Abstract
Brain atlases are spatial references for integrating, processing, and analyzing brain features gathered from different individuals, sources, and scales. Here we introduce a collection of joint surface–volume atlases that chart postnatal development of the human brain in a spatiotemporally dense manner from two weeks to two years of age. Our month-specific atlases chart normative patterns and capture key traits of early brain development and are therefore conducive to identifying aberrations from normal developmental trajectories. These atlases will enhance our understanding of early structural and functional development by facilitating the mapping of diverse features of the infant brain to a common reference frame for precise multifaceted quantification of cortical and subcortical changes.
Similar content being viewed by others
Main
Human brain development is a complex and protracted process that begins during the third gestational week and extends through adulthood1. Throughout the late prenatal period and early infancy, the human brain undergoes a myriad of cellular processes, including neurogenesis, neuronal migration, astrogliogenesis, oligodendrogenesis, synaptogenesis, myelination, and synaptic pruning2 (Supplementary Fig. 1). These cellular events drive the rapid growth of the infant brain during the first two years of life, resulting in structural changes and reorganization of neural circuits3. The intracranial volume of the newborn brain doubles during the first postnatal year, attaining approximately 65% of the adult brain volume4. The gray matter (GM) volume increases more (~71%) than the white matter (WM) volume (~20%) in the first year. A precise quantification of early brain growth will be conducive to a step change in our understanding of developments that lead to maturation of cognitive functions3. However, endeavors in this direction have so far been hindered by the dearth of brain atlases for mapping features of early brain development to common spaces necessary for fine-grained spatiotemporal quantification of brain changes.
Unlike the long-established growth charts utilized by pediatricians to quantify year-to-year maturation in terms of a child’s height, weight, and head circumference in relation to standardized curves derived from healthy growing children5, normative references for neurodevelopment are virtually nonexistent. Recent efforts have been dedicated toward constructing brain charts that capture normative patterns of cerebral development4 in terms of volumetric growth of GM, WM, and cerebrospinal fluid (CSF), and cortical growth captured by surface area and cortical thickness6. Although these brain charts standardize brain morphological measurements, they do not define a common coordinate system for mapping brain features, particularly from multiple imaging modalities, to a unified reference space. An additional limitation of existing brain charts is that they rely on the demarcation of the brain into adjoint but separate brain areas with hypothetically uniform functions, while in reality an extensive body of evidence suggests a more gradual transition of areal boundaries, particularly in association cortices7. These limitations call for the construction of atlases that offer standardized coordinate frameworks for concurrent analysis of multimodal data at multiple levels of granularity.
Existing atlases of the baby brain are typically limited to specific prenatal or postnatal periods8,9,10,11,12 (Supplementary Fig. 2 and Supplementary Table 1). Brain atlases densely covering the entirety of the first two years of life are lacking owing to challenges in collecting longitudinal brain magnetic resonance imaging (MRI) data. Adding to the difficulties in constructing these much-needed atlases are the rapid changes in the sizes and shapes of cerebral structures (Supplementary Fig. 3), and the evolving and often insufficient tissue contrast between WM and GM13. Moreover, existing cortical surface and volumetric atlases are typically constructed independently, resulting in the following problems:
-
(i)
misalignment between tissue interfaces of the volumetric atlas and the white and pial surfaces of the cortical atlas;
-
(ii)
fuzzy cortical structures, as volumetric displacements are estimated without taking into account the complex convolutions of the cortical surface;
-
(iii)
anatomically implausible displacements owing to cortical surface registration that is based only on surface attributes but ignoring the volumetric data14;
-
(iv)
volumetric and cortical surface attributes are not defined in the same space, making it difficult to analyze the two entities concurrently and consistently15; and
-
(v)
diminishing of real but subtle changes owing to inconsistent and inaccurate alignment16.
In this work, we present a set of month-specific surface–volume longitudinal brain atlases of infants from 2 weeks to 24 months of age. These atlases facilitate the precise mapping of fine-grained changes in both space and time, and are therefore a valuable resource for studying postnatal human brain development, identifying early roots of neurological disorders, and quantifying development-related malformations. We demonstrate that our high-fidelity atlases capture the normative evolutionary landscape of cortical surface features and tissue volumetric characteristics of the infant brain during the first two years of brain development.
Results
Surface–volume consistency
Our atlases allow multifaceted analyses of volumetric and surface data in a common space. As a reference, we first construct the infant brain atlas (IBA) at 12 months, for volume (IBA12-V) and surface (IBA12-S), in a surface–volume consistent manner17 using high-quality magnetic resonance (MR) images of 37 infant subjects scanned around 12 months of age as part of the Baby Connectome Project (BCP)18. The cortical surface and volumetric data of these subjects are simultaneously normalized in space19 and then fused to form
-
(i)
the white and pial cortical surfaces of IBA12-S and
-
(ii)
the T1-weighted (T1w) intensity image, T2-weighted (T2w) intensity image, and the WM, GM, and CSF tissue maps of IBA12-V.
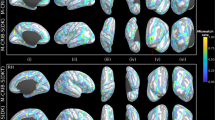
In contrast to atlases constructed with Spherical Demons20 and ANTs21, IBA12-V, and IBA12-S exhibit sharp anatomical details despite being an average of a few tens of subjects (Fig. 1), thanks to the alignment of GM–WM boundaries via explicit registration of cortical surfaces. In fact, IBA12-V resembles MR images of one-year-olds with substantially less fuzziness than the atlas constructed with ANTs without leveraging the geometry of cortical surfaces. This is evident from the close-ups of the temporal and parietal lobes (Fig. 1a,b). Subcortical structures, including the thalamus, caudate nucleus, and putamen, are well defined, indicating that the surface–volume consistent atlas construction process is conducive to preserving both cortical and subcortical anatomical details. While the T1w IBA12-V exhibits adult-like contrast, the T2w IBA12-V is close to isointense with substantial overlap in intensity distributions between WM and GM, consistent with T2w images of one-year-olds22. This is particularly noticeable at the superior frontal and rostral middle frontal cortices (Fig. 1b).
a,b, Transverse sections of T1w and T2w atlases and the T1w and T2w images of an individual subject. c, White and pial cortical surface atlases overlaid onto the corresponding tissue segmentation atlases. The cortical surfaces of the individual subject are overlaid onto its tissue segmentation map. d, Subject and atlas average convexity maps projected onto a sphere. Lateral and medial views of the IBA12-S white surface colored by average convexity are shown for reference. e, Subject and atlas mean curvature maps projected onto a sphere. Lateral and medial views of the IBA12-S white surface colored by mean curvature are shown for reference.
The white and pial cortical surfaces of IBA12-S are consistently aligned with the GM–WM and WM–CSF interfaces as delineated by the tissue segmentation maps of IBA12-V (Fig. 1c). This is confirmed by the zoomed-in views of, for example, the lateral occipital, inferior parietal, cuneus, precuneus, medial orbitofrontal, and superior parietal cortices. On the other hand, the ANTs atlas shows inconsistent alignment of the cortical surfaces with tissue interfaces in the volumetric space.
The cortical surfaces of both hemispheres in IBA12-S preserve cortical convolutions with distinct gyral and sulcal patterns (Fig. 1d,e). For greater clarity, we map the average convexity and mean curvature of the white surface onto a sphere. Mapping is only performed for IBA12-S and ANTs as Spherical Demons outputs only spherical atlases of cortical features. Average convexity captures coarse-scale geometric features of primary sulcal folds23, whereas mean curvature captures fine-scale geometric features of secondary and tertiary folds. The average convexity maps of the Spherical Demons and IBA12-S atlases are consistent with that of an individual subject. On the other hand, the average convexity map of the ANTs atlas indicates atypical narrowing of the gyral and sulcal folds. Only IBA12-S exhibits typical mean curvature patterns. Spherical Demons gives a fuzzy mean curvature map that fails to capture fine-grained details of cortical folds. ANTs gives an atypical mean curvature map, indicating alteration of surface topology. These results indicate that primary gyri and sulci, including the precentral and postcentral gyri and sulci and the central sulcus, are captured by all three atlases, but localized gyral and sulcal details are only preserved in IBA12-S.
Surface atlases across infancy
Early brain development is characterized by dynamic changes in cortical folding patterns. We constructed month-specific cortical atlases for the first two years of life by longitudinally warping IBA12-S. Major cortical folds of white surfaces—including the central sulcus, precentral and postcentral gyri, inferior parietal lobule, temporal gyrus, superior and inferior temporal sulci, superior frontal gyrus, cingulate gyrus and sulcus, and parieto-occipital sulcus—are consistent across time points (Fig. 2a and Extended Data Figs. 1–3). Mean curvature mapped onto the inflated white surface atlases indicates subtle developments of secondary and tertiary gyri and sulci (Fig. 2b). Cortical thickness measured between the white and pial cortical surfaces of the atlases, projected onto the inflated white surface atlases (Fig. 2c), indicates that the cortical thickness of the prefrontal cortex, temporal lobe, and insula increases. By contrast, the thickness of the visual and sensorimotor cortices increases at a slower pace.
Volumetric atlases across infancy
We generated IBA-V, T1w and T2w atlases from infants 2 weeks to 24 months of age (Fig. 3a,b and Extended Data Figs. 4 and 5). The image contrast evolves month-to-month during year one and becomes adult-like in year two. The T1w and T2w atlases are isointense around 3–6 months and 9–12 months, respectively. The atlases preserve distinct boundaries at the GM–WM interface as evident from the close-ups of the precuneus, cuneus, and inferior parietal cortex (Fig. 3a,b).
Age-specific white and pial cortical surface atlases overlapped on the tissue segmentation atlases (Fig. 3c and Extended Data Fig. 6) indicate that the cortical surface atlases are well aligned with the tissue boundaries of the volumetric atlases, particularly at the superior and middle frontal gyri of both the hemispheres, supramarginal gyrus, inferior parietal cortex, insula, and precentral and postcentral gyri. Good alignment is confirmed by zoomed-in views of the atlases at selected time points (Fig. 3c).
Surface and volumetric development
The IBA captures the developmental features (Supplementary Fig. 4) of typically developing infants in the first two years of life. Compared with the atlases constructed with Spherical Demons and ANTs, the IBA more faithfully reflects the individuals, as evidenced by the smaller mean absolute error between the growth curve for each surface or volumetric feature of the IBA and the population curve obtained by fitting the generalized additive mixture model (GAMM)24 (Fig. 4a).
a, Violin plots for absolute error of the developmental trajectories of surface and volumetric features, computed between the atlases and the individual subjects. The mean absolute error is marked by a green crossbar. A single star indicates that the mean error for the IBA is significantly different from ANTs (two-tailed paired t-test, Pa). A double star indicates that the mean error for the IBA is significantly different from both Spherical Demons (two-tailed paired t-test, Psd) and ANTs (two-tailed paired t-test, Pa). b, Developmental trajectories (adjusted R2 = 0.99) of white surface features and tissue volumes of the IBA. c, Surface and volumetric changes every six months.
We studied the surface and volumetric features captured by the IBA (Fig. 4b,c). During the first and second postnatal years, the IBA-S increases in cortical thickness by 25.7% and 4.3%, increases in surface area by 56.1% and 8.5%, increases in average convexity by 25.5% and 3.6%, and decreases in mean curvature by 20.9% and 3.9% (growth rates are reported in terms of percentage change). During the same period, the IBA-V increases in GM volume by 97.2% and 13.8%, increases in WM volume by 92.9% and 15.9%, increases in ventricular CSF volume by 89.8% and 10.5%, and increases in whole-brain volume (WBV = GM + WM) by 95.6% and 14.6%. We also report the velocity curves along with peak growth ages, indicating maximal growth rates, for these surface and volumetric features (Supplementary Fig. 5).
Regional analysis indicates spatially heterogeneous development. We measured the thickness of 34 cortical regions-of-interest (ROIs) delineated via FreeSurfer using the Desikan–Killiany atlas25 (Supplementary Fig. 6). Developmental trajectories of regional cortical thickness show that the insula, frontal cortex, and superior, middle, and inferior temporal cortices are consistently thicker during infancy (Fig. 5). On the other hand, the precentral and postcentral gyri, occipital and inferior parietal cortices, and banks of superior temporal sulcus are thinner throughout the study period. Regional growth rates vary from 21.2% to 32.0% and 2.3% to 6.7% for the first and second postnatal years (Extended Data Fig. 7). The growth rates (Year 1, Year 2) are (22.6%, 2.3%) for the primary visual cortex, (25.1%, 3.3%) for the primary somatosensory cortex, (24.5%, 4.3%) for the primary motor cortex, (29.5%, 4.0%) for the primary auditory cortex, (27.7%, 4.4%) for the temporal association cortex, (27.6%, 4.4%) for the parietal association cortex, and (23.9%, 4.8%) for the prefrontal association cortex (Extended Data Fig. 7a).
Growth curves of cortical thickness for the IBA cortical regions. Shaded regions indicate whether cortical thickness is higher or lower than the whole-brain average.
We show the developmental trajectories of regional surface area in Extended Data Fig. 8 and growth rates for the first and second postnatal years in Extended Data Fig. 9. High-expansion regions include rostral anterior cingulate gyrus, medial orbitofrontal gyrus, superior frontal gyrus, rostral middle frontal gyrus, inferior and middle temporal gyri, and superior and inferior parietal gyri. Low-expansion regions include insula, pericalcarine, cuneus, entorhinal, temporal pole, and parahippocampal gyrus. Regional growth rates vary from 20.5% to 49.7% in year 1 and 2.9% to 11.1% in year 2. The growth rates (Year 1, Year 2) are (42.4%, 3.4%) for the primary visual cortex, (36.0%, 5.6%) for the primary somatosensory cortex, (34.1%, 6.0%) for the primary motor cortex, (42.3%, 5.3%) for the primary auditory cortex, (39.8%, 6.9%) for the temporal association cortex, (38.9%, 5.8%) for the parietal association cortex, and (38.1%, 7.7%) for the prefrontal association cortex (Extended Data Fig. 9a).
The IBA captures the asymmetry of cortical features. We show the hemispheric lateralization of regional cortical features via the laterality index, LI = (left − right)/(left + right), computed for each ROI. There is significant asymmetry (two-tailed t-test; P < 0.01) in cortical thickness (Extended Data Fig. 10) and surface area (Supplementary Fig. 7) for majority of the ROIs. The corresponding P values, t-scores, and degrees of freedom are reported for cortical thickness in the source data of Extended Data Fig. 10 and for surface area in Supplementary Data File 2. The ROI-specific mean LI for cortical thickness and surface area are shown in Extended Data Figs. 7b, 9b. Results for other attributes are presented in Supplementary Note 1.
Cortical T1w/T2w ratio
We investigated cortical myelination across infancy by T1w/T2w ratio mapping of the cortical ribbon to the white surface of each individual subject26. The GAMM-fitted myelin maps (Fig. 6) indicate increasing myelination in both cerebral hemispheres at average rates of 57.7% and 7.9% in the first and second postnatal years, respectively. Myelination is spatially heterogeneous with heavy myelination in the primary visual, motor, and somatosensory cortices and light myelination in the prefrontal, parietal, and temporal association cortices.
Cortical T1w/T2w ratio, mapped onto the inflated white surfaces of the IBA.
Discussion
Charting normative structural and functional changes during early brain development is key to understanding aberrations associated with neurodevelopmental disorders such as attention-deficit/hyperactivity disorder, dyslexia, and cerebral palsy27,28,29. We have presented a set of longitudinal, unified surface–volume atlases of the infant brain covering every month of the first two postnatal years. These atlases represent a resource that will facilitate the joint investigation of changes in cortical geometries and brain tissues during a period of rapid and critical brain development. Our atlases are substantially better in preserving anatomical details and surface geometric features than ANTs21,30 and Spherical Demons20,31.
The human brain undergoes complex gyrification that begins after mid-gestation32, nudging the initially smooth cerebral surface into a highly convoluted structure. A widely used macroscopic morphological measure is cortical thickness, which is systematically related to the cytoarchitecture and hierarchical structural organization of the cortex33. The neurobiological mechanisms underlying developmental changes in cortical thickness are complex and involve processes such as synaptogenesis, proliferation of dendrites and dendritic spines, axonal sprouting, and vascular development34. The IBA shows that cortical thickness increases rapidly during the first year and more slowly in the second year. Greater cortical thickness during early brain development is in general positively associated with intelligence and cognitive skills in later stages of life35. The IBA also indicates that cortical thickness changes heterogeneously across the cortex, in line with previous studies36. The association cortices in the temporal, parietal, and prefrontal lobes are thicker compared with the primary visual and sensorimotor cortices, which agrees with the fact that thicker cortices are in general less mature. This is consistent with the functional development of the infant brain where vision, motor, and sensory systems are more mature than executive functions. More discussion on the cortical attributes of the IBA is included in Supplementary Note 2.
In addition to folding geometry, we quantitatively assessed brain tissue volume changes during infancy. Early neurodevelopment is underpinned by cellular and molecular processes that drive the growth and maturation of brain tissues. Cell bodies, axon terminal branches, dendrites, and spines residing in GM contribute to the early growth of GM37. Axonal tracts accounting for WM form inter-hemispheric, cortico-cortical, limbic, brainstem, and cortico-spinal connections, providing an efficient network of structural connectivity38,39. Furthermore, CSF, apart from cushioning the brain, contributes to brain maturation and function by delivering growth factors and signaling molecules to progenitor cells that proliferate into immature neurons, which later migrate to different areas of the cerebral cortex40,41. IBA growth curves for GM, WM, ventricular CSF, and whole-brain volume indicate that volumes of all tissues increase from birth through two years of age, albeit at rates different from previous studies. Knickmeyer et al.4 reported changes in GM volume by 149% in the first year and 14% in the second year. More moderate changes were reported for the WM volume at 11% in the first year and 19% in the second year. On the other hand, the IBA increases in GM volume by 97.2% and 13.8%, and increase in WM volume by 92.9% and 15.9%, during first and second postnatal years. These discrepancies can be due to differences in segmentation methods, percentage change definitions, and what were considered as WM and GM during volume calculation.
Overall, qualitative and quantitative analyses confirm that the surface–volume consistent IBA faithfully reflects the growth trajectories of infants with rich anatomical details. These atlases capture monthly changes in brain shape and size, cortical geometry, tissue contrast, volume, and microstructural characteristics of typically developing infants. A trait that sets the IBA apart from currently available atlases is that it covers each month during the first two postnatal years, providing a dense spatiotemporal depiction of this critical period of development. We hope that these atlases will become an invaluable common coordinate framework and facilitate the discovery of new insights into developmental processes underpinning child cognition and social behavior.
Methods
Dataset and preprocessing
Longitudinal T1w and T2w MRI scans of 37 subjects (20 females and 17 males) enrolled as part of the UNC/UMN Baby Connectome Project (BCP)18 were used in this work. Participants were recruited from existing registries at UNC and UMN based on state-wide birth records as well as from broader community resources (for example, community centers and targeted day-care centers) to ensure the sample approximates the racial/ethnic and socio-economic diversity of the US census. Informed consent was obtained from the parents of all subjects. The subjects were divided into six cohorts (A1, A2, A3, B1, B2, and B3) with first visits scheduled at 2 weeks, 1, 2, 9, 10, and 11 months, respectively. The subjects in A1, A2, and A3 were scheduled to be scanned every 3 months in the first year and then at 24 months; whereas, the subjects in B1, B2, and B3 were scanned every 3 months for the first 2 years. The total number of scans for each subject is different since not all subjects could be scanned at all expected time points. A total of 108 scans for each imaging modality were used. The study protocols were approved by the Institutional Review Board of the School of Medicine of The University of North Carolina at Chapel Hill.
The images were acquired using 3T Siemens Prisma MRI scanners equipped with Siemens 32-channel head coils. T1w MR images were acquired with 208 sagittal slices, TR/TE = 2,400/2.24 ms, flip angle = 8°, acquisition matrix = 320 × 320, and resolution = (0.8 mm)3. T2w MR images were acquired with 208 sagittal slices, TR/TE = 3,200/564 ms, variable flip angles, acquisition matrix = 320 × 320, and resolution = (0.8 mm)3.
MR images were quality-controlled using an automated algorithm42 and then preprocessed using an infant-centric processing pipeline (iBEAT v.2.0; available at https://ibeat.wildapricot.org) consisting of the following steps:
-
(i)
rigid alignment of T1w and T2w images using FLIRT43;
-
(ii)
skull stripping by a learning-based method44;
-
(iii)
intensity inhomogeneity correction by N345;
-
(iv)
brain tissue segmentation by an infant dedicated learning-based method46;
-
(v)
hemisphere separation and subcortical filling; and
-
(vi)
topologically-correct cortical surface reconstruction47.
Example results of the processing steps are shown in Supplementary Fig. 15.
Surface–volume atlas construction
Our atlas construction method (Supplementary Fig. 16a) involves two main tasks:
-
(i)
construction of the 12-month surface–volume atlas via surface-constrained groupwise registration; and
-
(ii)
construction of longitudinal atlases for 2 weeks to 24 months by propagating the 12-month atlas through parallel transported longitudinal deformations.
The steps involved in each task are detailed below.
(i) Construction of reference atlas—IBA12. We first construct the 12-month surface–volume reference atlas, that is, IBA12, and use it to generate the atlases at the other time points. The IBA12 lies in the middle of the two-year time span and is hence ideal to capture developmental patterns between birth and two years of age.
Surface-constrained groupwise registration. The low intensity contrast of infant brain MRI renders image registration and atlas construction challenging. Here we use a dynamic elasticity model with surface constraint (SC-DEM19) for groupwise registration using tissue segmentation maps instead of intensity images. Groupwise registration allows a population of tissue segmentation maps to be registered simultaneously to a common space as demonstrated in our previous work17. In our case, this is achieved by first selecting a reference based on a subject scan that is most similar in appearance to the whole set of images. Then, the reference tissue segmentation map is iteratively updated by fusing all tissue segmentation maps that are registered to it. SC-DEM improves the alignment of tissue boundaries using predetermined surface transformations. Before SC-DEM groupwise registration, the tissue segmentation maps are affine-aligned with the reference tissue segmentation map using FLIRT43. From here on, affine transform is assumed to have taken place before SC-DEM registration unless otherwise stated.
For cortical surface registration, the white surface is inflated and mapped onto the unit sphere via metric distortion minimization23. The surfaces in the spherical space are represented by two folding attributes: average convexity and mean curvature. The spherical surfaces of the moving scans are registered to that of the reference scan using Spherical Demons20. The resulting vertex-wise correspondences are propagated to the white and pial cortical surfaces by leveraging the one-to-one vertex mapping between the spherical surfaces and the white and pial cortical surfaces.
SC-DEM employs a dynamic elasticity model48 to characterize large nonlinear displacements. For the i-th subject scanned at time \(\tau \in {{{{\mathcal{W}}}}}_{12}\) (in terms of chronological age), where \({{{{\mathcal{W}}}}}_{\rho }=[(\rho -1.5),(\rho +1.5)]\) months, we estimate displacement field with respect to a reference \({\phi }_{(i,\tau )\to {{{\rm{ref}}}}}(\overrightarrow{x})\) by solving the wave equation:
where ∇2 is the vector Laplacian operator and \({f}_{(i,\tau )\to {{{\rm{ref}}}}}^{\,{{{\rm{vol}}}}}(\overrightarrow{x})\) and \({f}_{(i,\tau )\to {{{\rm{ref}}}}}^{\,{{{\rm{surf}}}}}(\overrightarrow{x})\) are respectively the volumetric and surface force fields. The volumetric force field \({f}_{(i,\tau )\to {{{\rm{ref}}}}}^{\,{{{\rm{vol}}}}}(\overrightarrow{x})\) captures the misalignment between the warped moving tissue segmentation map \({I}_{(i,\tau )}(\overrightarrow{x}+{\phi }_{(i,\tau )\to {{{\rm{ref}}}}}(\overrightarrow{x}))\) and the reference tissue segmentation map \({I}_{{{{\rm{ref}}}}}(\overrightarrow{x})\):
The surface force field \({f}_{(i,\tau )\to {\,{{\rm{ref}}}}}^{{{{\rm{surf}}}}}(\overrightarrow{x})\) is computed on the basis of the differences between the volumetric displacements and the predetermined surface displacements for white and pial surfaces for both left and right hemispheres. The parameter α controls deformation smoothness and parameters β and γ balance the two force fields and control the rate of convergence. Registration halts when the two force fields are negligibly small, thereby consistently aligning the surface and the tissue segmentation map.
Cortical surface atlas. The cortical surface atlas comprising white and pial surfaces of both left and right hemispheres is constructed by using the registered cortical surfaces \({\{{\hat{S}}_{(i,\tau )}\}}_{(i,\tau )}\), each associated with a weight w(τ, 12), computed using \(w({\tau }_{1},{\tau }_{2})=\frac{1}{\sigma \sqrt{2\pi }}\exp \left(\frac{-{({\tau }_{1}-{\tau }_{2})}^{2}}{2{\sigma }^{2}}\right)\), where parameter σ controls temporal smoothness and is set to 0.7 months. The 12-month cortical surface atlas \({A}_{12}^{{{{\rm{surf}}}}}\) is constructed by weighted averaging of the vertex coordinates for \(\tau \in {{{{\mathcal{W}}}}}_{12}\): \(\frac{{\sum }_{(i,\tau )}w(\tau ,12){\hat{S}}_{(i,\tau )}}{{\sum }_{(i,\tau )}w(\tau ,12)}\). Next, we use \({A}_{12}^{{{{\rm{surf}}}}}\) to construct the corresponding volumetric atlas such that the two atlases are defined in the same coordinate space.
Volumetric atlas. The tissue segmentation volumetric atlas \({A}_{12}^{{{{\rm{tissue}}}}}\) is constructed by correcting the displacement fields \({\{{\phi }_{(i,\tau )}(\overrightarrow{x})\}}_{(i,\tau )}\) for \(\tau \in {{{{\mathcal{W}}}}}_{12}\) on the basis of the surface atlas \({A}_{12}^{{{{\rm{surf}}}}}\); ensuring the alignment of the volumetric WM–GM and GM–CSF interfaces in accordance with the surface atlas. This involves updating the volumetric displacement fields by considering surface misalignment as described below:
-
1.
Compute for each vertex with coordinates \(\overrightarrow{y}\) on the surface atlas the vertex-wise surface displacement \({{\Delta }}{\psi }_{(i,\tau )}(\overrightarrow{y})\) between \({\hat{S}}_{(i,\tau )}\) and \({A}_{12}^{{{{\rm{surf}}}}}\).
-
2.
Find in \({A}_{12}^{{{{\rm{surf}}}}}\) the triangle with vertices \({\{{\overrightarrow{y}}_{p}\}}_{p = 1}^{3}\) closest to a query voxel \(\overrightarrow{x}\).
-
3.
Compute the corrective volumetric displacement field \({{\Delta }}{\phi }_{(i,\tau )}(\overrightarrow{x})\) as
$${{\Delta }}{\phi }_{(i,\tau )}(\overrightarrow{x})=\left\{\begin{array}{ll}\frac{\mathop{\sum }\nolimits_{p = 1}^{3}w(\overrightarrow{x},{\overrightarrow{y}}_{p}){{\Delta }}{\psi }_{(i,\tau )}({\overrightarrow{y}}_{p})}{\mathop{\sum }\nolimits_{p = 1}^{3}w(\overrightarrow{x},{\overrightarrow{y}}_{p})}\quad &{{{\rm{if}}}}\ \parallel \overrightarrow{x}-{\overrightarrow{y}}_{p}\parallel \le \delta ,\quad \forall p;\\ 0\quad &{{{\rm{otherwise}}}},\end{array}\right.$$(3)where \(w(\overrightarrow{x},\overrightarrow{y})=\frac{1}{{\sigma }_{g}\sqrt{2\pi }}\exp \left(\frac{-{\parallel \overrightarrow{x}-\overrightarrow{y}\parallel }^{2}}{2{\sigma }_{g}^{2}}\right)\) and δ = 6 mm. We set σg = 3 mm for smooth displacement fields.
-
4.
Warp the tissue segmentation maps using the total displacement field \({\phi }_{(i,\tau )\to {A}_{12}^{{{{\rm{vol}}}}}}={\phi }_{(i,\tau )\to {{{\rm{ref}}}}}+{{\Delta }}{\phi }_{(i,\tau )}\) for better alignment and preservation of anatomical details.
-
5.
Determine the tissue label via majority voting for \(\tau \in {{{{\mathcal{W}}}}}_{12}\).
-
6.
Warp the intensity images (T1w and T2w) using the total displacement field \({\phi }_{(i,\tau )\to {A}_{12}^{{{{\rm{vol}}}}}}\) to obtain \({\{{\hat{I}}_{(i,\tau )}\}}_{(i,\tau )}\).
-
7.
Average the warped intensity images using the weights {w(τ,12)} to obtain 12-month T1w and T2w atlases: \({A}_{12}^{{{{\rm{int}}}}}=\frac{{\sum }_{(i,\tau )}w(\tau ,12){\hat{I}}_{(i,\tau )}}{{\sum }_{(i,\tau )}w(\tau ,12)}\).
(ii) Construction of longitudinal surface–volume atlases. The surface–volume atlases for 2 weeks to 24 months are generated by propagating the IBA12 to each month. To achieve this, we determine the average longitudinal change from each month to the 12-month atlas space. This is realized by transporting the longitudinal deformations to the atlas space via inter-subject deformations (Supplementary Fig. 16b).
Parallel transport of longitudinal deformations. The longitudinal growth of a subject is characterized by the displacement field \({\phi }_{(i,\tau ^{\prime} )\to (i,\tau )}\) from time point \(\tau ^{\prime}\) to time point \(\tau \in {{{{\mathcal{W}}}}}_{12}\), estimated via affine and SC-DEM registration. Intra-subject deformation fields \({{{\Phi }}}_{(i,\tau ^{\prime} )\to (i,\tau )}=\overrightarrow{x}+{\phi }_{(i,\tau ^{\prime} )\to (i,\tau )}\) are spatially normalized to the 12-month atlas space by parallel transport49—disentangling inter-subject variability from longitudinal growth. The transported deformation field from the subject space to the 12-month atlas space is given as \({{{\Phi }}}_{(i,\tau ^{\prime} )\to (i,\tau )\to {A}_{12}^{{{{\rm{vol}}}}}}^{\parallel }={{{\Phi }}}_{(i,\tau )\to {A}_{12}^{{{{\rm{vol}}}}}}^{-1}\circ {{{\Phi }}}_{(i,\tau ^{\prime} )\to (i,\tau )}\circ {{{\Phi }}}_{(i,\tau )\to {A}_{12}^{{{{\rm{vol}}}}}}\).
Kernel regression. To construct age-specifc surface–volume consistent atlases, we warp the 12-month atlas to each specific time point τ0 with the weighted average of the transported displacement fields \({\{{\phi }_{(i,\tau ^{\prime} )\to (i,\tau )\to {A}_{12}^{{{{\rm{vol}}}}}}^{\parallel }\}}\,_{\tau ^{\prime} \in {{{{\mathcal{W}}}}}_{{\tau }_{0}},\tau \in {{{{\mathcal{W}}}}}_{12}}\):
The transported displacement fields are weighted based on whether \(\tau ^{\prime}\) and τ are close to τ0 and 12, respectively (Supplementary Fig. 16c). The displacement field \({\phi }_{{\tau }_{0}\to {A}_{12}^{{{{\rm{vol}}}}}}^{\parallel }\) is used to warp the 12-month cortical surface and tissue segmentation maps atlases to each time point. Intensity images (T1w and T2w) are warped with total deformation fields \({{{\Phi }}}_{(i,\tau ^{\prime} )\to (i,\tau )}\circ {{{\Phi }}}_{(i,\tau )\to {A}_{12}^{{{{\rm{vol}}}}}}\circ {{{{\Phi }}}^{\parallel }}_{{\tau }_{0}\to {A}_{12}^{{{{\rm{vol}}}}}}^{-1}\) to obtain \({\{{\hat{I}}_{(i,\tau ^{\prime} )}\}}_{(i,\tau ^{\prime} )}\), which are averaged to generate T1w and T2w atlases: \({A}_{{\tau }_{0}}^{{{{\rm{int}}}}}=\frac{{\sum }_{i}w(\tau ^{\prime} ,{\tau }_{0})w(\tau ,12)\ {\hat{I}}_{(i,\tau ^{\prime} )}}{{\sum }_{i}w(\tau ^{\prime} ,{\tau }_{0})w(\tau ,12)}\).
Developmental trajectories of the infant population
To characterize infant brain maturation, we estimated the developmental trajectories of brain tissues volume, cortical thickness, surface area, average convexity, mean curvature, and cortical T1w/T2w ratio. For subject i at scan time t, the age effects on feature fi(t) are modeled using GAMM24: fi(t) = s(t) + γi. Here, s(⋅) is a smooth nonlinear function represented by cubic regression splines and γi is the subject-specific random intercept. The degree of smoothness is selected using the restricted maximum likelihood criterion in R (https://www.r-project.org/).
Developmental trajectories of the IBA
To characterize developmental pattern captured by the IBA, we estimated the growth trajectories of surface and volumetric features. For the IBA at time point t, the age effects on feature f(t) are modeled using the generalized additive model (GAM): f(t) = s(t). Here, s(⋅) is a smooth nonlinear function represented by cubic regression splines. The degree of smoothness is selected using the restricted maximum likelihood criterion in R.
Statistical analysis for laterality
For each cortical ROI, we performed a one-sample two-tailed t-test at 1% significance level to determine whether the surface geometric features are significantly lateralized across the first two years of life.
Atlas construction with compared methods
For comparison purposes, we constructed (i) cortical surface atlases with Spherical Demons20 and (ii) volume and surface atlases with ANTs21. We used developer-optimized parameters20,50.
(i) Spherical Demons atlas construction. The cortical surface atlases at each month τ0 are generated by groupwise registration of white surfaces of the subjects scanned at time \(\tau ^{\prime} \in {{{{\mathcal{W}}}}}_{{\tau }_{0}}\). The white cortical surfaces are mapped onto the unit sphere and then spatially aligned via Spherical Demons, which uses average convexity and mean curvature to drive surface registration. During groupwise registration, individual cortical surfaces are aligned to the iteratively updated mean cortical surface. Finally, average convexity and mean curvature maps are averaged to obtain the surface atlases.
(ii) ANTs atlas construction. We first generate the 12-month surface and volume atlases, and use these to obtain atlases at the other time points. For the 12-month atlas, the tissue segmentation maps of the subjects scanned at time \(\tau \in {{{{\mathcal{W}}}}}_{12}\) are spatially aligned via groupwise registration with ANTs. The cortical surfaces and intensity images of the registered subjects are then averaged with weights {w(τ, 12)}. The warped tissue segmentation maps are fused via majority voting. Next, we propagate these 12-month surface and volume atlases to each month τ0 via parallel transport of intra-subject deformation fields estimated using affine and ANTs registration. Warped intensity images are weighted-averaged to obtain the T1w and T2w atlases.
Reporting summary
Further information on research design is available in the Nature Portfolio Reporting Summary linked to this article.
Data availability
The infant brain atlases are available at Zenodo (https://doi.org/10.5281/zenodo.7044932) under the Creative Commons Attribution Non Commercial Share Alike 4.0 International license. The MRI data used in this work can be obtained from the National Institute of Mental Health Data Archive (https://nda.nih.gov/) or by contacting the investigative team18. Source data for quantitative results in the Figures 4, 5, and 6, Extended Data Figs. 8 and 10, and Supplementary Figs. 5, 7, 8, 10, 12, 13, and 17 are provided as Excel spreadsheets with this paper.
Code availability
Software packages used for atlas construction include iBEAT v.2.0, FreeSurfer v.7.2, Spherical Demons v.1.4, FSL v.6.0.5, and RStudio v.1.2.1335. Additional code facilitating atlas construction is available under the MIT license via our project webpage (https://iba.yaplab.io).
References
Stiles, J. & Jernigan, T. L. The basics of brain development. Neuropsychol. Rev. 20, 327–348 (2010).
Silbereis, J. C., Pochareddy, S., Zhu, Y., Li, M. & Sestan, N. The cellular and molecular landscapes of the developing human central nervous system. Neuron 89, 248–268 (2016).
Gilmore, J. H., Knickmeyer, R. C. & Gao, W. Imaging structural and functional brain development in early childhood. Nat. Rev. Neurosci. 19, 123–137 (2018).
Knickmeyer, R. C. et al. A structural MRI study of human brain development from birth to 2 years. J. Neurosci. 28, 12176–12182 (2008).
Tanner, J. M., Whitehouse, R. H. & Takaishi, M. Standards from birth to maturity for height, weight, height velocity, and weight velocity: British children, 1965. i. Arch. Dis. Child. 41, 454–471 (1966).
Bethlehem, R. et al. Brain charts for the human lifespan. Nature 604, 525–533 (2022).
Margulies, D. S. et al. Situating the default-mode network along a principal gradient of macroscale cortical organization. Proc. Natl Acad. Sci. USA 113, 12574–12579 (2016).
Oishi, K., Chang, L. & Huang, H. Baby brain atlases. Neuroimage 185, 865–880 (2019).
Bozek, J. et al. Construction of a neonatal cortical surface atlas using multimodal surface matching in the developing human connectome project. Neuroimage 179, 11–29 (2018).
Wu, Z. et al. 4D infant cortical surface atlas construction using spherical patch-based sparse representation. In Medical Image Computing and Computer Assisted Intervention (eds Descoteaux, M. et al.) 57–65 (HHS, 2017).
Oishi, K. et al. Multi-contrast human neonatal brain atlas: application to normal neonate development analysis. Neuroimage 56, 8–20 (2011).
Shi, F. et al. Infant brain atlases from neonates to 1- and 2-year-olds. PLoS ONE 6, 1–11 (2011).
Wang, L. et al. 4D multi-modality tissue segmentation of serial infant images. PLoS ONE 7, 1–9 (2012).
Villalon, J., Joshi, A. A., Toga, A. W. & Thompson, P. Comparison of volumetric registration algorithms for tensor-based morphometry. In IEEE International Symposium on Biomedical Imaging 1536 – 1541 (IEEE, 2011).
Wu, J. et al. Accurate nonlinear mapping between MNI volumetric and FreeSurfer surface coordinate systems. Hum. Brain Mapp. 39, 3793–3808 (2018).
Lecoeur, J. et al. Automated longitudinal registration of high resolution structural MRI brain sub-volumes in non-human primates. J. Neurosci. Methods 202, 99–108 (2011).
Ahmad, S. et al. Surface–volume consistent construction of longitudinal atlases for the early developing brain. In Medical Image Computing and Computer Assisted Intervention (eds Shen, D. et al.) 815–822 (Springer, 2019).
Howell, B. R. et al. The UNC/UMN Baby Connectome Project (BCP): an overview of the study design and protocol development. Neuroimage 185, 891–905 (2019).
Ahmad, S. et al. Surface-constrained volumetric registration for the early developing brain. Med. Image Anal. 58, 101540 (2019).
Yeo, B. T. T. et al. Spherical demons: Fast diffeomorphic landmark-free surface registration. IEEE Trans. Med. Imaging 29, 650–668 (2010).
Avants, B., Epstein, C., Grossman, M. & Gee, J. Symmetric diffeomorphic image registration with cross-correlation: evaluating automated labeling of elderly and neurodegenerative brain. Med. Image Anal. 12, 26–41 (2008).
Severino, M. et al. Definitions and classification of malformations of cortical development: practical guidelines. Brain 143, 2874–2894 (2020).
Fischl, B., Sereno, M. I. & Dale, A. M. Cortical surface-based analysis: II: Inflation, flattening, and a surface-based coordinate system. Neuroimage 9, 195–207 (1999).
Lin, X. & Zhang, D. Inference in generalized additive mixed models by using smoothing splines. J. Royal Stat. Soc. Series B Stat. Methodol. 61, 381–400 (1999).
Fischl, B. Freesurfer. Neuroimage 62, 774–781 (2012).
Glasser, M. F. & Van Essen, D. C. Mapping human cortical areas in vivo based on myelin content as revealed by T1- and T2-weighted MRI. J. Neurosci. 31, 11597–11616 (2011).
Johnson, M. H., Gliga, T., Jones, E. & Charman, T. Annual research review: Infant development, autism, and ADHD — early pathways to emerging disorders. J. Child Psychol. Psychiat. 56, 228–247 (2015).
Hadders-Algra, M. Early diagnosis and early intervention in cerebral palsy. Frontiers Neurol. 5, 185 (2014).
Langer, N. et al. White matter alterations in infants at risk for developmental dyslexia. Cereb. Cortex 27, 1027–1036 (2015).
Yu, B. et al. HybraPD atlas: towards precise subcortical nuclei segmentation using multimodality medical images in patients with Parkinson disease. Hum. Brain Mapp. 42, 4399–4421 (2021).
Wu, Z. et al. Construction of 4D infant cortical surface atlases with sharp folding patterns via spherical patch-based group-wise sparse representation. Hum. Brain Mapp. 40, 3860–3880 (2019).
Girard, N., Raybaud, C., Gambarelli, D. & Figarella-Branger, D. Fetal brain MR imaging. Magn. Reson. Imaging Clin. N. Am. 9, 19–56 (2001).
Valk, S. L. et al. Shaping brain structure: Genetic and phylogenetic axes of macroscale organization of cortical thickness. Sci. Adv. 6, eabb3417 (2020).
Fjell, A. M. et al. Development and aging of cortical thickness correspond to genetic organization patterns. Proc. Natl Acad. Sci. USA 112, 15462–15467 (2015).
Karama, S. et al. Childhood cognitive ability accounts for associations between cognitive ability and brain cortical thickness in old age. Mol. Psych. 19, 555–559 (2014).
Wang, F. et al. Developmental topography of cortical thickness during infancy. Proc. Natl Acad. Sci. USA 116, 15855–15860 (2019).
Purves, D. et al. Neuroscience (Oxford Univ. Press, 2017).
Yu, Q. et al. Differential white matter maturation from birth to 8 years of age. Cereb. Cortex 30, 2674–2690 (2019).
Fields, R. D. Change in the brain’s white matter. Science 330, 768–769 (2010).
Shen, M. D. Cerebrospinal fluid and the early brain development of autism. J. Neurodev. Dis. 10, 39 (2018).
Lehtinen, M. K. et al. The cerebrospinal fluid provides a proliferative niche for neural progenitor cells. Neuron 69, 893–905 (2011).
Liu, S., Thung, K.-H., Lin, W., Yap, P.-T. & Shen, D. Real-time quality assessment of pediatric MRI via semi-supervised deep nonlocal residual neural networks. IEEE Trans. Image Process. 29, 7697–7706 (2020).
Smith, S. M. et al. Advances in functional and structural MR image analysis and implementation as FSL. Neuroimage 23, S208–S219 (2004).
Zhang, Q. et al. Frnet: flattened residual network for infant MRI skull stripping. In 2019 IEEE 16th International Symposium on Biomedical Imaging 999–1002 (IEEE, 2019).
Sled, J. G., Zijdenbos, A. P. & Evans, A. C. A nonparametric method for automatic correction of intensity nonuniformity in MRI data. IEEE Trans. Med. Imaging 17, 87–97 (1998).
Wang, L. et al. Volume-based analysis of 6-month-old infant brain MRI for autism biomarker identification and early diagnosis. In Medical Image Computing and Computer Assisted Intervention (eds Frangi, A. F. et al.) 411–419 (Springer, 2018).
Li, G. et al. Mapping region-specific longitudinal cortical surface expansion from birth to 2 years of age. Cereb. Cortex 23, 2724–2733 (2013).
Ahmad, S. & Khan, M. F. Dynamic elasticity model for inter-subject non-rigid registration of 3D MRI brain scans. Biomed. Signal Process. Control 33, 346–357 (2017).
Lorenzi, M. & Pennec, X. Efficient parallel transport of deformations in time series of images: from schild’s to pole ladder. J. Math. Imaging Vis. 50, 5–17 (2014).
Klein, A. et al. Evaluation of 14 nonlinear deformation algorithms applied to human brain MRI registration. Neuroimage 46, 786–802 (2009).
Acknowledgements
This work was supported in part by National Institutes of Health (NIH) grants (EB008374 and MH125479 to P.-T.Y.; MH116225 to G.L.; MH117943 to G.L. and L.W.; and MH110274 to W.L.) and the efforts of the UNC/UMN Baby Connectome Project Consortium. Figures were in part created with BioRender (https://biorender.com).
Author information
Authors and Affiliations
Contributions
S.A.: methodology, software, investigation, writing—original draft, writing—review and editing. Y.W.: writing—review and editing. Z.W.: data curation. K.-H.T.: data curation. S.L.: resources. W.L.: resources. G.L.: resources. L.W: resources. P.-T.Y.: conceptualization, supervision, funding acquisition, validation, writing—review and editing.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Peer review
Peer review information
Nature Methods thanks Ulas Bagci, James Shine, and the other, anonymous, reviewer for their contribution to the peer review of this work. Primary Handling editor: Nina Vogt, in collaboration with the Nature Methods team.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Extended data
Extended Data Fig. 1 Cortical atlases of the white surface.
The age-specific cortical atlases of the white surface for both hemispheres, colored by average convexity (millimeter).
Extended Data Fig. 2 Cortical atlases of the pial surface.
Dorsal views of the age-specific cortical atlases of the pial surface for both hemispheres spanning 2 weeks to 24 months, colored by mean curvature (millimeter−1).
Extended Data Fig. 3 Mean curvature maps of the longitudinal infant brain atlases.
The inflated cortical atlases of the white surface (left hemisphere, lateral view) superimposed with mean curvature (millimeter−1) spanning 2 weeks to 24 months.
Extended Data Fig. 4 T1w atlases of the infant brain.
Transverse sections of the T1w atlases depicting dynamic changes in tissues contrast, size, and shape of anatomical structures at each month between 2 weeks and 24 months.
Extended Data Fig. 5 T2w atlases of the infant brain.
Transverse sections of the T2w atlases depicting dynamic changes in tissues contrast, size, and shape of anatomical structures at each month between 2 weeks and 24 months.
Extended Data Fig. 6 Spatial consistency between age-specific cortical surface and volumetric atlases.
Left and right cortical atlases of the white (blue) and pial (red) surfaces are superimposed onto the tissue segmentation atlases.
Extended Data Fig. 7 Analysis of cortical thickness.
a, Regional growth rates in terms of cortical thickness for the first (top row) and second (bottom row) postnatal years. b, ROI-specific mean laterality index for cortical thickness.
Extended Data Fig. 8 Regional developmental trajectories of surface area.
Growth curves of surface area for the IBA cortical regions. Shaded regions indicate whether surface area is higher or lower than the whole-brain average.
Extended Data Fig. 9 Analysis of surface area.
a, Regional growth rates in terms of surface area for the first (top row) and second (bottom row) postnatal years. b, ROI-specific mean laterality index for surface area.
Extended Data Fig. 10 Hemispheric asymmetry of cortical thickness.
Region-specific laterality index for cortical thickness of the IBA. Positive laterality is associated with left lateralization (two-tailed t-test: p < 0.01) and negative laterality is associated with right lateralization (two-tailed t-test: p < 0.01).
Supplementary information
Supplementary Information
Supplementary Figs 1–17, Supplementary Tables 1–2, Supplementary Notes 1–4, and Supplementary References.
Supplementary Data 1
Source data for Supplementary Figure 5.
Supplementary Data 2
Source data for Supplementary Fig. 7.
Supplementary Data 3
Source data for Supplementary Fig. 8.
Supplementary Data 4
Source data for Supplementary Fig. 10.
Supplementary Data 5
Source data for supplementary Fig. 12.
Supplementary Data 6
Source data for supplementary Fig. 13.
Supplementary Data 7
Source data for supplementary Fig. 17.
Source data
Source Data Fig. 4
Error values, fitted curves, and growth rates.
Source Data Fig. 5
Cortical thickness values of all ROIs across infancy.
Source Data Fig. 6
Cortical T1w/T2w ratio for different cortical lobes/hemispheres.
Source Data Extended Data Fig. 8
Surface area values of all ROIs across infancy.
Source Data Extended Data Fig. 10
Laterality index and statistical tests for cortical thickness.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Ahmad, S., Wu, Y., Wu, Z. et al. Multifaceted atlases of the human brain in its infancy. Nat Methods 20, 55–64 (2023). https://doi.org/10.1038/s41592-022-01703-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41592-022-01703-z
This article is cited by
-
A multimodal submillimeter MRI atlas of the human cerebellum
Scientific Reports (2024)