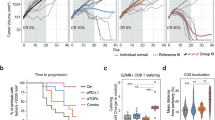
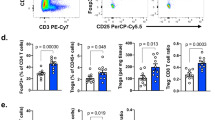
Abstract
Regulatory T (Treg) cells have an immunosuppressive function and highly express the immune checkpoint receptor PD-1 in the tumor microenvironment; however, the function of PD-1 in tumor-infiltrating (TI) Treg cells remains controversial. Here, we showed that conditional deletion of PD-1 in Treg cells delayed tumor progression. In Pdcd1fl/flFoxp3eGFP−Cre-ERT2(+/−) mice, in which both PD-1-expressing and PD-1-deficient Treg cells coexisted in the same tissue environment, conditional deletion of PD-1 in Treg cells resulted in impairment of the proliferative and suppressive capacity of TI Treg cells. PD-1 antibody therapy reduced the TI Treg cell numbers, but did not directly restore the cytokine production of TI CD8+ T cells in TC-1 lung cancer. Single-cell analysis indicated that PD-1 signaling promoted lipid metabolism, proliferation and suppressive pathways in TI Treg cells. These results suggest that PD-1 ablation or inhibition can enhance antitumor immunity by weakening Treg cell lineage stability and metabolic fitness in the tumor microenvironment.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout







Similar content being viewed by others
Data availability
Data generated in the study are included within the paper (and its extended data) or are available from the corresponding author upon reasonable request. scRNA-seq and scVDJ-seq data that support the findings of this study have been deposited in the Gene Expression Omnibus (https://www.ncbi.nlm.nih.gov/geo/) under accession no. GSE164033. Hallmark gene sets related to oxidative phosphorylation, fatty acid metabolism are available from the MSigDB (https://www.gsea-msigdb.org/gsea/msigdb/). Source data are provided with this paper.
References
Fontenot, J. D., Gavin, M. A. & Rudensky, A. Y. Foxp3 programs the development and function of CD4+CD25+ regulatory T cells. Nat. Immunol. 4, 330–336 (2003).
Josefowicz, S. Z., Lu, L. F. & Rudensky, A. Y. Regulatory T cells: mechanisms of differentiation and function. Annu. Rev. Immunol. 30, 531–564 (2012).
Tanaka, A. & Sakaguchi, S. Regulatory T cells in cancer immunotherapy. Cell Res. 27, 109–118 (2017).
Wang, H. et al. CD36-mediated metabolic adaptation supports regulatory T cell survival and function in tumors. Nat. Immunol. 21, 298–308 (2020).
Galluzzi, L., Chan, T.A., Kroemer, G., Wolchok, J.D. & Lopez-Soto, A. The hallmarks of successful anticancer immunotherapy. Sci. Transl. Med. https://doi.org/10.1126/scitranslmed.aat7807 (2018).
Park, S. M. & Youn, J. I. Role of myeloid-derived suppressor cells in immune checkpoint inhibitor therapy in cancer. Arch. Pharm. Res. 42, 560–566 (2019).
Grinberg-Bleyer, Y. et al. NF-κB c-Rel is crucial for the regulatory T cell immune checkpoint in cancer. Cell 170, 1096–1108 (2017).
Overacre-Delgoffe, A. E. et al. Interferon-γ drives Treg fragility to promote anti-tumor immunity. Cell 169, 1130–1141 (2017).
Wang, D. et al. Targeting EZH2 reprograms intratumoral regulatory T cells to enhance cancer immunity. Cell Rep. 23, 3262–3274 (2018).
Wherry, E. J. T cell exhaustion. Nat. Immunol. 12, 492–499 (2011).
Kamphorst, A. O. et al. Rescue of exhausted CD8 T cells by PD-1-targeted therapies is CD28-dependent. Science 355, 1423–1427 (2017).
Gordon, S. R. et al. PD-1 expression by tumour-associated macrophages inhibits phagocytosis and tumour immunity. Nature 545, 495–499 (2017).
Lucca, L. E. & Dominguez-Villar, M. Modulation of regulatory T cell function and stability by co-inhibitory receptors. Nat. Rev. Immunol. https://doi.org/10.1038/s41577-020-0296-3 (2020).
Strauss, L. et al. Targeted deletion of PD-1 in myeloid cells induces antitumor immunity. Sci. Immunol. 5, eaay1863 (2020).
Asano, T. et al. PD-1 modulates regulatory T-cell homeostasis during low-dose interleukin-2 therapy. Blood 129, 2186–2197 (2017).
Park, H. J. et al. PD-1 upregulated on regulatory T cells during chronic virus infection enhances the suppression of CD8+ T cell immune response via the interaction with PD-L1 expressed on CD8+ T cells. J. Immunol. 194, 5801–5811 (2015).
Yoshida, K. et al. Anti-PD-1 antibody decreases tumour-infiltrating regulatory T cells. BMC Cancer 20, 25 (2020).
Lim, C. J. et al. Multidimensional analyses reveal distinct immune microenvironment in hepatitis B virus-related hepatocellular carcinoma. Gut 68, 916–927 (2019).
Kim, H. R. et al. Tumor microenvironment dictates regulatory T cell phenotype: upregulated immune checkpoints reinforce suppressive function. J. Immunother. Cancer 7, 339 (2019).
Lim, S. A. et al. Lipid signalling enforces functional specialization of Treg cells in tumours. Nature 591, 306–311 (2021).
Stathopoulou, C. et al. PD-1 inhibitory receptor downregulates asparaginyl endopeptidase and maintains Foxp3 transcription factor stability in induced regulatory T cells. Immunity 49, 247–263 (2018).
Kamada, T. et al. PD-1(+) regulatory T cells amplified by PD-1 blockade promote hyperprogression of cancer. Proc. Natl Acad. Sci. USA 116, 9999–10008 (2019).
Kumagai, S. et al. The PD-1 expression balance between effector and regulatory T cells predicts the clinical efficacy of PD-1 blockade therapies. Nat. Immunol. 21, 1346–1358 (2020).
Tan, C. L. et al. PD-1 restraint of regulatory T cell suppressive activity is critical for immune tolerance. J. Exp. Med. 218, e20182232 (2021).
Higashi, A. Y. et al. Direct hematological toxicity and illegitimate chromosomal recombination caused by the systemic activation of CreERT2. J. Immunol. 182, 5633–5640 (2009).
Schmidt-Supprian, M. & Rajewsky, K. Vagaries of conditional gene targeting. Nat. Immunol. 8, 665–668 (2007).
Anz, D. et al. CD103 is a hallmark of tumor-infiltrating regulatory T cells. Int J. Cancer 129, 2417–2426 (2011).
Cretney, E. et al. The transcription factors Blimp-1 and IRF4 jointly control the differentiation and function of effector regulatory T cells. Nat. Immunol. 12, 304–311 (2011).
Wing, K. et al. CTLA-4 control over Foxp3+ regulatory T cell function. Science 322, 271–275 (2008).
Zhou, X. et al. Instability of the transcription factor Foxp3 leads to the generation of pathogenic memory T cells in vivo. Nat. Immunol. 10, 1000–1007 (2009).
El-Behi, M. et al. The encephalitogenicity of T(H)17 cells is dependent on IL-1- and IL-23-induced production of the cytokine GM-CSF. Nat. Immunol. 12, 568–575 (2011).
Odoardi, F. et al. T cells become licensed in the lung to enter the central nervous system. Nature 488, 675–679 (2012).
Delacher, M. et al. Precursors for nonlymphoid-tissue Treg cells reside in secondary lymphoid organs and are programmed by the transcription factor BATF. Immunity 52, 295–312 (2020).
Wu, D. et al. Lkb1 maintains Treg cell lineage identity. Nat. Commun. 8, 15876 (2017).
Patsoukis, N. et al. PD-1 alters T-cell metabolic reprogramming by inhibiting glycolysis and promoting lipolysis and fatty acid oxidation. Nat. Commun. 6, 6692 (2015).
Charbonnier, L. M. et al. Functional reprogramming of regulatory T cells in the absence of Foxp3. Nat. Immunol. 20, 1208–1219 (2019).
Liu, X. et al. Reprogramming lipid metabolism prevents effector T cell senescence and enhances tumor immunotherapy. Sci. Transl. Med. https://doi.org/10.1126/scitranslmed.aaz6314 (2021).
Watson, M. J. et al. Metabolic support of tumour-infiltrating regulatory T cells by lactic acid. Nature 591, 645–651 (2021).
Mootha, V. K. et al. PGC-1α-responsive genes involved in oxidative phosphorylation are coordinately downregulated in human diabetes. Nat. Genet. 34, 267–273 (2003).
Subramanian, A. et al. Gene set enrichment analysis: a knowledge-based approach for interpreting genome-wide expression profiles. Proc. Natl Acad. Sci. USA 102, 15545–15550 (2005).
Kallies, A., Zehn, D. & Utzschneider, D. T. Precursor exhausted T cells: key to successful immunotherapy? Nat. Rev. Immunol. 20, 128–136 (2020).
Lee, J. B., Kim, H. R. & Ha, S. J. Immune checkpoint inhibitors in 10 years: contribution of basic research and clinical application in cancer immunotherapy. Immune Netw. 22, e2 (2022).
Angelin, A. et al. Foxp3 reprograms T cell metabolism to function in low-glucose, high-lactate environments. Cell Metab. 25, 1282–1293 (2017).
Gerriets, V. A. et al. Foxp3 and Toll-like receptor signaling balance Treg cell anabolic metabolism for suppression. Nat. Immunol. 17, 1459–1466 (2016).
Gruta, N. L. L., Driel, I. R. V. & Gleeson, P. A. Peripheral T cell expansion in lymphopenic mice results in a restricted T cell repertoire. Eur. J. Immunol. 30, 3380–3386 (2000).
Marleau, A. M. & Sarvetnick, N. T cell homeostasis in tolerance and immunity. J. Leukoc. Biol. 78, 575–584 (2005).
Kim, K. H. et al. Immunological characteristics of hyperprogressive disease in patients with non-small cell lung cancer treated with anti-PD-1/PD-L1 Abs. Immune Netw. 20, e48 (2020).
Chen, X. et al. PD-1 regulates extrathymic regulatory T-cell differentiation. Eur. J. Immunol. 44, 2603–2616 (2014).
Blank, C. et al. Absence of programmed death receptor 1 alters thymic development and enhances generation of CD4/CD8 double-negative TCR-transgenic T cells. J. Immunol. 171, 4574–4581 (2003).
Francisco, L. M. et al. PD-L1 regulates the development, maintenance, and function of induced regulatory T cells. J. Exp. Med. 206, 3015–3029 (2009).
Son, J. et al. Tumor-infiltrating regulatory T-cell accumulation in the tumor microenvironment is mediated by IL33/ST2 Signaling. Cancer Immunol. Res. 8, 1393–1406 (2020).
Ahmed, R., Salmi, A., Butler, L. D., Chiller, J. M. & Oldstone, M. B. Selection of genetic variants of lymphocytic choriomeningitis virus in spleens of persistently infected mice. Role in suppression of cytotoxic T lymphocyte response and viral persistence. J. Exp. Med. 160, 521–540 (1984).
Butler, A., Hoffman, P., Smibert, P., Papalexi, E. & Satija, R. Integrating single-cell transcriptomic data across different conditions, technologies, and species. Nat. Biotechnol. 36, 411–420 (2018).
Stuart, T. et al. Comprehensive integration of single-cell data. Cell 177, 1888–1902 (2019).
Macosko, E. Z. et al. Highly parallel genome-wide expression profiling of individual cells using nanoliter droplets. Cell 161, 1202–1214 (2015).
Blondel, V.D., Guillaume, J.-L., Lambiotte, R. & Lefebvre, E. Fast unfolding of communities in large networks. J. Stat. Mech. Theory Exp. https://doi.org/10.1088/1742-5468/2008/10/P10008 (2008).
Bassez, A. et al. A single-cell map of intratumoral changes during anti-PD1 treatment of patients with breast cancer. Nat. Med. 27, 820–832 (2021).
Hanzelmann, S., Castelo, R. & Guinney, J. GSVA: gene set variation analysis for microarray and RNA-seq data. BMC Bioinf. 14, 7 (2013).
Yu, G., Wang, L. G., Han, Y. & He, Q. Y. clusterProfiler: an R package for comparing biological themes among gene clusters. OMICS 16, 284–287 (2012).
Kolde, R. & Kolde, M. R. Package ‘pheatmap’. R Package 1, 790 (2015).
Acknowledgements
This study was supported by National Research Foundation of Korea grants funded by the Korean government (2017R1A5A1014560, 2019M3A9B6065221 to S-.J.H.; 2018R1A5A2025079, 2019M3A9B6065192 to I.L.). This study was also supported by the Korean Health Technology R&D Project (HV20C0144, HN21C1410 to S-.J.H.) through the Korean Health Industry Development Institute funded by the Ministry of Health & Welfare. The funders had no role in the study design, data collection and analysis, decision to publish or preparation of the manuscript.
Author information
Authors and Affiliations
Contributions
M.J.K. conceived the project, designed the experiments, developed the experimental models, performed the experiments, analyzed the FACS data, wrote the manuscript and edited the manuscript. K.K. analyzed the scRNA-seq and scVDJ-seq data and edited the manuscript. H.J.P. developed the experimental models. G.-R.K. performed the EAE experiments and provided advice about the EAE experiments. K.H.H. performed the experiments. J.H.O. and J.S. provided advice for this project. D.J.P. and D.K. assisted with experiments. J.-M.C. supervised the EAE experiments and provided advice for this project. I.L. supervised the single-cell data analysis and edited the manuscript. S.-J.H. conceived the project, designed the experiments, developed the experimental models, supervised the project, wrote the manuscript and edited the manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Immunology thanks the anonymous reviewers for their contribution to the peer review of this work. Primary Handling Editor: Ioana Visan, in collaboration with the Nature Immunology team.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Extended data
Extended Data Fig. 1 Establishment of mice with a conditional deletion of PD-1 in Treg cells.
a. Genetic constructs of Pdcd1fl/fl mice. b. Experimental scheme. c. Representative flow cytometry plots of CD8+ and CD4+ T cells in the PBMCs of PD-1Ctr and PD-1cKO mice treated intraperitoneally with vehicle and TAM at day 1, 3, 7, 11 and 24 post-TAM treatment. d. Kinetics of PD-1, TIM3, TIGIT, LAG3 and CTLA4 expression in T cells in the PBMCs of PD-1Ctr (n = 5) and PD-1cKO (n = 5) mice as in c. e. Experimental scheme. f. Quantification of the percentages and absolute numbers of T cells in the spleen (S) and lung (L) of PD-1Ctr (n = 6) and PD-1cKO (n = 6) mice at day 12 after vehicle and TAM treatment. g. Quantification of the percentages of PD-1, TIM3, TIGIT, and LAG3 expression in CD8+ T cells, CD4+ Tconv cells, and Treg cells in the spleen and lung of PD-1Ctr (n = 6) and PD-1cKO (n = 6) mice as in f. The data were analyzed by the two-tailed unpaired Student’s t-test (f, g). All bar graphs show the mean ± s.e.m. The data are representative of two independent experiments (b-g).
Extended Data Fig. 2 Comparison of the phenotype of Treg cells between Foxp3cKO and PD-1Ctr mice.
a. Representative histograms of T cells in the spleen and lung of PD-1Ctr and PD-1cKO mice at day 12 after vehicle and TAM treatment. b. Experimental scheme. c. Quantification of the tumor nodules in the lungs of TAM-treated Foxp3cKO (n = 15) mice and vehicle-treated PD-1Ctr (n = 11) mice injected intravenously with TC-1 cells at day 20 after injection. d. Representative flow cytometry plot of CD4+ T cells in the tumor of TC-1 tumor-bearing Foxp3cKO mice and PD-1Ctr mice as in c. Quantification of the percentages of Treg cells in the tumor of TC-1 tumor-bearing Foxp3cKO (n = 15) mice and PD-1Ctr (n = 11) mice as in c. e. Representative histograms of Ki67 expression in Treg cells in the tumor of TC-1 tumor-bearing Foxp3cKO mice and PD-1Ctr mice as in c. Quantification of the percentages of Ki67+ cells in Treg cells in the tumor of TC-1 tumor-bearing Foxp3cKO (n = 15) mice and PD-1Ctr (n = 6) mice as in c. The data were analyzed by the two-tailed unpaired Student’s t-test (c, d, e). All bar graphs show the mean ± s.e.m. The data are representative of two independent experiments (a). The data were pooled from two independent experiments (c, d, e).
Extended Data Fig. 3 Conditional deletion of PD-1 in Treg cells enhanced viral control during LCMV-Clone13 infection.
a. Experimental scheme. b. Quantification of viral titer of serum of the LCMV-Cl13-infected PD-1Ctr (n = 10) and PD-1cKO (n = 10) mice as in a at day 8 and 15 after infection. Quantification of viral titer of spleen, lung, and kidney of the LCMV-Cl13-infected PD-1Ctr (n = 5) and PD-1cKO (n = 5) mice as in a at day 8 and 15 after infection. The dashed line indicates the viral detection limit. c. Representative flow cytometry plots of CD8+ T cells in the PBMC, spleen, and lung of LCMV-Cl13-infected PD-1Ctr and PD-1cKO mice as in a at the indicated time point. d. Kinetics of the percentage of H-2DbGP33+CD8+ T cells and the MFIs of PD-1 expression in H-2DbGP33+CD8+ T cells in the PBMCs of LCMV-Cl13-infected PD-1Ctr (n = 5) and PD-1cKO (n = 5) mice as in a. Quantification of the percentage of H-2DbGP33+CD8+ T cells and the MFIs of PD-1 expression in H-2DbGP33+CD8+ T cells in the spleen, and lung of LCMV-Cl13-infected PD-1Ctr (n = 5) and PD-1cKO (n = 5) mice as in a at day 21 after infection. e. Representative flow cytometry plots of CD4+ T cells in the PBMC, spleen, and lung of LCMV-Cl13-infected PD-1Ctr and PD-1cKO mice as in a at the indicated time point. f. Kinetics of the percentages of Treg cells and the MFIs of Foxp3 expression in Treg cells in the PBMCs of LCMV-Cl13-infected PD-1Ctr (n = 5) and PD-1cKO (n = 5) mice as in a. Quantification of the percentages of Treg cells and the MFIs of Foxp3 expression in Treg cells in the spleen, and lung of LCMV-Cl13-infected PD-1Ctr (n = 5) and PD-1cKO (n = 5) mice as in a at day 21 after infection. g. Representative histograms of Ki67+ and annexinV+ cells in Treg cells in the spleen and lung of LCMV-Cl13-infected PD-1Ctr (n = 5) and PD-1cKO (n = 5) mice as in a at day 21 after infection. h. Quantification of the percentages of Ki67+ and annexinV+ cells in Treg cells in the spleen and lung of LCMV-Cl13-infected PD-1Ctr (n = 5) and PD-1cKO (n = 5) mice as in g. i. Representative flow cytometry plots of CD8+ T cells in the spleen and lung of LCMV-Cl13-infected PD-1Ctr and PD-1cKO mice as in a at day 15 after infection. j. Quantification of the percentages of IFN-γ+TNF+ cells in CD8+ T cells and the ratio of IFN-γ+TNF+CD8+ T cells/IFN-γ+CD8+ T cells in the spleen and lung of LCMV-Cl13-infected PD-1Ctr (n = 5) and PD-1cKO (n = 5) mice as in i. k. Representative flow cytometry plots of CD8+ T cells in the spleen and lung of LCMV-Cl13-infected PD-1Ctr and PD-1cKO mice as in a at day 21 after infection. l. Quantification of the percentages of IFN-γ+TNF+ cells in CD8+ T cells and the ratio of IFN-γ+TNF+CD8+ T cells/IFN-γ+CD8+ T cells in the spleen and lung of LCMV-Cl13-infected PD-1Ctr (n = 5) and PD-1cKO (n = 5) mice as in k. The numbers in the plots and histograms indicate the percentage of the population (c, e, g, i, k). The data were analyzed by the two-tailed unpaired Student’s t test (b, d, f, h, j, l) and two-way ANOVA with Sidak’s post hoc test (d, f). All bar graphs show the mean ± s.e.m. The data are representative of two independent experiments. The data were pooled from two independent experiments (b, left).
Extended Data Fig. 4 TI CD8+ T cells and CD4+ Tconv cells in PD-1cKO mice are less exhausted than those in PD-1Ctr mice.
a. Experimental scheme. b. Representative histograms of PD-1 expression in CD3- cells and Treg cells and Foxp3 expression of CD4+ Tconv cells and Treg cells in the spleen and tumor of PD-1Ctr and PD-1cKO mice injected intravenously with TC-1 cells at day 20 after injection. c. Representative histograms of Treg cells in the spleen and tumor of TC-1 tumor-bearing PD-1Ctr and PD-1cKO mice as in a. d. Representative flow cytometry plots of TI CD8+ T cells and CD4+ Tconv cells of TC-1 tumor-bearing PD-1Ctr and PD-1cKO mice as in a. e. Quantification of the percentages of annexin V+7-AAD+ and Ki67+ cells in TI CD8+ T cells and CD4+ Tconv cells of TC-1 tumor-bearing PD-1Ctr (n = 9) and PD-1cKO (n = 9) mice as in a. f. Representative flow cytometry plots of TI CD8+ T cells of TC-1 tumor-bearing PD-1Ctr and PD-1cKO mice as in a. Quantification of the percentages of PD-1+CD44+ and PD-1+Eomes+ cells in TI CD8+ T cells of TC-1 tumor-bearing PD-1Ctr (n = 9) and PD-1cKO (n = 9) mice as in a. g. Representative flow cytometry plots of TI CD4+ Tconv cells of TC-1 tumor-bearing PD-1Ctr and PD-1cKO mice as in a. Quantification of the percentages of PD-1+CD44+ and PD-1+Eomes+ cells in TI CD4+ Tconv cells of TC-1 tumor-bearing PD-1Ctr (n = 9) and PD-1cKO (n = 9) mice as in a. The numbers in the plots indicate the percentage of the population (d, f, g). The numbers in the histograms indicate the percentage of positive populations or the MFIs (b, c, d). The data were analyzed by the two-tailed unpaired Student’s t-test (e, f, g). All bar graphs show the mean ± s.e.m. The data are representative of three independent experiments (b, c). The data were pooled from two independent experiments (e, f, g).
Extended Data Fig. 5 Graphical description of PD-1Cre+/− and PD-1cKORosa26tdTomato mice.
a. Graphical description of PD-1Cre+/− mice. b. Graphical description of PD-1cKORosa26tdTomato mice.
Extended Data Fig. 6 TAM-titrated TC-1 tumor-bearing Pdcd1fl/flFoxp3eGFP−Cre-ERT2 mice for ex vivo suppression assay.
a. Experimental scheme. b. Representative flow cytometry plots of CD4+ T cells in the spleen and tumor of naïve and TC-1 tumor-bearing (as in a.) Pdcd1fl/flFoxp3eGFP−Cre-ERT2 mice at day 20 post-TC-1 intravenous injection on day 8 post-1mg of TAM treatment. c. The purity of sorted CD25+PD-1+ and CD25+PD-1- Treg cells in the tumor of TC-1 tumor-bearing Pdcd1fl/flFoxp3eGFP−Cre-ERT2 mice as in a.
Extended Data Fig. 7 PD-1 expression before tumor inoculation is crucial for maintaining the proliferation of TI Treg cells.
a. Experimental scheme. b. Quantification of tumor nodules and the ratio of CD8+ T cells/Treg cells in the tumor of the indicated TC-1 tumor-bearing PD-1Ctr and PD-1cKO mice as in a at day 20 after injection. c. Representative flow cytometry plots of CD4+ T cells in the spleen and tumor of the indicated TC-1 tumor-bearing PD-1Ctr and PD-1cKO mice as in a. The numbers in the plots indicate the percentage of the population. Quantification of the percentages of Treg cells in the spleen and tumor of the indicated TC-1 tumor-bearing PD-1Ctr and PD-1cKO mice as in a. d. Representative histograms of Ki67 expression in Treg cells in the spleen and tumor of the indicated TC-1 tumor-bearing PD-1Ctr and PD-1cKO mice as in a. The numbers in the histograms indicate the percentage of positive populations. Quantification of the percentages of Ki67+ cells in Treg cells in the spleen and tumor of the indicated TC-1 tumor-bearing PD-1Ctr and PD-1cKO mice as in a. Tx → TC-1 group; PD-1Ctr mice; n = 5, PD-1cKO mice; n = 5 ((b, left), d). Tx → TC-1 group; PD-1Ctr mice; n = 9, PD-1cKO mice; n = 9 ((b, right), c). TC-1 → Tx D2 ~ D6 group; PD-1Ctr mice; n = 6, PD-1cKO mice; n = 3, TC-1 → Tx D9 ~ D13 group; PD-1Ctr mice; n = 5, PD-1cKO mice; n = 5 (b, c, d). The data were analyzed by the two-tailed unpaired Student’s t-test (b, c, d). All bar graphs show the mean ± s.e.m. The data of Tx → TC-1 were pooled from two independent experiments (b, c). The data are representative of three (a-d) independent experiments.
Extended Data Fig. 8 PD-1 expression is important for naïve Treg cells activation and proliferation.
a. Representative flow cytometry plots and histograms of Treg cells in the spleen of naïve TAM-treated PD-1Cre+/− mice at day 12 after treatment cultured with CD3 antibodies in the presence of dendritic cells for 3 days. b. Quantification of the percentage of CTV- and Ki67+ cells in PD-1WT and PD-1KO Treg cells (n = 3) cultured as in a. c. Representative histograms of Treg cells cultured as in a. d. Quantification of the percentage of TIM3, TIGIT, LAG3 (n = 3), CD103, and CD69 (n = 4) expression and the MFIs of Foxp3, CD25, CD44 (n = 3), CTLA4, and ICOS (n = 4) expression in cultured Treg cells. e. Representative histograms of Treg cells cultured as in a. f. Quantification of the MFIs of Blimp1 and GATA3 expression and the percentage of T-bet and TCF1 expression in cultured Treg cells (n = 4). g. Representative flow cytometry plots of Treg cells cultured as in a with isotype and PD-1 antibody. Quantification of the percentage of CTV- cells in the indicated Treg cells. The numbers in the plots and histograms indicate the percentage of the population or the MFIs. The data were analyzed by the two-tailed unpaired Student’s t-test (b, d, f, g). All bar graphs show the mean ± s.e.m. The data are representative of two independent experiments.
Extended Data Fig. 9 Loss of PD-1 in Treg cells delays EAE progression by inducing excessive lung inflammation.
a, b. The disease score (a) assessed by ref. 24 and ref. 31. and body weight (b) of PD-1Ctr (n = 5) and PD-1cKO (n = 5) mice immunized with MOG peptide in CFA emulsion at day 12 post-TAM treatment. c. Representative flow cytometry plots of CD4+ T cells and quantification of the percentages of CD4+ T cells and Treg cells and absolute number of CD4+ T cells, CD4+ Tconv cells, and Treg cells in the spinal cord of PD-1Ctr and PD-1cKO mice as in a at day 19 after immunization. PD-1Ctr (n = 12), PD-1cKO (n = 14) mice. d. Representative histograms of Foxp3 expression in Treg cells quantification of the MFI of Foxp3 expression in Treg cells in the spinal cord of PD-1Ctr and PD-1cKO mice as in c. PD-1Ctr (n = 5), PD-1cKO (n = 5) mice. The numbers in the histogram indicate the MFI. e. Representative flow cytometry plots of CD4+ Tconv cells and quantification of the percentages and absolute number of Tetramer+CD4+ Tconv cells in the spinal cord of PD-1Ctr and PD-1cKO mice as in c. PD-1Ctr (n = 5), PD-1cKO (n = 5) mice. f. Representative flow cytometry plots of CD4+ Tconv cells and quantification of the percentage of IFN-γ+ and/or IL-17+ cells in CD4+ Tconv cells in the spinal cord of PD-1Ctr and PD-1cKO mice as in c. PD-1Ctr (n = 5), PD-1cKO (n = 5) mice. g. Images of H&E staining of the lung of PD-1Ctr (n = 5) and PD-1cKO (n = 5) mice as in c. Scale bars: 200 μm. h. Representative flow cytometry plots of CD8+ and CD4+ T cells and quantification of the percentages of CD8+ T cells, CD4+ Tconv cells, and Treg cells in the lung of PD-1Ctr and PD-1cKO mice as in c. PD-1Ctr (n = 5), PD-1cKO (n = 5) mice. i. Representative flow cytometry plots of CD62L and CD44 expression in CD4+ Tconv cells and quantification of the percentages of CD44+ cells in CD4+ Tconv cells in the lung of PD-1Ctr and PD-1cKO mice as in c. PD-1Ctr (n = 5), PD-1cKO (n = 5) mice. j. Representative flow cytometry plots of CD4+ Tconv cells and quantification of the percentages and absolute number of Tetramer+CD4+ Tconv cells in the lung of PD-1Ctr and PD-1cKO mice as in c. PD-1Ctr (n = 5), PD-1cKO (n = 5) mice. k. Representative flow cytometry plots of CD4+ Tconv cells and quantification of the percentage of IFN-γ+ and/or IL-17+ cells in CD4+ Tconv cells in the lung of PD-1Ctr and PD-1cKO mice as in c. PD-1Ctr (n = 5), PD-1cKO (n = 5) mice. The numbers in the plots indicate the percentage of the population (c, e, f, h, i, j, k). The data were analyzed by the two-tailed unpaired Student’s t-test (c, d, e, f, h, i, j, k) and two-way ANOVA with Sidak’s post hoc test (a, b). All bar graphs show the mean ± s.e.m. The data were pooled from two independent experiments (c). The data are representative of two independent experiments (a, b, d–k).
Extended Data Fig. 10 Deletion of PD-1 in TI Treg cells decreases lipid uptake and mitochondrial mass.
a. Experimental scheme of single-cell analysis. b. UMAP visualization for TI Treg cells of PD-1Cre+/− mice at day 14 post-intravenous injection of TC-1 cells on day 12 post-TAM treatment from the replicate scRNAseq experiments. Different subsets of TI Treg cells in PD-1Cre+/− mice as in b are indicated by color codes. c. UMAP visualization and Contour plot for TI Treg cells in PD-1Cre+/− mice as in b from the replicate scRNAseq experiments. Different subsets of TI Treg cells in PD-1Cre+/− mice as in b are indicated by color codes. d. UMAP visualization of TI Treg cells in PD-1Cre+/− mice as in b from the replicate scRNAseq experiments with an indication of a unique or expanded clonotype. e. The proportion of expanded clonotypes in each TI Treg cell subset in PD-1Cre+/− mice as in b from the replicate scRNAseq experiments. f. Volcano plot of Gene set enrichment analysis (GSEA) between isotype PD-1WT and isotype PD-1KO Treg cells in PD-1Cre+/− mice as in b using MSigDB hallmark gene sets. g. The expression of the indicated gene sets in isotype PD-1WT and isotype PD-1KO Treg cells h. Representative flow cytometry plots of Treg cells in the tumors of PD-1Cre+/− mice injected intravenously with TC-1 cells at day 20 injection. The numbers in the histograms indicate the MFIs. Quantification of the MFIs of lipid uptake and mitochondrial mass in Treg cells in the TC-1 tumor-bearing PD-1Cre+/− mice (n = 6). The data were analyzed by two-tailed unpaired Student’s t-test (h). All bar graphs show the mean ± s.e.m. The data are representative of three independent experiments.
Supplementary information
Supplementary Table
The list of differentially expressed genes between PD-1WT and PD-1KO Treg cells in the same tumor of isotype-treated TC-1 tumor-bearing (as in Extended Data Fig. 10a) PD-1Cre+/− mice at day 14 after injection. Significance of difference in this table was determined by two-sided Wilcoxon rank-sum test.
Source data
Source Data Fig. 1
Statistical Source Data.
Source Data Fig. 2
Statistical Source Data.
Source Data Fig. 3
Statistical Source Data.
Source Data Fig. 4
Statistical Source Data.
Source Data Fig. 5
Statistical Source Data.
Source Data Fig. 6
Statistical Source Data.
Source Data Fig. 7
Statistical Source Data.
Source Data Extended Data Fig. 1
Statistical Source Data.
Source Data Extended Data Fig. 2
Statistical Source Data.
Source Data Extended Data Fig. 3
Statistical Source Data.
Source Data Extended Data Fig. 4
Statistical Source Data.
Source Data Extended Data Fig. 7
Statistical Source Data.
Source Data Extended Data Fig. 8
Statistical Source Data.
Source Data Extended Data Fig. 9
Statistical Source Data.
Source Data Extended Data Fig. 10
Statistical Source Data.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Kim, M.J., Kim, K., Park, H.J. et al. Deletion of PD-1 destabilizes the lineage identity and metabolic fitness of tumor-infiltrating regulatory T cells. Nat Immunol 24, 148–161 (2023). https://doi.org/10.1038/s41590-022-01373-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41590-022-01373-1
This article is cited by
-
Reprogramming of lipid metabolism in the tumor microenvironment: a strategy for tumor immunotherapy
Lipids in Health and Disease (2024)
-
Potential anti-tumor effects of regulatory T cells in the tumor microenvironment: a review
Journal of Translational Medicine (2024)
-
Lipid metabolism in tumor-infiltrating regulatory T cells: perspective to precision immunotherapy
Biomarker Research (2024)
-
Targeting JMJD1C to selectively disrupt tumor Treg cell fitness enhances antitumor immunity
Nature Immunology (2024)
-
Regulatory T cell-mediated immunosuppression orchestrated by cancer: towards an immuno-genomic paradigm for precision medicine
Nature Reviews Clinical Oncology (2024)