B., Bonnard, G. & Merchant, S. Molecular mechanisms of cytochrome c biogenesis: three distinct systems. Mol. Microbiol. 29, 383–396 (1998).
Methé, B. A. et al. Genome of Geobacter sulfurreducens: metal reduction in subsurface environments. Science 302, 1967–1969 (2003).
Babbitt, S. E., Sutherland, M. C., Francisco, B. S., Mendez, D. L. & Kranz, R. G. Mitochondrial cytochrome c biogenesis: no longer an enigma. Trends Biochem. Sci. 40, 446–455 (2015).
Babbitt, S. E. et al. Mechanisms of mitochondrial holocytochrome c synthase and the key roles played by cysteines and histidine of the heme attachment site, Cys-XX-Cys-His. J. Biol. Chem. 289, 28795–28807 (2014).
Stevens, J. M., Uchida, T., Daltrop, O. & Ferguson, S. J. Covalent cofactor attachment to proteins: cytochrome c biogenesis. Biochem. Soc. Trans. 33, 792–795 (2005).
Sanders, C., Turkarslan, S., Lee, D. W. & Daldal, F. Cytochrome c biogenesis: the Ccm system. Trends Microbiol. 18, 266–274 (2010).
Thöny-Meyer, L. Cytochrome c maturation: a complex pathway for a simple task? Biochem. Soc. Trans. 30, 633–638 (2002).
Schulz, H., Fabianek, R. A., Pellicioli, E. C., Hennecke, H. & Thöny-Meyer, L. Heme transfer to the heme chaperone CcmE during cytochrome c maturation requires the CcmC protein, which may function independently of the ABC-transporter CcmAB. Proc. Natl Acad. Sci. USA 96, 6462–6467 (1999).
Baysse, C. et al. Co-ordination of iron acquisition, iron porphyrin chelation and iron–protoporphyrin export via the cytochrome c biogenesis protein CcmC in Pseudomonas fluorescens. Microbiology 149, 3543–3552 (2003).
Page, M. D. & Ferguson, S. J. Mutational analysis of the Paracoccus denitrificans c-type cytochrome biosynthetic genes ccmABCDG: disruption of ccmC has distinct effects suggesting a role for CcmC independent of CcmAB. Microbiology 145, 3047–3057 (1999).
Richard-Fogal, C. L., Frawley, E. R. & Kranz, R. G. Topology and function of CcmD in cytochrome c maturation. J. Bacteriol. 190, 3489–3493 (2008).
Sanders, C. et al. The cytochrome c maturation components CcmF, CcmH, and CcmI form a membrane-integral multisubunit heme ligation complex. J. Biol. Chem. 283, 29715–29722 (2008).
Ahuja, U., Rozhkova, A., Glockshuber, R., Thöny-Meyer, L. & Einsle, O. Helix swapping leads to dimerization of the N-terminal domain of the c-type cytochrome maturation protein CcmH from Escherichia coli. FEBS Lett. 582, 2779–2786 (2008).
Fabianek, R. A., Hofer, T. & Thöny-Meyer, L. Characterization of the Escherichia coli CcmH protein reveals new insights into the redox pathway required for cytochrome c maturation. Arch. Microbiol. 171, 92–100 (1999).
San Francisco, B., Sutherland, M. C. & Kranz, R. G. The CcmFH complex is the system I holocytochrome c synthetase: engineering cytochrome c maturation independent of CcmABCDE. Mol. Microbiol. 91, 996–1008 (2014).
Lee, J. H., Harvat, E. M., Stevens, J. M., Ferguson, S. J. & Saier, M. H. Evolutionary origins of members of a superfamily of integral membrane cytochrome c biogenesis proteins. Biochim. Biophys. Acta Biomembr. 1768, 2164–2181 (2007).
Richard-Fogal, C. L. et al. A conserved heme redox and trafficking pathway for cofactor attachment. EMBO J. 28, 2349–2359 (2009).
San Francisco, B., Bretsnyder, E. C., Rodgers, K. R. & Kranz, R. G. Heme ligand identification and redox properties of the cytochrome c synthetase, CcmF. Biochemistry 50, 10974–10985 (2011).
San Francisco, B. & Kranz, R. G. Interaction of holoCcmE with CcmF in heme trafficking and cytochrome c biosynthesis. J. Mol. Biol. 426, 570–585 (2014).
Shoemaker, K. R., Kim, P. S., York, E. J., Stewart, J. M. & Baldwin, R. L. Tests of the helix dipole model for stabilization of α-helices. Nature 326, 563–567 (1987).
Lomize, M. A., Pogozheva, I. D., Joo, H., Mosberg, H. I. & Lomize, A. L. OPM database and PPM web server: resources for positioning of proteins in membranes. Nucleic Acids Res. 40, D370–D376 (2012).
Schulz, H., Hennecke, H. & Thöny-Meyer, L. Prototype of a heme chaperone essential for cytochrome c maturation. Science 281, 1197–1200 (1998).
Enggist, E., Thöny-Meyer, L., Guntert, P. & Pervushin, K. NMR structure of the heme chaperone CcmE reveals a novel functional motif. Structure 10, 1551–1557 (2002).
Arnesano, F. et al. Solution structure and characterization of the heme chaperone CcmE. Biochemistry 41, 13587–13594 (2002).
Ferreira, G. C. et al. Structure and function of ferrochelatase. J. Bioenerg. Biomembr. 27, 221–229 (1995).
Dailey, T. A. & Dailey, H. A. Identification of [2Fe-2S] clusters in microbial ferrochelatases. J. Bacteriol. 184, 2460–2464 (2002).
Richard-Fogal, C. & Kranz, R. G. The CcmC:heme:CcmE complex in heme trafficking and cytochrome c biosynthesis. J. Mol. Biol. 401, 350–362 (2010).
Ahuja, U. & Thöny-Meyer, L. CcmD is involved in complex formation between CcmC and the heme chaperone CcmE during cytochrome c maturation. J. Biol. Chem. 280, 236–243 (2005).
Verissimo, A. F., Mohtar, M. A. & Daldal, F. The heme chaperone apoCcmE forms a ternary complex with CcmI and apocytochrome c. J. Biol. Chem. 288, 6272–6283 (2013).
Verissimo, A. F. & Daldal, F. Cytochrome c biogenesis system I: an intricate process catalyzed by a maturase supercomplex? Biochim. Biophys. Acta 1837, 989–998 (2014).
Verissimo, A. F. et al. The thioreduction component CcmG confers efficiency and the heme ligation component CcmH ensures stereo-specificity during cytochrome c maturation. J. Biol. Chem. 292, 13154–13167 (2017).
Frawley, E. R. & Kranz, R. G. CcsBA is a cytochrome c synthetase that also functions in heme transport. Proc. Natl Acad. Sci. USA 106, 10201–10206 (2009).
Gibson, D. G. et al. Enzymatic assembly of DNA molecules up to several hundred kilobases. Nat. Methods 6, 343–345 (2009).
Kabsch, W. XDS. Acta Crystallogr. D Biol. Crystallogr. 66, 125–132 (2010).
Vonrhein, C. et al. Data processing and analysis with the autoPROC toolbox. Acta Crystallogr. D Biol. Crystallogr. 67, 293–302 (2011).
Terwilliger, T. C. et al. Decision-making in structure solution using Bayesian estimates of map quality: the PHENIX AutoSol wizard. Acta Crystallogr. D Biol. Crystallogr. 65, 582–601 (2009).
Adams, P. D. et al. PHENIX: a comprehensive Python-based system for macromolecular structure solution. Acta Crystallogr. D Biol. Crystallogr. 66, 213–221 (2010).
Emsley, P., Lohkamp, B., Scott, W. G. & Cowtan, K. Features and development of Coot. Acta Crystallogr. D Biol. Crystallogr. 66, 486–501 (2010).
BUSTER v. 2.10.3 (Global Phasing Ltd., 2010).
Chen, V. B. et al. MolProbity: all-atom structure validation for macromolecular crystallography. Acta Crystallogr. D Biol. Crystallogr. 66, 12–21 (2010).
Acknowledgements
We thank F. Kersten and S. Andrade for support and helpful discussions. This work was supported by the European Research Council (grant 310656) and Deutsche Forschungsgemeinschaft (CRC 1381, project ID 403222702, and RTG 2202, project ID 46710898). We thank the beam line staff at the Swiss Light Source, Villigen, Switzerland, for excellent assistance with data collection.
Author information
Authors and Affiliations
Contributions
A.B. and O.E. designed the experiments. A.B. and L.I. produced protein and generated crystals. A.B. solved the crystal structure. A.B. and L.Z. built and refined the crystal structure. A.B. and O.E. analyzed the data and wrote the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Peer review information Nature Chemical Biology thanks T. Iverson and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Extended data
Extended Data Fig. 1 The heme cofactor and c-type cytochromes.
a, Fe-protoporphyrin IX, the widely used tetrapyrrole cofactor heme, with IUPAC numbering of the carbon atoms in the aromatic ligand. Two vinyl side chains are located at position 3 and 8, and the negatively charged propionate side chains at positions 13 and 17 are relevant for the translocation of the cofactor across a lipid bilayer. b, in cytochromes of type c, the heme cofactor is covalently linked to two cysteine residues of the protein chain via thioether bonds. This linkage is catalyzed by heme lyases and allows for a high cofactor/protein ratio. c, heme cofactors are bound to signature CXXCH motifs in the protein sequence, with the two cysteine residues of the motif forming the thioether linkages, and the subsequent histidine acting as a proximal axial ligand to the iron ion of the cofactor. Figure made from cytochrome c nitrite reductase (PDB ID 1FS7)1.
Extended Data Fig. 2 The tryptophan-rich signature motif (WXWD).
In all classes of heme lyases, a tryptophan-rich motif is suggested to be directly involved in the handling of the heme cofactor. It is found in human cytochrome c synthase (HCCS), as well as in the lyase CcsA of system II and the components CcmC and CcmF of system I.
Extended Data Fig. 3 B-factor distribution in TtCcmF.
Elevated B-factors provide a measure of structural flexibility within the structure of CcmF. The cytoplasmic face of the protein shows high B-factors only in the loop connecting helices h8 and h14 near the C-terminus. In contrast, the periplasmic face of the heme lyase features multiple regions with increased flexibility, notably including the periplasmic domain in the loop connection helices h14 and h15.
Extended Data Fig. 4 Surface representations of TtCcmF.
a, Stereo representation of a low-resolution molecular surface of CcmF and its orientation within the membrane. The membrane is represented by a red disc for the periplasmic boundary and a blue disc for the cytoplasmic boundary. b, Three different views of the surface of CcmF with bound lipids in stick representation, highlighting the extensive periplasmic protrusion that is made up predominantly by the C-terminal periplasmic domain.
Extended Data Fig. 5 Environment of the accessory heme group in CcmF.
The stereo figure shows the b-type heme group liganded by residues H259 in helix h7 and H493 in helix h14. The open space above the accessory heme is the vestibule suggested to accommodate the substrate heme group for attachment to an apocytochrome chain. The refined 2Fo–Fc electron density map is contoured at the 1 σ level.
Extended Data Fig. 6 Docking model for a substrate heme group in CcmF.
The stereo figure details the docking result for a second b-type heme cofactor as a substrate heme within the cavity located above the accessory heme, seen from the opening of this vestibule towards the inner leaflet of the membrane. In order to accommodate the substrate heme, the two tryptophane residues of the WXWD motif in loop 6, W238 and W240, had to relocate, interacting with the bound heme moiety via π-stacking interactions.
Extended Data Fig. 7 Orientation of b-type heme groups in membrane proteins.
Membrane-integral heme groups are most commonly canonical Fe-protoporphyrin IX (heme b). Even bound within the protein matrix, they consistently orient with their propionate sidechains towards the hydrophilic surface of the membrane, revealing this to be a preferred orientation in either leaflet of the membrane. Panels show the orientation of protein complexes in the membrane above a detail of the orientation of the membrane-integral heme b groups. In the respiratory complexes cytochrome bc1 (a) from oxidative phosphorylation and cytochrome b6f (b) from oxygenic photosynthesis, the low- and high-potential heme b moieties have both propionates facing the hydrophilic phase. c, in TtCcmF, the single heme b cofactor is located at the boundary of membrane and cytoplasm, with an approximate 40° rotation with respect to a and b, but in a highly similar arrangement as the heme group in the cytoplasmic leaflet of formate dehydrogenase (d) and nitrate reductase (e).
Supplementary information
Supplementary Information
Supplementary Figs. 1–3 and Table 1.
Rights and permissions
About this article
Cite this article
Brausemann, A., Zhang, L., Ilcu, L. et al. Architecture of the membrane-bound cytochrome c heme lyase CcmF. Nat Chem Biol 17, 800–805 (2021). https://doi.org/10.1038/s41589-021-00793-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41589-021-00793-8
This article is cited by
-
Architecture of the Heme-translocating CcmABCD/E complex required for Cytochrome c maturation
Nature Communications (2023)
-
AF2Complex predicts direct physical interactions in multimeric proteins with deep learning
Nature Communications (2022)
-
Structures of the CcmABCD heme release complex at multiple states
Nature Communications (2022)
-
Cryo-EM of CcsBA reveals the basis for cytochrome c biogenesis and heme transport
Nature Chemical Biology (2022)
-
Handling heme with care
Nature Chemical Biology (2021)