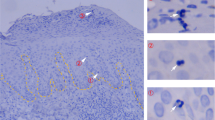
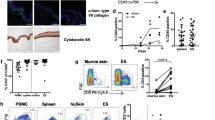
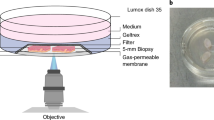
Abstract
Emerging data show that tissue-resident memory T (TRM) cells play an important protective role at murine and human barrier sites. TRM cells in the epidermis of mouse skin patrol their surroundings and rapidly respond when antigens are encountered. However, whether a similar migratory behavior is performed by human TRM cells is unclear, as technology to longitudinally follow them in situ has been lacking. To address this issue, we developed an ex vivo culture system to label and track T cells in fresh skin samples. We validated this system by comparing in vivo and ex vivo properties of murine TRM cells. Using nanobody labeling, we subsequently demonstrated in human ex vivo skin that CD8+ TRM cells migrated through the papillary dermis and the epidermis, below sessile Langerhans cells. Collectively, this work allows the dynamic study of resident immune cells in human skin and provides evidence of tissue patrol by human CD8+ TRM cells.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
Data availability
Publicly available source data were not used in this study. Data supporting the findings of this study are available from the corresponding author upon reasonable request.
Code availability
The custom developed macro for measuring circularity of fluorescent cells is publicly available at https://github.com/bvandenbroek/NKI_ImageJ_Macros/tree/master/Measure_cell_circularity/.
Change history
24 March 2020
An amendment to this paper has been published and can be accessed via a link at the top of the paper.
References
Mueller, S. N. & Mackay, L. K. Tissue-resident memory T cells: local specialists in immune defence. Nat. Rev. Immunol. 16, 79–89 (2016).
Gebhardt, T., Palendira, U., Tscharke, D. C. & Bedoui, S. Tissue-resident memory T cells in tissue homeostasis, persistent infection, and cancer surveillance. Immunol. Rev. 283, 54–76 (2018).
Thome, J. J. & Farber, D. L. Emerging concepts in tissue-resident T cells: lessons from humans. Trends Immunol. 36, 428–435 (2015).
Clark, R. A. Resident memory T cells in human health and disease. Sci. Transl. Med. 7, 269rv1 (2015).
Mackay, L. K. et al. The developmental pathway for CD103+CD8+ tissue-resident memory T cells of skin. Nat. Immunol. 14, 1294–1301 (2013).
Sathaliyawala, T. et al. Distribution and compartmentalization of human circulating and tissue-resident memory T cell subsets. Immunity 38, 187–197 (2013).
Kumar, B. V. et al. Human tissue-resident memory T cells are defined by core transcriptional and functional signatures in lymphoid and mucosal sites. Cell Rep. 20, 2921–2934 (2017).
Wu, H. et al. Pathogenic role of tissue-resident memory T cells in autoimmune diseases. Autoimmun. Rev. 17, 906–911 (2018).
Park, C. O. & Kupper, T. S. The emerging role of resident memory T cells in protective immunity and inflammatory disease. Nat. Med. 21, 688–697 (2015).
Matos, T. R. et al. Clinically resolved psoriatic lesions contain psoriasis-specific IL-17-producing αβ T cell clones. J. Clin. Invest. 127, 4031–4041 (2017).
Edwards, J. et al. CD103+ tumor-resident CD8+ T cells are associated with improved survival in immunotherapy-naive melanoma patients and expand significantly during anti-PD-1 treatment. Clin. Cancer Res. 24, 3036–3045 (2018).
Webb, J. R., Milne, K., Watson, P., Deleeuw, R. J. & Nelson, B. H. Tumor-infiltrating lymphocytes expressing the tissue resident memory marker CD103 are associated with increased survival in high-grade serous ovarian cancer. Clin. Cancer Res. 20, 434–444 (2014).
Djenidi, F. et al. CD8+CD103+ tumor-infiltrating lymphocytes are tumor-specific tissue-resident memory T cells and a prognostic factor for survival in lung cancer patients. J. Immunol. 194, 3475–3486 (2015).
Ganesan, A. P. et al. Tissue-resident memory features are linked to the magnitude of cytotoxic T cell responses in human lung cancer. Nat. Immunol. 18, 940–950 (2017).
Duhen, T. et al. Co-expression of CD39 and CD103 identifies tumor-reactive CD8 T cells in human solid tumors. Nat. Commun. 9, 2724 (2018).
Ariotti, S. et al. Tissue-resident memory CD8+ T cells continuously patrol skin epithelia to quickly recognize local antigen. Proc. Natl Acad. Sci. USA 109, 19739–19744 (2012).
Zaid, A. et al. Persistence of skin-resident memory T cells within an epidermal niche. Proc. Natl Acad. Sci. USA 111, 5307–5312 (2014).
Beura, L. K. et al. Intravital mucosal imaging of CD8+ resident memory T cells shows tissue-autonomous recall responses that amplify secondary memory. Nat. Immunol. 19, 173–182 (2018).
Park, S. L. et al. Local proliferation maintains a stable pool of tissue-resident memory T cells after antiviral recall responses. Nat. Immunol. 19, 183–191 (2018).
Ariotti, S. et al. Skin-resident memory CD8+ T cells trigger a state of tissue-wide pathogen alert. Science 346, 101–105 (2014).
Cheuk, S. et al. CD49a expression defines tissue-resident CD8+ T cells poised for cytotoxic function in human skin. Immunity 46, 287–300 (2017).
Mort, R. L., Hay, L. & Jackson, I. J. Ex vivo live imaging of melanoblast migration in embryonic mouse skin. Pigment Cell Melanoma Res. 23, 299–301 (2010).
Masters, B. R. et al. Mitigating thermal mechanical damage potential during two-photon dermal imaging. J. Biomed. Opt. 9, 1265–1270 (2004).
Li, J. L. et al. Intravital multiphoton imaging of immune responses in the mouse ear skin. Nat. Protoc. 7, 221–234 (2012).
Ng, L. G. et al. Visualizing the neutrophil response to sterile tissue injury in mouse dermis reveals a three-phase cascade of events. J. Invest. Dermatol. 131, 2058–2068 (2011).
Pasparakis, M., Haase, I. & Nestle, F. O. Mechanisms regulating skin immunity and inflammation. Nat. Rev. Immunol. 14, 289–301 (2014).
Larsen, C. P. et al. Migration and maturation of Langerhans cells in skin transplants and explants. J. Exp. Med. 172, 1483–1493 (1990).
Clark, R. A. et al. The vast majority of CLA+ T cells are resident in normal skin. J. Immunol. 176, 4431–4439 (2006).
Watanabe, R. et al. Human skin is protected by four functionally and phenotypically discrete populations of resident and recirculating memory T cells. Sci. Transl. Med. 7, 279ra239 (2015).
Pozzi, A., Yurchenco, P. D. & Iozzo, R. V. The nature and biology of basement membranes. Matrix Biol. 57–58, 1–11 (2017).
Schenkel, J. M. et al. Resident memory CD8 T cells trigger protective innate and adaptive immune responses. Science 346, 98–101 (2014).
McNamara, H. A. et al. Up-regulation of LFA-1 allows liver-resident memory T cells to patrol and remain in the hepatic sinusoids. Sci. Immunol. 2, eaaj1996 (2017).
Cepek, K. L. et al. Adhesion between epithelial cells and T lymphocytes mediated by E-cadherin and the αEβ7 integrin. Nature 372, 190–193 (1994).
Cheuk, S. et al. Epidermal Th22 and Tc17 cells form a localized disease memory in clinically healed psoriasis. J. Immunol. 192, 3111–3120 (2014).
Shirshin, E. A. et al. Two-photon autofluorescence lifetime imaging of human skin papillary dermis in vivo: assessment of blood capillaries and structural proteins localization. Sci. Rep. 7, 1171 (2017).
Omelyanenko, N. P., Slutsky, L. I. & Mironov, S. P. Connective Tissue: Histophysiology, Biochemistry, Molecular Biology (Taylor & Francis, 2013).
Oakford, M. E. et al. Migration of immunocytes across the basement membrane in skin: the role of basement membrane pores. J. Invest. Dermatol. 131, 1950–1953 (2011).
Bins, A. D. et al. A rapid and potent DNA vaccination strategy defined by in vivo monitoring of antigen expression. Nat. Med. 11, 899–904 (2005).
Oosterhuis, K., Aleyd, E., Vrijland, K., Schumacher, T. N. & Haanen, J. B. Rational design of DNA vaccines for the induction of human papillomavirus type 16 E6- and E7-specific cytotoxic T-cell responses. Hum. Gene Ther. 23, 1301–1312 (2012).
Antos, J. M. et al. Site-specific protein labeling via sortase-mediated transpeptidation. Curr. Protoc. Protein Sci. 89, 15.3.1–15.3.9 (2017).
Stronen, E. et al. Targeting of cancer neoantigens with donor-derived T cell receptor repertoires. Science 352, 1337–1341 (2016).
Kvistborg, P. et al. Anti-CTLA-4 therapy broadens the melanoma-reactive CD8+ T cell response. Sci. Transl. Med. 6, 254ra128 (2014).
Beltman, J. B., Maree, A. F. & de Boer, R. J. Analysing immune cell migration. Nat. Rev. Immunol. 9, 789–798 (2009).
Fürth, R. Die brownsche Bewegung bei Berücksichtigung einer Persistenz der Bewegungsrichtung. Mit Anwendungen auf die Bewegung lebender In- fusorien. [Brownian motion under consideration of a persistence of the direction of movement. With applications to the movement of living Infusoria.]. Z. Phys. 2, 244–256 (1920).
Schindelin, J. et al. Fiji: an open-source platform for biological-image analysis. Nat. Methods 9, 676–682 (2012).
Acknowledgements
Plasmid sequences for anti-mouse and anti-human CD8 nanobodies were kindly provided by 121Bio with support of M. Gostissa and G. Grotenbreg (Agenus subsequently acquired substantially all the assets of 121Bio). We thank H. Ploegh (Harvard University) for providing the sortase expression vector and D. Elatmioui and H. Ovaa (LUMC) for the GGGC peptide. We would like to acknowledge P.G.L. Koolen (Rode Kruis Ziekenhuis), T. Venema (Slotervaart Ziekenhuis) and W.G. van Selms (Onze Lieve Vrouwe Gasthuis West) and staff of the plastic surgery departments for providing human skin tissue. We thank M. Hoekstra for illustrations, M. Willemsen, L. Brocks, L. Oomen, T. Rademakers, the NKI flow cytometry and animal facility for technical support, and S. Ariotti and members of the Schumacher and Haanen laboratories for discussions. This work was supported by an ΕΑDV Research Fellowship (to T.R.M.) and ERC AdG Life-His-T (to T.N.S.).
Author information
Authors and Affiliations
Contributions
F.E.D. performed experiments and analyzed data. M.H. performed multiphoton imaging and J.B.B. analyzed migration parameters. M.T. produced fluorescently labeled nanobodies and performed in vitro T cell activation experiments. F.E.D., M.M. and B.v.d.B. designed imaging analysis. J.-Y.S. evaluated immunohistochemistry data. T.R.M. and M.B.M.T. organized human skin material. F.E.D., T.R.M., M.H., M.T., D.W.V., M.B.M.T., R.M.L., J.B.B. and T.N.S. contributed to experimental design. F.E.D., J.B.B. and T.N.S. prepared the manuscript with input from all co-authors.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Integrated supplementary information
Supplementary Figure 1 Validation of ex vivo imaging technology.
a, Estimated motility coefficient (left) and estimated persistence time (middle), with error bars indicating 95% confidence interval (the range q0.025-q0.975) based on bootstrapping of the data for in vivo (n = 3 mice) and ex vivo (n = 3 mice) murine skin-resident TRM cells (black dots represent median). Right: black dots indicate median turning angles of individual tracks for in vivo (n = 208) and ex vivo (n = 177) murine skin-resident TRM cells (n = 3 mice each), with red lines indicating medians of all tracks. b, Top: confocal maximum intensity projections of OT-I-GFP TRM cells in skin of C57BL/6 albino mice recorded in vivo (left) or ex vivo (middle) and multiphoton (MP) section view of OT-I-GFP TRM cells in skin of C57BL/6 albino mouse recorded ex vivo (right; SHG, blue). Images are representative of n = 3 mice per condition. Scale bars indicate 50 μm and 10 μm for overviews and zoomed images, respectively. Bottom: plots of mean square displacement (μm2) versus time (min) using a 15 min time window to estimate motility coefficient (M) and persistence time (Pt) (individual mice are shown in different colors, dots represent data points and lines the best fits). Data are based on 1–4 h recordings, n = 3 mice. c, Speeds of individual movement steps over time (black dots) of representative confocal (top) or MP (bottom) imaging of ex vivo OT-I-GFP TRM cells in skin of C57BL/6 black (left) or albino mice (right). Red line indicates running average of 10 movement steps. d, Median speed of individual tracks of gBT-GFP (green dots, pre: n = 110, post: n = 146) and OT-I-mTmG (red dots, pre: n = 75, post: n = 56) ex vivo skin-resident TRM cells before and after OVA257-264 peptide addition. Black line indicates median of all tracks. Data is based on n = 3 mice, based on 1-2 h recordings. In a and d, the statistical test performed is a two-tailed Mann-Whitney U-test, and FD indicates fold difference.
Supplementary Figure 2 Validation of anti-mCD8 nanobody labeling of ex vivo murine skin-resident TRM cells.
a, Confocal maximum intensity projection of OT-I-GFP (green) T cells stained with anti-hCD8-AF594 (red) nanobody. Scale bar indicates 50 μm. Representative of n = 2 mice. b, Top: ex vivo (left, median speed, n = 185; right, turning angle, n = 177) and ex vivo nanobody labeled (left, median speed, n = 107; right, turning angle, n = 102) skin-resident TRM cells. Black dots represent individual tracks, red lines indicate median of cell population. Statistical differences were determined using a two-tailed Mann-Whitney U-test. FD indicates fold difference. Bottom: estimated motility coefficient (left) and persistence time (right) for ex vivo and ex vivo nanobody labeled skin-resident TRM cells. Error bars indicate 95% confidence interval (the range q0.025-q0.975) based on bootstrapping of the data (dots represent median) (n = 3 mice, 4 h-recordings). c, Representative confocal maximum projection of OT-I-GFP TRM cells (green) stained with anti-mCD8-AF594 (red). White arrow indicates direction of movement of cell for 6 migration steps. Note that AF594 signal is enriched at the rear side of the cell. Scale bar indicates 10 μm, based on n = 3 mice. d, Top: H&E staining of murine tissue fixed either directly after harvest (t = 0, left), after ex vivo nanobody labeling and a 4 h-confocal imaging session performed within 24 h after mounting (middle), or after ex vivo nanobody labeling and ex vivo culture for 72 h (right). Scale bar indicates 100 μm. Representative of n = 4 mice per condition. Bottom: enumeration of GFP+ cells by IHC of tissue fixed after indicated treatments. Symbols represent individual mice, solid red line indicates median, dashed red line indicates median at t = 0, n = 4 mice. e, Left: histogram of unstimulated OT-I cells showing specific staining of the anti-mCD8-AF594 nanobody. Cells are gated on single/live/CD8β+ lymphocytes. Middle: exemplary flow cytometry plot of T cells stimulated with 10 nM OVA257-264 peptide. Right: Percentage of CD25+CD69+ cells of unlabeled (gray), anti-hCD8-AF594 (black) or anti-mCD8-AF594 (red) labeled T cells upon in vitro overnight stimulation with indicated amounts of OVA257-264 peptide. Cells are gated on single/live/CD8β+Vβ5+ lymphocytes. Symbols indicate technical replicates, lines connect means. Data are representative of 2 experiments.
Supplementary Figure 3 Validation of anti-hCD8 nanobody labeling of ex vivo human skin-resident TRM cells.
a, Four left plots: percentage of CD137+ cells of unlabeled (gray), a-mCD8-AF594 labeled (black) or a-hCD8-AF594 (red) labeled TCR-transduced CD8+ T cells (clone #8, left; clone #17, right) in response to in vitro overnight stimulation with indicated peptide-loaded cells. Symbols indicate technical replicates, lines connect means. Top right: histogram of unstimulated cells showing specific staining of the a-hCD8-AF594 nanobody. Cells are gated on single/live/CD8+ lymphocytes. Bottom right: exemplary histogram of CD137+ T cells stimulated with 10 nM CDK4mut peptide. Cells are gated on single/live/CD8+mTCRβ+ lymphocytes. Data of clone #8 is representative of 1 and clone #17 of 2 experiments. b, Representative flow cytometry plot (of n = 2 individuals) of ex vivo human tissue stained in situ with anti-hCD8 nanobody that was subsequently digested into single cell suspensions of epidermis and dermis, and then stained with anti-hCD8 antibody. Cells are gated on single/live lymphocytes. c, Speeds of individual movement steps during a representative 4 h-recording of human ex vivo skin-resident TRM cells (red line indicates the running average of 10 movement steps). d, Mean square displacement (μm2) versus time (min) plot using a 60 min time window to estimate motility coefficient (M) and persistence time (Pt) of the indicated conditions (individual replicates are shown in different colors, dots represent data points and lines the best fits) (based on 3.5-4 h recordings). e, Top: box and whiskers (representing 1.5 times the interquartile range) plot showing distribution of speed-steps of dermal CD8+ TRM cells in collagen type I-poor (‘SHG–’, n = 887) or -rich (‘SHG+’, n = 4372) areas (n = 4 individuals). Low and high ends of the box represent the 0.25 (q0.25; that is, the speed where 25% of observations lies below and 75% of observations above) and 0.75 (q0.75) quantile and the line in between represents the median; lower whisker represents q0.25—1.5 * IQR (interquartile range) and upper whisker q0.75 + 1.5 * IQR; black dots represent outliers. Note that the distribution of the SHG– speed-steps is more skewed than that of the SHG+ steps (for example, q0.75 is higher in the SHG-group). Bottom: estimated 95% confidence interval (CI) for q0.75 based on bootstrapping of the data (n = 4 individuals), showing that the speed distributions of SHG– and SHG+ groups are different, as indicated by non-overlapping CIs. Dots represent medians of all bootstrapped q0.75 values and error bars represent 95% CIs (the range q0.025-q0.975). 95% CIs for 0.25 and 0.5 quantiles were also determined and resulted in smaller differences between CIs of SHG– and SHG+ (data not shown).
Supplementary Figure 4 Gating strategies of murine and human T cells.
a, Analysis of in vitro stimulated murine T cells. Panel shows a sample of unlabeled OT-I splenocytes after overnight culture with 100 pM OVA257-264 peptide. Lymphocyte population was selected by FSC-A/SSC-A gating. Doublets were then gated out by FSC-A/FSC-H and additional gating on SSC-A/SSC-H. Dead cells were excluded by gating on DAPI– cells. CD8+ OT-I T cells were then gated out on CD8b and Vb5 positivity. Subsequently, reactive T cells were gated out by selecting CD25 and CD69 double positive cells (quantification plotted in Supplementary Fig. 2e) (representative of 2 experiment). b, Analysis of in vitro stimulated human T cells. Panel shows a sample of unlabeled CDK4mut reactive TCR clone #8 T cells after overnight coculture with 10 nM CDK4mut-peptide loaded JI cells. Lymphocyte population was selected by FSC-A/SSC-A gating. Doublets were then gated out by FSC-A/FSC-H and additional gating on SSC-A/SSC-H. Dead cells were excluded by gating on IR– cells. TCR modified cells were then gated out based on mTCRb and CD8 positivity. Subsequently, reactive cells were selected by positivity for CD137 (quantification plotted in Supplementary Fig. 3a) (representative of 1 experiment). c, Analysis of human CD8+ skin-resident TRM cells. Panel shows a single cell suspension of the epidermis. Lymphocyte population was selected by FSC-A/SSC-A gating. Doublets were then gated out by FSC-A/FSC-H and additional gating on SSC-A/SSC-H. Dead cells were excluded by gating on IR– cells. Cells were subsequently gated on CD8, followed by CD69 versus CD103 (quantification plotted in Fig. 3c) (representative of n = 3 individuals).
Supplementary information
Supplementary Information
Supplementary Figures 1–4 and Supplementary Table 1
Supplementary Video 1
Murine skin-resident TRM cell migration in vivo. Confocal maximum projection depicting migration of OT-I–GFP CD8+ TRM cells (green) in the skin of C57BL/6J mice in vivo (representative of n = 3 mice).
Supplementary Video 2
Murine skin-resident TRM cell migration ex vivo. Confocal maximum projection depicting migration of OT-I–GFP CD8+ TRM cells (green) in skin of C57BL/6J mice ex vivo (air exposed) (representative of n = 3 mice).
Supplementary Video 3
Murine skin-resident TRM cell migration ex vivo (non air exposed). Confocal maximum projection depicting lack of migration of OT-I–GFP CD8+ TRM cells (green) in the skin of C57BL/6J mice ex vivo (non air exposed) (representative of n = 1 mouse).
Supplementary Video 4
CD8+ TRM cell migration in murine albino skin in confocal imaging. Confocal maximum projection depicting migration of OT-I–GFP CD8+ TRM cells (green) in the skin of C57BL/6J albino mice, either in vivo (first segment), or ex vivo (second segment) (both representative of n = 3 mice).
Supplementary Video 5
CD8+ TRM cell migration in murine albino skin in MP imaging. MP perspective top view depicting migration of OT-I–GFP CD8+ TRM cells (white dots indicate cells, green stripes indicate dragon tail tracks) in the skin of C57BL/6J albino mice ex vivo (SHG signal is depicted in blue) (representative of n = 3 mice).
Supplementary Video 6
OVA257–264 recognition by OT-I–mTmG and not by gBT–GFP CD8+ skin-resident TRM cells ex vivo. First segment: confocal maximum projection depicting OT-I–mTmG (red) and gBT–GFP (green) cells showing steady-state patrolling behavior. Second segment: confocal maximum projection showing the rounding up and stalling of OT-I–mTmG skin-resident TRM cells after OVA257–264 addition, whereas gBT–GFP cells continue to patrol the skin. Green and red double-positive structures indicate autofluorescent hairs (representative of n = 3 mice).
Supplementary Video 7
OVA257–264 recognition by anti-mCD8 nanobody-labeled OT-I–GFP CD8+ TRM cells ex vivo. First segment: confocal maximum projection depicting anti-mCD8 nanobody single-positive (red) and OT-I–GFP skin-resident TRM cells (green) co-stained with anti-mCD8 nanobody that show steady-state patrolling behavior. Second segment: confocal maximum projection showing the rounding up and stalling of anti-mCD8 nanobody stained OTI–GFP skin-resident TRM cells after OVA257–264 addition, whereas an anti-mCD8 nanobody single-positive cell continues to migrate throughout the recording (representative of n = 2 mice).
Supplementary Video 8
Migration of CD8+ TRM cells in healthy human skin tissue. MP perspective top view of anti-hCD8 nanobody-labeled CD8+ skin-resident TRM cells (red; white dots indicate cells, red stripes indicate dragon tail tracks) migrating in full-thickness healthy human skin biopsy; SHG indicates collagen type I positive dermis (blue) (representative of n = 4 individuals).
Supplementary Video 9
Migration of human CD8+ TRM cells through surrounding epidermal cells. MP recordings showing a detailed and overview section view of anti-hCD8 nanobody-labeled CD8+ skin-resident TRM cells (red) migrating amid surrounding nucleated epidermal skin cells (Hoechst, gray; SHG, blue) (representative of n = 4 individuals).
Supplementary Video 10
Migration of human epidermal CD8+ TRM cells along dermal projections and in close proximity to basement membrane. First segment: 3D surface rendering of an MP recording of an anti-hCD8 nanobody-labeled CD8+ skin-resident TRM cell (red) migrating on top of finger-like dermal projections (SHG, blue) (representative of n = 4 individuals). Second segment: section view and 3D surface rendering of an MP recording of an anti-hCD8 nanobody-labeled CD8+ skin-resident TRM cell (red) migrating in close proximity to basement membrane (collagen type IV, green) (SHG, blue) (representative of n = 3 individuals).
Supplementary Video 11
Migration of epidermal CD8+CD103− and CD8+CD103+ TRM cells in human skin. MP section view depicting anti-hCD8 nanobody-labeled CD8+ skin-resident TRM cells (red), either negative or positive for CD103 (green), migrating through the tissue (SHG, blue) (representative of n = 3 individuals).
Supplementary Video 12
Migration of human CD8+ TRM cells below sessile Langerhans cells. First segment: MP section view depicting anti-hCD8 nanobody-labeled CD8+ skin-resident TRM cells (red) migrating in dermis (SHG, blue) and in epidermis below sessile Langerhans cells (CD1a+, green) (representative of n = 4 individuals). Second segment: 3D surface rendering; third and fourth segments: section view of CD8+ TRM cells migrating in close proximity to Langerhans cells (representative of n = 3 individuals).
Supplementary Video 13
Migration of human dermal CD8+ TRM cells along the perimeter of collagen type I-poor areas. First segment: MP virtual sectioning; second segment: 3D surface rendering; third segment: perspective top view depicting anti-hCD8 nanobody-labeled CD8+ skin-resident TRM cells (red) migrating along perimeter of a collagen type I-poor area (SHG, blue). Cylinders indicate tracks (representative of n = 4 individuals).
Supplementary Video 14
Migration of human CD8+ TRM cells along dermal vessels. First segment: MP perspective view animation of full-thickness skin biopsy showing anti-hCD8 nanobody-positive TRM cells (red), and also depicting collagen type IV (green) and SHG (blue). Note that collagen type I-poor areas contain basement membrane-positive vessels. Third segment: 3D surface rendering; second and fourth segments: section view depicting anti-hCD8 nanobody-labeled CD8+ (red) skin-resident TRM cells migrating along collagen type IV positive vessels (representative of n = 3 individuals).
Supplementary Video 15
Migration of human CD8+ TRM cells in and out of the dermal compartment. 3D surface renderings and perspective side view depicting anti-hCD8 nanobody-labeled CD8+ TRM cells (red) migrating in and out of collagen type I-positive areas (SHG, blue) (representative of n = 3 individuals).
Rights and permissions
About this article
Cite this article
Dijkgraaf, F.E., Matos, T.R., Hoogenboezem, M. et al. Tissue patrol by resident memory CD8+ T cells in human skin. Nat Immunol 20, 756–764 (2019). https://doi.org/10.1038/s41590-019-0404-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41590-019-0404-3
This article is cited by
-
FGL2-targeting T cells exhibit antitumor effects on glioblastoma and recruit tumor-specific brain-resident memory T cells
Nature Communications (2023)
-
Persistent virus-specific and clonally expanded antibody-secreting cells respond to induced self-antigen in the CNS
Acta Neuropathologica (2023)
-
New definitions of human lymphoid and follicular cell entities in lymphatic tissue by machine learning
Scientific Reports (2022)
-
The precursors of CD8+ tissue resident memory T cells: from lymphoid organs to infected tissues
Nature Reviews Immunology (2022)
-
Human T lymphocytes at tumor sites
Seminars in Immunopathology (2022)