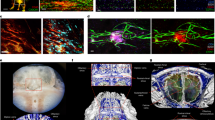
Abstract
Meningeal lymphatics near the cribriform plate undergo lymphangiogenesis during neuroinflammation to drain excess fluid. Here, we hypothesized that lymphangiogenic vessels may acquire an altered phenotype to regulate immunity. Using single-cell RNA sequencing of meningeal lymphatics near the cribriform plate from healthy and experimental autoimmune encephalomyelitis in the C57BL/6 model, we report that neuroinflammation induces the upregulation of genes involved in antigen presentation such as major histocompatibility complex class II, adhesion molecules including vascular cell adhesion protein 1 and immunoregulatory molecules such as programmed cell death 1 ligand 1, where many of these changes are mediated by interferon-γ. The inflamed lymphatics retain CD11c+ cells and CD4 T cells where they capture and present antigen, creating an immunoregulatory niche that represents an underappreciated interface in the regulation of neuroinflammation. We also found discontinuity of the arachnoid membrane near the cribriform plate, which provides unrestricted access to the cerebrospinal fluid. These findings highlight a previously unknown function of local meningeal lymphatics in regulating immunity that has only previously been characterized in draining lymph nodes.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout







Similar content being viewed by others
Data availability
The data generated for this study are deposited at the Gene Expression Omnibus under GSE175802. Source data are are provided with this paper.
References
Aspelund, A. et al. A dural lymphatic vascular system that drains brain interstitial fluid and macromolecules. J. Exp. Med. 212, 991–999 (2015).
Louveau, A. et al. Structural and functional features of central nervous system lymphatic vessels. Nature 523, 337–341 (2015).
Ahn, J. H. et al. Meningeal lymphatic vessels at the skull base drain cerebrospinal fluid. Nature 572, 62–66 (2019).
Hsu, M. et al. Neuroinflammation-induced lymphangiogenesis near the cribriform plate contributes to drainage of CNS-derived antigens and immune cells. Nat. Commun. 10, 229 (2019).
Louveau, A. et al. CNS lymphatic drainage and neuroinflammation are regulated by meningeal lymphatic vasculature. Nat. Neurosci. 21, 1380–1391 (2018).
De Mesquita, S. et al. Functional aspects of meningeal lymphatics in ageing and Alzheimer’s disease. Nature 560, 185–191 (2018).
Bolte, A. C. et al. Meningeal lymphatic dysfunction exacerbates traumatic brain injury pathogenesis. Nat. Commun. 11, 4524 (2020).
Hu, X. et al. Meningeal lymphatic vessels regulate brain tumor drainage and immunity. Cell Res. 30, 229–243 (2020).
Song, E. et al. VEGF-C-driven lymphatic drainage enables immunosurveillance of brain tumours. Nature 577, 689–694 (2020).
Xiang, M. et al. A single-cell transcriptional roadmap of the mouse and human lymph node lymphatic vasculature. Front. Cardiovasc. Med. 7, 52 (2020).
Takeda, A. et al. Single-cell survey of human lymphatics unveils marked endothelial cell heterogeneity and mechanisms of homing for neutrophils. Immunity 51, 561–572.e5 (2019).
Tewalt, E. F. et al. Lymphatic endothelial cells induce tolerance via PD-L1 and lack of costimulation leading to high-level PD-1 expression on CD8 T cells. Blood 120, 4772–4782 (2012).
Tewalt, E. F., Cohen, J. N., Rouhani, S. J. & Engelhard, V. H. Lymphatic endothelial cells—key players in regulation of tolerance and immunity. Front. Immunol. 3, 305 (2012).
Tamburini, B. A., Burchill, M. A. & Kedl, R. M. Antigen capture and archiving by lymphatic endothelial cells following vaccination or viral infection. Nat. Commun. 5, 3989 (2014).
Lucas, E. D. et al. Type 1 IFN and PD-L1 coordinate lymphatic endothelial cell expansion and contraction during an inflammatory immune response. J. Immunol. 201, 1735–1747 (2018).
Rouhani, S. J. et al. Roles of lymphatic endothelial cells expressing peripheral tissue antigens in CD4 T-cell tolerance induction. Nat. Commun. 6, 6771 (2015).
Lucas, E. D. & Tamburini, B. A. J. Lymph node lymphatic endothelial cell expansion and contraction and the programming of the immune response. Front. Immunol. 10, 36 (2019).
Santambrogio, L., Berendam, S. J. & Engelhard, V. H. The antigen processing and presentation machinery in lymphatic endothelial cells. Front. Immunol. 10, 1033 (2019).
Herz, J., Louveau, A., Da Mesquita, S. & Kipnis, J. Morphological and functional analysis of CNS-associated lymphatics. Methods Mol. Biol. 1846, 141–151 (2018).
Kretschmer, S. et al. Autofluorescence multiphoton microscopy for visualization of tissue morphology and cellular dynamics in murine and human airways. Lab. Invest. 96, 918–931 (2016).
Maruyama, K. et al. Inflammation-induced lymphangiogenesis in the cornea arises from CD11b-positive macrophages. J. Clin. Invest. 115, 2363–2372 (2005).
Zumsteg, A. et al. Myeloid cells contribute to tumor lymphangiogenesis. PLoS ONE 4, e7067 (2009).
Kerjaschki, D. The crucial role of macrophages in lymphangiogenesis. J. Clin. Invest. 115, 2316–2319 (2005).
Trapnell, C. et al. The dynamics and regulators of cell fate decisions are revealed by pseudotemporal ordering of single cells. Nat. Biotechnol. 32, 381–386 (2014).
Qiu, X. et al. Single-cell mRNA quantification and differential analysis with Census. Nat. Methods 14, 309–315 (2017).
Qiu, X. et al. Reversed graph embedding resolves complex single-cell trajectories. Nat. Methods 14, 979–982 (2017).
Volk-Draper, L. D., Hall, K. L., Wilber, A. C. & Ran, S. Lymphatic endothelial progenitors originate from plastic myeloid cells activated by Toll-like receptor-4. PLoS ONE 12, e0179257 (2017).
Ran, S. & Volk-Draper, L. Lymphatic endothelial cell progenitors in the tumor microenvironment. Adv. Exp. Med. Biol. 1234, 87–105 (2020).
Giladi, A. et al. Dissecting cellular crosstalk by sequencing physically interacting cells. Nat. Biotechnol. 38, 629–637 (2020).
Bendall, S. C. Diamonds in the doublets. Nat. Biotechnol. 38, 559–561 (2020).
Paddock, C., Zhou, D., Lertkiatmongkol, P., Newman, P. J. & Zhu, J. Structural basis for PECAM-1 homophilic binding. Blood 127, 1052–1061 (2016).
Harris, M. G. et al. Immune privilege of the CNS is not the consequence of limited antigen sampling. Sci. Rep. 4, 4422 (2014).
Rayasam, A. et al. Regional distribution of CNS antigens differentially determines T-cell mediated neuroinflammation in a CX3CR1-dependent manner. J. Neurosci. 38, 7058–7071 (2018).
Marelli-Berg, F. M., Clement, M., Mauro, C. & Caligiuri, G. An immunologist’s guide to CD31 function in T-cells. J. Cell Sci. 126, 2343–2352 (2013).
Acton, S. E. et al. Podoplanin-rich stromal networks induce dendritic cell motility via activation of the C-type lectin receptor CLEC-2. Immunity 37, 276–289 (2012).
Johnson, L. A. et al. Dendritic cells enter lymph vessels by hyaluronan-mediated docking to the endothelial receptor LYVE-1. Nat. Immunol. 18, 762–770 (2017).
Torzicky, M. et al. Platelet endothelial cell adhesion molecule-1 (PECAM-1/CD31) and CD99 are critical in lymphatic transmigration of human dendritic cells. J. Invest. Dermatol. 132, 1149–1157 (2012).
Fletcher, J. M., Lalor, S. J., Sweeney, C. M., Tubridy, N. & Mills, K. H. G. T cells in multiple sclerosis and experimental autoimmune encephalomyelitis. Clin. Exp. Immunol. 162, 1–11 (2010).
Astarita, J. L., Acton, S. E. & Turley, S. J. Podoplanin: emerging functions in development, the immune system, and cancer. Front. Immunol. 3, 283 (2012).
Lane, R. S. et al. IFNγ-activated dermal lymphatic vessels inhibit cytotoxic T cells in melanoma and inflamed skin. J. Exp. Med. 215, 3057–3074 (2018).
Sosa, R. A., Murphey, C., Robinson, R. R. & Forsthuber, T. G. IFN-γ ameliorates autoimmune encephalomyelitis by limiting myelin lipid peroxidation. Proc. Natl Acad. Sci. USA 112, E5038–E5047 (2015).
Arellano, G., Ottum, P. A., Reyes, L. I., Burgos, P. I. & Naves, R. Stage-specific role of interferon-gamma in experimental autoimmune encephalomyelitis and multiple sclerosis. Front. Immunol. 6, 492 (2015).
Yao, S. et al. PD-1 on dendritic cells impedes innate immunity against bacterial infection. Blood 113, 5811–5818 (2009).
Proulx, S. T. Cerebrospinal fluid outflow: a review of the historical and contemporary evidence for arachnoid villi, perineural routes, and dural lymphatics. Cell. Mol. Life Sci. 78, 2429–2457 (2021).
Weller, R. O., Sharp, M. M., Christodoulides, M., Carare, R. O. & Møllgård, K. The meninges as barriers and facilitators for the movement of fluid, cells and pathogens related to the rodent and human CNS. Acta Neuropathol. 135, 363–385 (2018).
Norwood, J. N. et al. Anatomical basis and physiological role of cerebrospinal fluid transport through the murine cribriform plate. eLife 8, e44278 (2019).
Rustenhoven, J. et al. Functional characterization of the dural sinuses as a neuroimmune interface. Cell 184, 1000–1016.e27 (2021).
Russo, E., Nitschké, M. & Halin, C. Dendritic cell interactions with lymphatic endothelium. Lymphat. Res. Biol. 11, 172–182 (2013).
Clarkson, B. D. et al. CCR7 deficient inflammatory dendritic cells are retained in the central nervous system. Sci. Rep. 7, 42856 (2017).
Bianchi, R. et al. Postnatal deletion of podoplanin in lymphatic endothelium results in blood filling of the lymphatic system and impairs dendritic cell migration to lymph nodes. Arterioscler. Thromb. Vasc. Biol. 37, 108–117 (2017).
Johnson, L. A. et al. An inflammation-induced mechanism for leukocyte transmigration across lymphatic vessel endothelium. J. Exp. Med. 203, 2763–2777 (2006).
Rinaldi, E. & Baggi, F. LYVE-1 is ‘on stage’ now: an emerging player in dendritic cell docking to lymphatic endothelial cells. Cell. Mol. Immunol. 15, 663–665 (2018).
Keir, M. E., Butte, M. J., Freeman, G. J. & Sharpe, A. H. PD-1 and its ligands in tolerance and immunity. Annu. Rev. Immunol. 26, 677–704 (2008).
Park, S. J. et al. Negative role of inducible PD-1 on survival of activated dendritic cells. J. Leukoc. Biol. 95, 621–629 (2014).
Krempski, J. et al. Tumor-infiltrating programmed death receptor-1+ dendritic cells mediate immune suppression in ovarian cancer. J. Immunol. 186, 6905–6913 (2011).
Lim, T. S. et al. PD-1 expression on dendritic cells suppresses CD8+ T cell function and antitumor immunity. Oncoimmunology 5, e1085146 (2016).
Palle, P., Monaghan, K. L., Milne, S. M. & Wan, E. C. K. Cytokine signaling in multiple sclerosis and its therapeutic applications. Med. Sci. (Basel) 5, 23 (2017).
Clarkson, B. D. et al. CCR2-dependent dendritic cell accumulation in the central nervous system during early effector experimental autoimmune encephalomyelitis is essential for effector T cell restimulation in situ and disease progression. J. Immunol. 194, 531–541 (2015).
Hauglund, N. L., Kusk, P., Kornum, B. R. & Nedergaard, M. Meningeal lymphangiogenesis and enhanced glymphatic activity in mice with chronically implanted EEG electrodes. J. Neurosci. 40, 2371–2380 (2020).
Hsu, M., Sandor, M. & Fabry, Z. Current concepts on communication between the central nervous system and peripheral immunity via lymphatics: what roles do lymphatics play in brain and spinal cord disease pathogenesis? Biol. Futur. 72, 45–60 (2021).
Hsu, M., Laaker, C., Sandor, M. & Fabry, Z. Neuroinflammation-driven lymphangiogenesis in CNS diseases. Front. Cell. Neurosci. 15, 683676 (2021).
Raghunandan, A. et al. Bulk flow of cerebrospinal fluid observed in periarterial spaces is not an artifact of injection. eLife 10, e65958 (2021).
Smith, T., Heger, A. & Sudbery, I. UMI-tools: modeling sequencing errors in unique molecular identifiers to improve quantification accuracy. Genome Res. 27, 491–499 (2017).
Yu, G., Wang, L.-G., Han, Y. & He, Q.-Y. clusterProfiler: an R package for comparing biological themes among gene clusters. OMICS 16, 284–287 (2012).
Acknowledgements
We thank K. Maclivay for his expertise in flow cytometry, L. Schmitt-Brunold for her expertise in molecular biology and all members of our laboratory for insightful discussions and constructive criticisms of this work. We thank members of the University of Wisconsin Flow Core Facility for their assistance and expertise in FACS sorting, T. Duellman and S. Splinter BonDurant for their expertise and assistance with scRNA-seq and B. Rauch and E. Meyerand at the UW Small Animal Imaging Facility supported by a UWCCC grant no. P30 CA014520 for the use of its facilities and services. This work was supported by National Institutes of Health grant nos. NS108497 and NS103506 awarded to Z.F., grant no. HL128778 awarded to M.S., a Neuroscience Training Program grant no. T32-GM007507 to M.H. and C.L. and a 10X Genomics Pilot grant to Z.F.
Author information
Authors and Affiliations
Contributions
M.H., M.S. and Z.F. conceptualized the experiments. M.H. performed the experiments, generated the figures, analyzed the data and wrote the manuscript. A.M. performed the analysis and assisted in generating the figures from the scRNA-seq data. C.L. assisted with FACS for scRNA-seq and manuscript writing. C.L. and ME.H. (Melinda Herbath) assisted with the in vitro coculture experiments. Y.H.C. assisted with the MRI experiments. All authors edited the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Immunology thanks Britta Engelhardt and the other, anonymous, reviewer(s) for their contribution to the peer review of this work. Laurie Dempsey was the primary editor(s) on this article and managed its editorial process and peer review in collaboration with the rest of the editorial team. Peer reviewer reports are available.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Extended data
Extended Data Fig. 1 Characterization of cribriform plate cell suspension.
(a): Experimental design for generating a single cell suspension of the cribriform plate. (b – d): Gating strategy used to identify cell types from the cpLEC cell suspension between healthy (c) and EAE score 3.0 (d). Microglia are identified as CD45intermediate CD11b+, macrophages as CD45+ CD11b+ CD11c−, dendritic cells as CD45+ CD11b+ CD11c+, CD4 T cells as CD45+ CD11b− CD11c− CD4+, CD8 T cells as CD45+ CD11b− CD11c− CD8+, B cells as CD45+ CD11b− CD11c− CD4− CD8- B220+, blood endothelial cells as CD45low CD31+ Lyve-1- Podoplanin−, and lymphatic endothelial cells as CD45low CD31+ Lyve-1+ Podoplanin+. (e – l): Quantitation of the average cell numbers of microglia (e), macrophages (f), dendritic cells (g), CD4 T cells (h), CD8 T cells (i), B cells (j), blood endothelial cells (k), and lymphatic endothelial cells (l) between healthy and EAE score 3.0. n = 5 healthy mice, 4 EAE mice; data are represented as mean ± standard error of the mean. For microglia, p = 0.0001; for macrophages, p = 0.0091; for dendritic cells, p = 0.0141; for CD4 T cells, p = 0.0031; for CD8 T cells, p = 0.0011; for B cells, p = 0.1059; for blood endothelial cells, p = 0.2523; for lymphatic endothelial cells, p = 0.0045; unpaired Student’s t-test.
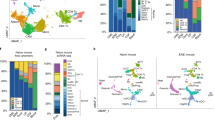
Extended Data Fig. 2 Volcano and CNET plots of scRNAseq.
(a): Volcano plot showing the top 50 most up-regulated and down-regulated genes in Cluster 1. (b): Cnet plot detailing the strength of association between representative GO enrichment terms for Cluster 1 for regulation of angiogenesis, regulation of protein catabolic process, regulation of cell-cell adhesion, and response to nutrient levels along with their associated genes. (c): Volcano plot showing the top 50 most up-regulated and down-regulated genes in Cluster 2. (d): Cnet plot detailing the strength of association between representative GO enrichment terms for Cluster 2 for antigen processing and presentation, response to interferon-gamma, leukocyte cell-cell adhesion, leukocyte chemotaxis, and leukocyte activation involved in immune response along with their associated genes. (e): Volcano plot showing the top 50 most up-regulated genes in Cluster 3. (f): Cnet plot detailing the strength of association between representative GO enrichment terms for Cluster 3 for nuclear division, positive regulation of cell cycle, DNA replication, and cell cycle checkpoint along with their associated genes.
Extended Data Fig. 3 Visualizing cpLEC trajectories.
(a): Single cell data was clustered using UMAP methodology and trajectories learned using default parameters by monacle3. Trajectories through pseudotime are shown for the three clusters; note that cluster 3 lies in between clusters 1 and 2 through pseudotime, and there are no direct connections between clusters 1 and 2. (b): 5 representative genes associated with the GO enrichment term adhesion/chemotaxis that is enriched in cluster 2 are shown through pseudotime. (c): 4 representative genes associated with the GO enrichment term response to IFN-γ that is enriched in cluster 2 are shown through pseudotime. (d): 3 representative genes associated with the GO enrichment term antigen processing/presentation that is enriched in cluster 2 are shown through pseudotime. (e): 4 representative genes associated with the GO enrichment term leukocyte activation that is enriched in cluster 2 are shown through pseudotime. Note that these genes tend to be up-regulated later through pseudotime relative to genes associated with the other 3 enrichment terms.
Extended Data Fig. 4 Characterizing cpLEC-leukocyte doublets.
(a): Gating strategy used to visualize leukocyte – LEC binding. Live Ghost UV450− doublets were gated for, and a leukocyte bound to a LEC were gated for both the leukocyte marker CD45 and LEC markers Podoplanin, Lyve-1, and CD31. Leukocytes were further gated for CD11b+ macrophages, CD11b+ CD11c+ dendritic cells, CD4+ T cells, CD8+ T cells, and B220+ B cells. (b-e): Representative confocal images taken of a healthy cribriform plate section immunolabeled with OVA-GFP (b), Podoplanin (c), CD11b (d), and merged (e). Scale bar = 50 µm. (f-i): Representative confocal images taken of an EAE score 3.0 cribriform plate section immunolabeled with OVA-GFP (f), Podoplanin (g), CD11b (h), and merged (i). Scale bar = 50 µm. (j): Quantitation of the average percent area of OVA-GFP within Podoplanin+ cpLECs after excluding CD11b+ area. n = 4 healthy mice, 6 EAE mice; data are represented as mean ± standard error of the mean. For percent area of OVA-GFP, p = 0.9079; unpaired Student’s t-test. (k): Quantitation of the absolute area of OVA-GFP within Podoplanin+ cpLECs. n = 4 healthy mice, 6 EAE mice; data are represented as mean ± standard error of the mean. For absolute area of OVA-GFP, p = 0.0380; unpaired Student’s t-test. (l): Quantitation of the average number of CD11b+ cells containing intracellular OVA-GFP within Podoplanin+ cpLECs. n = 4 healthy mice, 6 EAE mice; data are represented as mean ± standard error of the mean. For number of OVA-GFP+ CD11b+ cells, p = 0.0022; unpaired Student’s t-test. (m – o): Representative confocal images taken of the cribriform plate from healthy (m) or EAE score 3.0 at 15 days post-immunization (n) immunolabeled with VCAM-1 and Podoplanin. Scale bar = 200um. (o): Quantitation of the average percent area of Podoplanin+ meningeal lymphatic vessels near the cribriform plate that express Vcam-1. n = 6 mice per group; data are represented as mean ± standard error of the mean. For percent area of Vcam-1+ labeling within Podoplanin+ cells, p = 0.0152; unpaired Student’s t-test. (p – u): Representative confocal images taken of an EAE score 3.0 cribriform plate section immunolabeled with CD11c (p), F4/80 (q), Ly6G (r), Lyve-1 (s), and merged (t). Quantitation reveals that during EAE, the majority of CD11c+ cells in contact with Lyve-1+ cpLECs are F4/80− and Ly6G−, with a relatively minor subset of CD11c+ cells identified as F4/80+ macrophages or Ly6G+ neutrophils (u). Scale bar = 50 µm.
Extended Data Fig. 5 cpLECs can present OVA323-339 to OT-II T cells.
(a – f): Healthy or EAE score 3.0 cpLECs were FACS sorted as CD31+ Podoplanin+ after excluding CD45intermediate microglia, CD45+ leukocytes, Ghost+ dead cells, and doublets. Sorted cpLECs were then co-cultured with magnetically purified CD4 OT-II T cells from splenocytes using negative selection after Cell Trace Violet labeling in the presence of 100 µg/mL of OVA323-339 for either 24 hours (a – c) or 72 hours (d – f). (a – b): Gating strategy for measuring the early T cell activation marker CD69 by OT-II T cells after 24 hours of co-culture. (c): Quantitation of the average percentage of 2D2 T cells expressing CD69 after 24 hours of co-culture. n = 3 replicates per group, pooled from 4 healthy and 4 EAE mice; data are represented as mean ± standard error of the mean. For percent of CD69 OT-II cells, p < 0.0001. (d – e): Gating strategy for measuring T cell proliferation by the dilution of Cell Trace Violet by OT-II T cell after 72 hours of co-culture. (f): Quantitation of the average percent of proliferated OT-II T cells after 72 hours of co-culture. n = 3 replicates per group, pooled from 4 healthy and 4 EAE mice; data are represented as mean ± standard error of the mean. For percent of proliferated OT-II cells, p = 0.0012.
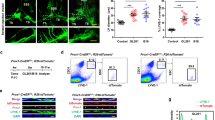
Extended Data Fig. 6 cpLECs upregulate CD31, Podoplanin, Lyve-1, and PD-L1 during EAE.
(a): Gating strategy used to confirm the up-regulation of CD31, Podoplanin, Lyve-1, and PDL-1 at the protein level during EAE score 3.0. (b – q): After gating for cpLECs as either Podoplanin+ CD31+ (b – e, n – q), Podoplanin+ Lyve-1+ (f – i), or Lyve-1+ CD31+ (j – m), the median fluorescence intensity (MFI) of Lyve-1 (b – e), CD31 (f – i), Podoplanin (j – m), and PDL-1 (n – q) by both singlet cpLECs and doublets in which a cpLEC is bound to a CD45+ leukocyte. n = 5 healthy cpLECs, 4 EAE cpLECs, and 4 EAE cpLECs + leukocytes; data are represented as mean ± standard error of the mean. For Lyve-1 MFI, healthy cpLECs vs. EAE cpLECs, p = 0.0003, healthy cpLECs vs. EAE cpLECs + leukocytes, p = 0.0001, EAE cpLECs vs. EAE cpLECs + leukocytes, p = 0.6590; for CD31 MFI, healthy cpLECs vs. EAE cpLECs, p = 0.0043, healthy cpLECs vs. EAE cpLECs + leukocytes, p < 0.0001, EAE cpLECs vs. EAE cpLECs + leukocytes, p < 0.0001; for Podoplanin MFI, healthy cpLECs vs. EAE cpLECs, p = 0.0112, healthy cpLECs vs. EAE cpLECs + leukocytes, p < 0.0001, EAE cpLECs + EAE cpLECs + leukocytes, p = 0.0028; for PD-L1 MFI, healthy cpLECs vs. EAE cpLECs, p = 0.0027, healthy cpLECs vs. EAE cpLECs + leukocytes, p < 0.0001, EAE cpLECs + EAE cpLECs + leukocytes, p < 0.0001; one-way ANOVA using Tukey’s multiple comparisons test.
Extended Data Fig. 7 Background doublet MFI is negligible compared to protein expression.
(a – d): FMO controls showing the increase in MFI of Podoplanin (a), CD31 (b), Lyve-1 (c), and PDL-1 (d) of doublets relative to singlets due to background is negligible relative to actual protein expression. n = 4 mice per group; data are represented as mean ± standard error of the mean. For Podoplanin MFI (a), singlet FMO vs. doublet FMO, p > 0.9999, singlet FMO vs. EAE singlets, p = 0.0055, singlet FMO vs. EAE doublets, p < 0.0001, doublet FMO vs. EAE singlets, p = 0.0057, doublet FMO vs. EAE doublets, p < 0.0001, EAE singlets vs. EAE doublets, p = 0.0016; for CD31 MFI (b), singlet FMO vs. doublet FMO, p = 0.0130, singlet FMO vs. EAE singlets, p < 0.0001, singlet FMO vs. EAE doublets, p < 0.0001, doublet FMO vs. EAE singlets, p < 0.0001, doublet FMO vs. EAE doublets, p < 0.0001, EAE singlets vs. EAE doublets, p < 0.0001; for Lyve-1 MFI (c), singlet FMO vs. doublet FMO, p = 0.0182, singlet FMO vs. EAE singlets, p < 0.0001, singlet FMO vs. EAE doublets, p < 0.0001, doublet FMO vs. EAE singlets, p < 0.0001, doublet FMO vs. EAE doublets, p < 0.0001, EAE singlets vs. EAE doublets, p = 0.7736; for PD-L1 MFI (d), singlet FMO vs. doublet FMO, p = 0.9828, singlet FMO vs. EAE singlets, p = 0.0003, singlet FMO vs. EAE doublets, p < 0.0001, doublet FMO vs. EAE singlets, p = 0.0005, doublet FMO vs. EAE doublets, p < 0.0001, EAE singlets vs. EAE doublets, p < 0.0001; one-way ANOVA with Tukey’s post-hoc multiple comparisons test.
Extended Data Fig. 8 Lymphangiogenesis does not require IFN-γ.
(a): Representative gating strategy used to characterize IFN-γ dependent regulation of Podoplanin and PDL-1. EAE was induced, and the expression of IFN-γ mediated Podoplanin, PDL-1, and CD31 was analyzed by flow cytometry at day 15 post-immunization at score 3.0 of EAE. Gating strategy taken from a representative wild-type EAE sample. (b – d): Gating strategy for identifying cpLECs between wild-type healthy (b), wild-type EAE score 3.0 (c), and IFN-γ-/- EAE score 3.0 (d). (e): Quantitation of the average number of cpLECs between wild-type healthy (b), wild-type EAE (c), and IFN-γ-/- EAE (d) reveal lymphangiogenesis in IFN-γ deficient transgenic mice. n = 5 wild-type healthy mice, 5 wild-type EAE mice, and 4 IFN-γ-/- mice; data are represented as mean ± standard error of the mean; for wild-type healthy vs. wild-type EAE, p = 0.0248, wild-type healthy vs. IFN-γ-/- EAE, p = 0.0062, wild-type EAE vs. IFN-γ-/- EAE, p = 0.5964; one-way ANOVA with Tukey’s post-hoc multiple comparisons test.
Supplementary information
Source data
Source Data Fig. 2
Statistical source data.
Source Data Fig. 4
Statistical source data.
Source Data Fig. 5
Statistical source data.
Source Data Fig. 7
Statistical source data.
Source Data Extended Data Fig. 1
Statistical source data.
Source Data Extended Data Fig. 4
Statistical source data.
Source Data Extended Data Fig. 5
Statistical source data.
Source Data Extended Data Fig. 6
Statistical source data.
Source Data Extended Data Fig. 7
Statistical source data.
Source Data Extended Data Fig. 8
Statistical source data.
Rights and permissions
About this article
Cite this article
Hsu, M., Laaker, C., Madrid, A. et al. Neuroinflammation creates an immune regulatory niche at the meningeal lymphatic vasculature near the cribriform plate. Nat Immunol 23, 581–593 (2022). https://doi.org/10.1038/s41590-022-01158-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41590-022-01158-6
This article is cited by
-
Advancing insights into in vivo meningeal lymphatic vessels with stereoscopic wide-field photoacoustic microscopy
Light: Science & Applications (2024)
-
Brain regulatory T cells
Nature Reviews Immunology (2024)
-
Identification of direct connections between the dura and the brain
Nature (2024)
-
Current views on meningeal lymphatics and immunity in aging and Alzheimer’s disease
Molecular Neurodegeneration (2023)
-
Systemic innate myeloid responses to acute ischaemic and haemorrhagic stroke
Seminars in Immunopathology (2023)