Abstract
The intestinal immune system interacts with commensal microbiota to maintain gut homeostasis. Furthermore, stress alters the microbiome composition, leading to impaired brain function; yet how the intestinal immune system mediates these effects remains elusive. Here we report that colonic γδ T cells modulate behavioral vulnerability to chronic social stress via dectin-1 signaling. We show that reduction in specific Lactobacillus species, which are involved in T cell differentiation to protect the host immune system, contributes to stress-induced social-avoidance behavior, consistent with our observations in patients with depression. Stress-susceptible behaviors derive from increased differentiation in colonic interleukin (IL)-17-producing γδ T cells (γδ17 T cells) and their meningeal accumulation. These stress-susceptible cellular and behavioral phenotypes are causally mediated by dectin-1, an innate immune receptor expressed in γδ T cells. Our results highlight the previously unrecognized role of intestinal γδ17 T cells in the modulation of psychological stress responses and the importance of dectin-1 as a potential therapeutic target for the treatment of stress-induced behaviors.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
Data availability
The raw data of the shotgun metagenomic sequencing of mouse fecal samples that support the findings of this study have been deposited to NCBI Sequence Read Archive under BioProject PRJNA758357. The 16S rRNA gene sequences obtained from human fecal samples have been deposited to the DNA DataBank of Japan (DDBJ) under the accession number DRA010810 (patients with MDD) and DRA012712 (healthy controls). Human data have been de-identified to protect confidentiality. The SILVA132 database can be downloaded at https://www.arb-silva.de/fileadmin/silva_databases/qiime/Silva_132_release.zip. Source data are provided with this paper.
References
Hodes, G. E. et al. Individual differences in the peripheral immune system promote resilience versus susceptibility to social stress. Proc. Natl Acad. Sci. USA 111, 16136–16141 (2014).
McKim, D. B. et al. Microglial recruitment of IL-1β-producing monocytes to brain endothelium causes stress-induced anxiety. Mol. Psychiatry 23, 1421–1431 (2018).
Nie, X. et al. The innate immune receptors TLR2/4 mediate repeated social defeat stress-induced social avoidance through prefrontal microglial activation. Neuron 99, 464–479 (2018).
Pearson-Leary, J. et al. The gut microbiome regulates the increases in depressive-type behaviors and in inflammatory processes in the ventral hippocampus of stress vulnerable rats. Mol. Psychiatry 25, 1068–1079 (2020).
Haroon, E., Raison, C. L. & Miller, A. H. Psychoneuroimmunology meets neuropsychopharmacology: translational implications of the impact of inflammation on behavior. Neuropsychopharmacology 37, 137–162 (2012).
Sakamoto, S. et al. Alterations in circulating extracellular vesicles underlie social stress-induced behaviors in mice. FEBS Open Bio 11, 2678–2692 (2021).
Biltz, R. G., Sawicki, C. M., Sheridan, J. F. & Godbout, J. P. The neuroimmunology of social-stress-induced sensitization. Nat. Immunol. 23, 1527–1535 (2022).
Ribot, J. C., Lopes, N. & Silva-Santos, B. γδ T cells in tissue physiology and surveillance. Nat. Rev. Immunol. 21, 221–232 (2021).
Jin, C. et al. Commensal microbiota promote lung cancer development via γδ T cells. Cell 176, 998–1013 (2019).
Shichita, T. et al. Pivotal role of cerebral interleukin-17-producing γδT cells in the delayed phase of ischemic brain injury. Nat. Med. 15, 946–950 (2009).
Dupraz, L. et al. Gut microbiota-derived short-chain fatty acids regulate IL-17 production by mouse and human intestinal γδ T cells. Cell Rep. 36, 109332 (2021).
Filiano, A. J. et al. Unexpected role of interferon-γ in regulating neuronal connectivity and social behaviour. Nature 535, 425–429 (2016).
Alves de Lima, K. et al. Meningeal γδ T cells regulate anxiety-like behavior via IL-17a signaling in neurons. Nat. Immunol. 21, 1421–1429 (2020).
Ribeiro, M. et al. Meningeal γδ T cell-derived IL-17 controls synaptic plasticity and short-term memory. Sci. Immunol. 4, eaay5199 (2019).
Benakis, C. et al. Commensal microbiota affects ischemic stroke outcome by regulating intestinal γδ T cells. Nat. Med. 22, 516–523 (2016).
Choi, G. B. et al. The maternal interleukin-17a pathway in mice promotes autism-like phenotypes in offspring. Science 351, 933–939 (2016).
Medina-Rodriguez, E. M. et al. Identification of a signaling mechanism by which the microbiome regulates Th17 cell-mediated depressive-like behaviors in mice. Am. J. Psychiatry 177, 974–990 (2020).
Brown, G. D. Dectin-1: a signalling non-TLR pattern-recognition receptor. Nat. Rev. Immunol. 6, 33–43 (2006).
Martin, B., Hirota, K., Cua, D. J., Stockinger, B. & Veldhoen, M. Interleukin-17-producing γδ T cells selectively expand in response to pathogen products and environmental signals. Immunity 31, 321–330 (2009).
Kamiya, T. et al. β-Glucans in food modify colonic microflora by inducing antimicrobial protein, calprotectin, in a Dectin-1-induced-IL-17F-dependent manner. Mucosal Immunol. 11, 763–773 (2018).
Tang, C. et al. Inhibition of Dectin-1 signaling ameliorates colitis by inducing Lactobacillus-mediated regulatory T cell expansion in the intestine. Cell Host Microbe 18, 183–197 (2015).
Zhu, X. et al. JHU-083 selectively blocks glutaminase activity in brain CD11b+ cells and prevents depression-associated behaviors induced by chronic social defeat stress. Neuropsychopharmacology 44, 683–694 (2019).
Burokas, A. et al. Targeting the microbiota–gut–brain axis: prebiotics have anxiolytic and antidepressant-like effects and reverse the impact of chronic stress in mice. Biol. Psychiatry 82, 472–487 (2017).
Gao, X. et al. Chronic stress promotes colitis by disturbing the gut microbiota and triggering immune system response. Proc. Natl Acad. Sci. USA 115, E2960–E2969 (2018).
Lee, Y. K., Menezes, J. S., Umesaki, Y. & Mazmanian, S. K. Proinflammatory T-cell responses to gut microbiota promote experimental autoimmune encephalomyelitis. Proc. Natl Acad. Sci USA 108, 4615–4622 (2011).
Atarashi, K. et al. Induction of colonic regulatory T cells by indigenous Clostridium species. Science 331, 337–341 (2011).
Yohn, C. N. et al. Chronic non-discriminatory social defeat is an effective chronic stress paradigm for both male and female mice. Neuropsychopharmacology 44, 2220–2229 (2019).
Harris, A. Z. et al. A novel method for chronic social defeat stress in female mice. Neuropsychopharmacology 43, 1276–1283 (2018).
Nielsen, M. M., Witherden, D. A. & Havran, W. L. γδ T cells in homeostasis and host defence of epithelial barrier tissues. Nat. Rev. Immunol. 17, 733–745 (2017).
Vantourout, P. & Hayday, A. Six-of-the-best: unique contributions of γδ T cells to immunology. Nat. Rev. Immunol. 13, 88–100 (2013).
Taylor, P. R. et al. Dectin-1 is required for β-glucan recognition and control of fungal infection. Nat. Immunol. 8, 31–38 (2007).
Iliev, I. D. et al. Interactions between commensal fungi and the C-type lectin receptor Dectin-1 influence colitis. Science 336, 1314–1317 (2012).
Goodridge, H. S. et al. Activation of the innate immune receptor Dectin-1 upon formation of a ‘phagocytic synapse’. Nature 472, 471–475 (2011).
Charlet, R., Bortolus, C., Sendid, B. & Jawhara, S. Bacteroides thetaiotaomicron and Lactobacillus johnsonii modulate intestinal inflammation and eliminate fungi via enzymatic hydrolysis of the fungal cell wall. Sci. Rep. 10, 11510 (2020).
Yuan, N. et al. An integrated pharmacology-based analysis for antidepressant mechanism of Chinese herbal formula Xiao-Yao-San. Front. Pharm. 11, 284 (2020).
Sutton, C. E. et al. Interleukin-1 and IL-23 induce innate IL-17 production from γδ T cells, amplifying Th17 responses and autoimmunity. Immunity 31, 331–341 (2009).
Guo, Y. et al. Antidepressant effects of rosemary extracts associate with anti-inflammatory effect and rebalance of gut microbiota. Front. Pharm. 9, 1126 (2018).
Tung, T. H. et al. Fish oil, but not olive oil, ameliorates depressive-like behavior and gut microbiota dysbiosis in rats under chronic mild stress. Biomolecules 9, 516 (2019).
Xie, R. et al. Oral treatment with Lactobacillus reuteri attenuates depressive-like behaviors and serotonin metabolism alterations induced by chronic social defeat stress. J. Psychiatr. Res. 122, 70–78 (2020).
Furusawa, Y. et al. Commensal microbe-derived butyrate induces the differentiation of colonic regulatory T cells. Nature 504, 446–450 (2013).
Park, S. G. et al. T regulatory cells maintain intestinal homeostasis by suppressing γδ T cells. Immunity 33, 791–803 (2010).
Golden, S. A., Covington, H. E. III, Berton, O. & Russo, S. J. A standardized protocol for repeated social defeat stress in mice. Nat. Protoc. 6, 1183–1191 (2011).
Klein, S. L. & Flanagan, K. L. Sex differences in immune responses. Nat. Rev. Immunol. 16, 626–638 (2016).
Salk, R. H., Hyde, J. S. & Abramson, L. Y. Gender differences in depression in representative national samples: meta-analyses of diagnoses and symptoms. Psychol. Bull. 143, 783–822 (2017).
Brachman, R. A., Lehmann, M. L., Maric, D. & Herkenham, M. Lymphocytes from chronically stressed mice confer antidepressant-like effects to naive mice. J. Neurosci. 35, 1530–1538 (2015).
Fan, K. Q. et al. Stress-induced metabolic disorder in peripheral CD4+ T cells leads to anxiety-like behavior. Cell 179, 864–879 (2019).
Papotto, P. H., Ribot, J. C. & Silva-Santos, B. IL-17+ γδ T cells as kick-starters of inflammation. Nat. Immunol. 18, 604–611 (2017).
Daley, D. et al. Dectin 1 activation on macrophages by galectin 9 promotes pancreatic carcinoma and peritumoral immune tolerance. Nat. Med. 23, 556–567 (2017).
Mikocka-Walus, A., Ford, A. C. & Drossman, D. A. Antidepressants in inflammatory bowel disease. Nat. Rev. Gastroenterol. Hepatol. 17, 184–192 (2020).
Lewis, K. et al. The prevalence and risk factors of undiagnosed depression and anxiety disorders among patients with inflammatory bowel disease. Inflamm. Bowel Dis. 25, 1674–1680 (2019).
Hamilton, M. A rating scale for depression. J. Neurol. Neurosurg. Psychiatry 23, 56–62 (1960).
Montgomery, S. A. & Asberg, M. A new depression scale designed to be sensitive to change. Br. J. Psychiatry 134, 382–389 (1979).
Hamilton, M. The assessment of anxiety states by rating. Br. J. Med. Psychol. 32, 50–55 (1959).
Buysse, D. J., Reynolds, C. F. III, Monk, T. H., Berman, S. R. & Kupfer, D. J. The Pittsburgh Sleep Quality Index: a new instrument for psychiatric practice and research. Psychiatry Res. 28, 193–213 (1989).
Ishii, C. et al. A metabologenomic approach reveals changes in the intestinal environment of mice fed on american diet. Int. J. Mol. Sci. 19, 4079 (2018).
Kim, S. W. et al. Robustness of gut microbiota of healthy adults in response to probiotic intervention revealed by high-throughput pyrosequencing. DNA Res. 20, 241–253 (2013).
Bolyen, E. et al. Reproducible, interactive, scalable and extensible microbiome data science using QIIME 2. Nat. Biotechnol. 37, 852–857 (2019).
Quast, C. et al. The SILVA ribosomal RNA gene database project: improved data processing and web-based tools. Nucleic Acids Res. 41, D590–596 (2013).
Yilmaz, P. et al. The SILVA and ‘All-species Living Tree Project (LTP)’ taxonomic frameworks. Nucleic Acids Res. 42, D643–D648 (2014).
Denou, E. et al. Gene expression of commensal Lactobacillus johnsonii strain NCC533 during in vitro growth and in the murine gut. J. Bacteriol. 189, 8109–8119 (2007).
Mindus, C., Ellis, J., van Staaveren, N. & Harlander-Matauschek, A. Lactobacillus-based probiotics reduce the adverse effects of stress in rodents: a meta-analysis. Front. Behav. Neurosci. 15, 642757 (2021).
Inoue, R., Otsuka, M., Nishio, A. & Ushida, K. Primary administration of Lactobacillus johnsonii NCC533 in weaning period suppresses the elevation of proinflammatory cytokines and CD86 gene expressions in skin lesions in NC/Nga mice. FEMS Immunol. Med. Microbiol. 50, 67–76 (2007).
Chu, M. P. et al. Pachyman treatment improves CD4+CD25+ Treg counts and serum interleukin 4 and interferon γ levels in a mouse model of Kawasaki disease. Mol. Med. Rep. 5, 1237–1240 (2012).
Niwa, M. et al. Knockdown of DISC1 by in utero gene transfer disturbs postnatal dopaminergic maturation in the frontal cortex and leads to adult behavioral deficits. Neuron 65, 480–489 (2010).
Hasegawa, Y. et al. Causal impact of local inflammation in the nasal cavity on higher brain function and cognition. Neurosci. Res. 172, 110–115 (2021).
Ottesen, A. et al. Enrichment dynamics of Listeria monocytogenes and the associated microbiome from naturally contaminated ice cream linked to a listeriosis outbreak. BMC Microbiol. 16, 275 (2016).
Ponnusamy, D. et al. Cross-talk among flesh-eating Aeromonas hydrophila strains in mixed infection leading to necrotizing fasciitis. Proc. Natl Acad. Sci. USA 113, 722–727 (2016).
Hasan, N. A. et al. Microbial community profiling of human saliva using shotgun metagenomic sequencing. PLoS ONE 9, e97699 (2014).
Lax, S. et al. Longitudinal analysis of microbial interaction between humans and the indoor environment. Science 345, 1048–1052 (2014).
Hatano, S. et al. Development of a new monoclonal antibody specific to mouse Vγ6 chain. Life Sci. Alliance 2, e201900363 (2019).
Wang, G. et al. Arf1-mediated lipid metabolism sustains cancer cells and its ablation induces anti-tumor immune responses in mice. Nat. Commun. 11, 220 (2020).
Acknowledgements
We thank S. Poynton and S. Madireddy for critical reading of the manuscript. We thank Y. Iwakura (Tokyo University of Science), R. Giger (University of Michigan) and T. Hohl (Sloan Kettering Institute) for providing us with Clec7a−/− mice. We thank the Johns Hopkins University School of Medicine (JHU SOM) Flow Cytometry Core Facility, the JHU SOM Microscopy Core Facility and the JHU SOM Behavioral Core Facility. We thank S. Duboux (Société des Produits Nestlé S.A.) and Y. Fukushima (Nestlé Japan Manufacturing, Tokyo, Japan) for their help with culturing L. johnsonii La1, which was supplied from NESTEC.LTD (Lausanne, Switzerland). Some elements in Fig. 4a and Extended Data Fig. 4a were created using BioRender.com (2021). We also thank D. Tamura for providing a gift fund. This work was supported by grants from the National Institute of Health Awards grant nos DA041208 (A.K.), AG065168 (A.K.), MH094268 (A.K.), MH128765 (A.K.), AT008547 (A.K.), AT010984 (X.Z.), NS041435 (P.A.C.) and MH113645 (S.-i.K.) as well as institutional and foundation grants from the JHU catalyst award (A.K.), JSCNP (S.S.), Kanae (Y.H.), JST ERATO (grant no. JPMJER1902; S.F.), AMED-CREST (grant no. JP22gm1010009; S.F.), JSPS KAKENHI (grant no. 22H03541; S.F.), the Food Science Institute Foundation (S.F.) and the Japan Dairy Association (J-milk) (K.S.).
Author information
Authors and Affiliations
Contributions
X.Z., S.S. and A.K. designed the study. X.Z. and S.S. performed cellular, behavioral and immunohistochemical experiments, and contributed to data analysis for all experiments. K.I., M.D.S., L.U., Y.H. and M.O. assisted X.Z. and S.S. with the cellular, behavioral and histochemical assays. X.Z. and S.S. performed shotgun metagenomic sequencing and analyzed taxonomic data. C.I. and S.F. performed 16S rRNA gene sequencing and analyzed taxonomic data together with S.S., X.Z. and K.S. S.K., T.K. and K.S. contributed to human data collection and analysis. H.L. and T.-H.W. contributed to the La1 culture, and helped X.Z. and M.O. perform in vivo La1 administration. S.H. and Y.Y. contributed to the production and characterization of antibody to Vγ6 as well as data interpretation of the flow cytometry experiments. P.A.C. and S.-i.K. contributed to study design, data analysis and interpretation, and provided technical assistance in the cellular experiments. A.K. contributed to the concept or design of the work, and drafting the manuscript with X.Z. and S.S. All authors have approved the final manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Immunology thanks the anonymous reviewers for their contribution to the peer review of this work. Primary Handling Editor: L. A. Dempsey, in collaboration with the Nature Immunology team.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Extended data
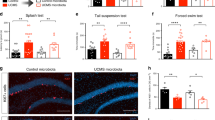
Extended Data Fig. 1 CSDS-induced social-avoidance phenotype assessed using a three-chamber social interaction test.
a, CSDS results in a spectrum of social-avoidance behavior, divided between susceptible and resilient phenotypes using their social interaction ratio (SIR) score. b, Time the mice spent in the chamber with an inanimate object versus with a stranger mouse (top left). Time the mice spent sniffing an inanimate object versus a stranger mouse (top right). Representative heatmaps depict movements of the control, resilient and susceptible mice (bottom). c, Body-weight change ratio of control, resilient and susceptible mice between before and after CSDS. a–c, n = 18 control mice, n = 16 resilient mice and n = 16 susceptible mice. **P < 0.01 (time spent in chambers: P = 0.0067, P < 0.0001, P < 0.0001; time spent sniffing: P < 0.0001, P = 0.0006 and P < 0.0001), determined using an unpaired two-tailed Student’s t-test. Error bars represent the mean ± s.e.m.
Extended Data Fig. 2 CSDS induces alteration of intestinal bacteria.
a, Bray–Curtis β-diversity index of grouped data of fecal bacteria at the species level (n = 5 per group). Analyzed by one-way ANOVA with Tukey’s post-hoc test; adjustments were made for multiple comparisons. b, Relative abundance of fecal bacteria at the genus level in control, resilient and susceptible mice (n = 5 per group). For details about altered species across conditions, refer to Supplementary Table 1.
Extended Data Fig. 3 The effect of CSDS on other T cell subtypes in the colon, small intestine and spleen.
a, Representative flow cytometry plots of CD3+ T cells, CD4+ T cells (CD3+CD4+), CD8+ T cells (CD3+CD8+), TH17 cells (CD3+CD4+IL-17+) and Treg cells (CD3+CD4+FOXP3+) in the LP of the colon. b–d, Percentages of CD3+ T cells in viable cells, CD4+ T cells in CD3+ T cells, CD8+ T cells in CD3+ T cells, TH17 cells in CD4+ T cells and Treg cells in CD4+ T cells in the LP of the colon (b; n = 5, 5 and 5), small intestine (c; n = 6, 6 and 5) and spleen (d; n = 6, 7 and 5) of control, resilient and susceptible mice. *P < 0.05 (P values are P = 0.031 and P = 0.0402), determined by one-way ANOVA with Tukey’s post-hoc test. Error bars represent the mean ± s.e.m.
Extended Data Fig. 4 CSDS-induced changes in T cells and social avoidance in female mice.
a, Schematic representation of chronic stress paradigm for female mice. b,c, Attack number (b) and latency (c) of CD-1 aggressors on male versus female mice. n = 100 (ten mice × 10 d) per group. d,e, Attack number (d) and latency (e) of CD-1 aggressors during the proestrus (n = 20), estrus (n = 33), metestrus (n = 18) and diestrus (n = 29) cycle of female mice. f, The percentage of CD-1 behaviors directed towards female mice over the 10-d chronic stress period (left) and over the course of the estrous cycle (middle and right). g, Time the mice spent in the chamber with an inanimate object versus a stranger female mouse (top left). Time the mice spent sniffing an inanimate object versus a stranger female mouse (top right). The color in the dots represents the estrous cycle of the test female mouse. Representative heatmaps depict mice movements (bottom). Control, resilient and susceptible mice (n = 5, 5, 5). h, Percentage of γδ T cells in CD3+ T cells, γδ17 T cells in γδ T cells, CD3+ T cells in viable cells, CD4+ T cells in CD3+ T cells, CD8+ T cells in CD3+ T cells, TH17 cells in CD4+ T cells and Treg cells in CD4+ T cells in the meninges of control, resilient and susceptible female mice (n = 5, 5, 5). b,g, *P < 0.05 and **P < 0.01 (b, P < 0.0001; g, time spent in chambers: P = 0.0039 and P = 0.0033; time spent sniffing: P = 0.0012, P = 0.0237 and P = 0.0049), determined using an unpaired two-tailed Student’s t-test. h, *P < 0.05 (percentage γδ T cells: P = 0.0158, P = 0.0126; percentage γδ17 T cells: P = 0.0203), determined by one-way ANOVA with Tukey’s post-hoc test. Error bars represent the mean ± s.e.m.
Extended Data Fig. 5 Quantification of La1 in the colonic fecal samples by quantitative real time PCR.
The graphs represent the La1 level in the fecal samples collected from the colon of ctr + vehicle and CSDS + vehicle groups of mice (n = 5, 5; left) as well as the colon of CSDS + vehicle and CSDS + La1 groups of mice (n = 5, 5; right). *P < 0.05 (P = 0.0039 and P = 0.0142), determined by an unpaired two-tailed Student’s t-test. Error bars represent the mean ± s.e.m.
Extended Data Fig. 6 Inhibition effects of i.p. injection of anti-TCR-γδ on γδ T cells in the spleen and meninges as well as CSDS-induced behaviors and the effect of genetic deletion of TCRd on CSDS-induced social-avoidance behavior.
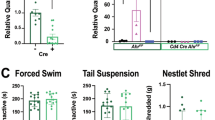
a, Representative flow cytometry plots of γδ T cells (CD3+TCR-β−TCR-γδ+) in the spleen of WT mice injected with IgG or anti-TCR-γδ (10, 50 and 250 µg per mouse, respectively) at post-injection day 12. b, Representative flow cytometry plots of γδ T cells in the spleen of WT mice injected with IgG or anti-TCR-γδ (50 µg per mouse) at post-injection days 3 and day 7, respectively. c, Representative flow cytometry plots of γδ T cells in the meninges of WT mice injected with IgG or anti-TCR-γδ (50 µg per mouse) at post-injection day 12. The boxes in the dot plots identify γδ T cells and represent the percentage of γδ T cells in CD3+ T cells in all groups of mice. d, Locomotion activity as assessed by total counts in the OFT of ctr + IgG, CSDS + IgG, ctr + anti-TCR-γδ and CSDS + anti-TCR-γδ groups of mice (n = 9, 9, 10 and 10). e, Percentage of time spent in the open arms during the elevated plus maze (EPM) test of mice: ctr + IgG, CSDS + IgG, ctr + anti-TCR-γδ and CSDS + anti-TCR-γδ (n = 9, 10, 9 and 10). f,g, The average of total fluid consumption (f) and the average percentage of sucrose consumption when given a choice between 1.5% sucrose and water (g) on days 5–8 of ctr + IgG, CSDS + IgG, ctr + anti-TCR-γδ and CSDS + anti-TCR-γδ groups of mice (n = 8, 9, 7, 9). h, Time test mice spend in the chambers (top left). Time test mice spent sniffing (top right). Representative heatmaps depicting mice movements (bottom). n = 10 for WT + CSDS and n = 7 for TCRd-KO + CSDS groups of mice. e,g, *P < 0.05 and **P < 0.01 (e, P = 0.0384 and P = 0.0045; g, P = 0.0207), determined by two-way ANOVA with Tukey’s post-hoc test. h, **P < 0.01 (time spent in chambers: P = 0.0041; time spent in sniffing: P < 0.0001), determined by an unpaired two-tailed Student’s t-test. Error bars represent the mean ± s.e.m.
Extended Data Fig. 7 No change in dectin-1 expression in the colonic CD3+, CD4+ and CD8+ T cells of C57BL/6 mice after CSDS.
Representative flow cytometry plots (left). Percentage of dectin-1+ cells in CD3+ (top right), CD4+ (middle right) and CD8+ (bottom right) T cells in the LP of the colon of control, resilient and susceptible mice (n = 4, 4, 4). The boxes in the dot plots identify dectin-1+ cells in each group of mice. Error bars represent the mean ± s.e.m.
Extended Data Fig. 8 The effects of genetic deletion of dectin-1 and treatment with pachyman on CSDS-induced phenotypes in colonic non-γδ T lymphocytes and sucrose preference test.
a–e, Percentage of CD3+ T cells in viable cells (a), CD4+ T cells in CD3+ cells (b), CD8+ T cells in CD3+ cells (c), TH17 cells in CD4+ cells (d) and Treg cells in CD4+ cells (e) in the LP of the colon of WT + ctr, WT + CSDS, dectin-1 KO + ctr and dectin-1 KO + CSDS groups of mice (n = 5, 5, 5, 5). f,g, The average of total fluid consumption (f) and the average percentage of sucrose consumption when given a choice between 1.5% sucrose and water (g) on days 5–8 for WT + ctr, WT + CSDS, dectin-1 KO + ctr and dectin-1 KO + CSDS groups of mice (n = 9, 9, 8, 9). h, Schematic of the experimental design. Eight-week-old TCRd-KO mice received i.v. injection of γδ T cells collected from Clec7a−/− (dectin-1 KO) mice or their WT littermates 1 d before and 5 d after the start of CSDS, followed by a social interaction test (SIT) and tissue collection. i, Schematic of the experimental design. Eight-week-old C57BL/6 mice were orally treated with the vehicle or pachyman daily during the CSDS period, followed by SIT and sucrose preference test (SPT; left). An independent cohort of mice was subjected to tissue harvest 1 d after CSDS (right). j–n, Percentage of CD3+ cells in viable cells (j), CD4+ cells in CD3+ cells (k), CD8+ cells in CD3+ cells (l), TH17 cells in CD4+ cells (m) and Treg cells in CD4+ cells (n) in the LP of the colon of ctr + vehicle, CSDS + vehicle, ctr + pachyman and CSDS + pachyman groups of mice (n = 5, 6, 6, 5). o,p, The average of total fluid consumption (o) and the average percentage of sucrose consumption when given a choice between 1.5% sucrose and water (p) on days 5–8 for four groups of mice: ctr + vehicle, CSDS + vehicle, ctr + pachyman and CSDS + pachyman (n = 10, 10, 10 and 10). *P < 0.05 and **P < 0.01 (c, P = 0.0429; g, P < 0.0001 and P < 0.0001; l, P = 0.0252; p, P < 0.0001 and P < 0.0001), determined by two-way ANOVA with Tukey’s post-hoc test. Error bars represent the mean ± s.e.m.
Supplementary information
Supplementary Information
Supplementary Tables 1–5.
Supplementary Data 1
Source data Supplementary Table 1.
Supplementary Data 2
Source data Supplementary Table 2.
Supplementary Data 3
Source data Supplementary Table 3.
Source data
Source Data Fig. 1
Statistical source data.
Source Data Fig. 2
Statistical source data.
Source Data Fig. 3
Statistical source data.
Source Data Fig. 4
Statistical source data.
Source Data Fig. 5
Statistical source data.
Source Data Fig. 6
Statistical source data.
Source Data Extended Data Fig. 1
Statistical source data.
Source Data Extended Data Fig. 2
Statistical source data.
Source Data Extended Data Fig. 3
Statistical source data.
Source Data Extended Data Fig. 4
Statistical source data,
Source Data Extended Data Fig. 5
Statistical source data.
Source Data Extended Data Fig. 6
Statistical source data.
Source Data Extended Data Fig. 7
Statistical source data.
Source Data Extended Data Fig. 8
Statistical source data.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Zhu, X., Sakamoto, S., Ishii, C. et al. Dectin-1 signaling on colonic γδ T cells promotes psychosocial stress responses. Nat Immunol 24, 625–636 (2023). https://doi.org/10.1038/s41590-023-01447-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41590-023-01447-8
This article is cited by
-
Gut microbiome-derived ammonia modulates stress vulnerability in the host
Nature Metabolism (2023)
-
Chiral nanoparticle-remodeled gut microbiota alleviates neurodegeneration via the gut–brain axis
Nature Aging (2023)
-
Central regulation of stress-evoked peripheral immune responses
Nature Reviews Neuroscience (2023)
-
γδ T cells: origin and fate, subsets, diseases and immunotherapy
Signal Transduction and Targeted Therapy (2023)
-
Dectin-1 on colonic γδ T cells mediates vulnerability to psychosocial stress
Nature Reviews Immunology (2023)