Abstract
GABA interneurons play a critical role in higher brain functions. Astrocytic glial cells interact with synapses throughout the whole brain and are recognized as regulatory elements of excitatory synaptic transmission. However, it is largely unknown how GABAergic interneurons and astrocytes interact and contribute to stable performance of complex behaviors. Here, we found that genetic ablation of GABAB receptors in medial prefrontal cortex astrocytes altered low-gamma oscillations and firing properties of cortical neurons, which affected goal-directed behaviors. Remarkably, working memory deficits were restored by optogenetic stimulation of astrocytes with melanopsin. Furthermore, melanopsin-activated astrocytes in wild-type mice enhanced the firing rate of cortical neurons and gamma oscillations, as well as improved cognition. Therefore, our work identifies astrocytes as a hub for controlling inhibition in cortical circuits, providing a novel pathway for the behaviorally relevant midrange time-scale regulation of cortical information processing and consistent goal-directed behaviors.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
Data availability
All data generated or analyzed during this study are either included in this published article or are available from the corresponding author upon reasonable request. Source data are provided with this paper.
Code availability
Analyses used in this study are largely standard approaches for this type of data. The code that supports these findings is available upon request from the corresponding author.
References
Kim, H., Ährlund-Richter, S., Wang, X., Deisseroth, K. & Carlén, M. Prefrontal parvalbumin neurons in control of attention. Cell 164, 208–218 (2016).
Cardin, J. A. et al. Driving fast-spiking cells induces gamma rhythm and controls sensory responses. Nature 459, 663–667 (2009).
Mainen, Z. F. & Seinowski, T. J. Reliability of spike timing in neocortical neurons. Science 268, 1503–1506 (1995).
Korotkova, T., Fuchs, E. C., Ponomarenko, A., von Engelhardt, J. & Monyer, H. NMDA receptor ablation on parvalbumin-positive interneurons impairs hippocampal synchrony, spatial representations, and working memory. Neuron 68, 557–569 (2010).
Euston, D. R., Gruber, A. J. & McNaughton, B. L. The role of medial prefrontal cortex in memory and decision making. Neuron 76, 1057–1070 (2012).
Atallah, B. V. & Scanziani, M. Instantaneous modulation of gamma oscillation frequency by balancing excitation with inhibition. Neuron 62, 566–577 (2009).
Ito, H. T., Zhang, S. J., Witter, M. P., Moser, E. I. & Moser, M. B. A prefrontal–thalamo–hippocampal circuit for goal-directed spatial navigation. Nature 522, 50–55 (2015).
Perea, G. & Araque, A. Astrocytes potentiate transmitter release at single hippocampal synapses. Science 317, 1083–1086 (2007).
Henneberger, C., Papouin, T., Oliet, S. H. R. & Rusakov, D. A. Long-term potentiation depends on release of d-serine from astrocytes. Nature 463, 232–236 (2010).
Santello, M., Toni, N. & Volterra, A. Astrocyte function from information processing to cognition and cognitive impairment. Nat. Neurosci. 22, 154–166 (2019).
Araque, A. et al. Gliotransmitters travel in time and space. Neuron 81, 728–739 (2014).
Martin-Fernandez, M. et al. Synapse-specific astrocyte gating of amygdala-related behavior. Nat. Neurosci. 20, 1540–1548 (2017).
Adamsky, A. et al. Astrocytic activation generates de novo neuronal potentiation and memory enhancement. Cell 174, 59–71.e14 (2018).
Boddum, K. et al. Astrocytic GABA transporter activity modulates excitatory neurotransmission. Nat. Commun. 7, 13572 (2016).
Matos, M. et al. Astrocytes detect and upregulate transmission at inhibitory synapses of somatostatin interneurons onto pyramidal cell. Nat. Commun. 9, 4254 (2018).
Perea, G. et al. Activity-dependent switch of GABAergic inhibition into glutamatergic excitation in astrocyte–neuron networks. eLife 5, e20362 (2016).
Kang, J., Jiang, L., Goldman, S. A. & Nedergaard, M. Astrocyte-mediated potentiation of inhibitory synaptic transmission. Nat. Neurosci. 1, 683–692 (1998).
Nagai, J. et al. Hyperactivity with disrupted attention by activation of an astrocyte synaptogenic cue. Cell 177, 1280–1292.e20 (2019).
Covelo, A. & Araque, A. Neuronal activity determines distinct gliotransmitter release from a single astrocyte. eLife 7, e32237 (2018).
Mariotti, L. et al. Interneuron-specific signaling evokes distinctive somatostatin-mediated responses in adult cortical astrocytes. Nat. Commun. 9, 82 (2018).
Perea, G., Yang, A., Boyden, E. S. & Sur, M. Optogenetic astrocyte activation modulates response selectivity of visual cortex neurons in vivo. Nat. Commun. 5, 3262 (2014).
Crosby, K. M. et al. Cholecystokinin switches the plasticity of GABA synapses in the dorsomedial hypothalamus via astrocytic ATP release. J. Neurosci. 38, 8515–8525 (2018).
Deemyad, T., Lüthi, J. & Spruston, N. Astrocytes integrate and drive action potential firing in inhibitory subnetworks. Nat. Commun. 9, 4336 (2018).
Dimidschstein, J. et al. A viral strategy for targeting and manipulating interneurons across vertebrate species. Nat. Neurosci. 19, 1743–1749 (2016).
Isaacson, J. S. & Scanziani, M. How inhibition shapes cortical activity. Neuron 72, 231–243 (2011).
Mederos, S. & Perea, G. GABAergic–astrocyte signaling: a refinement of inhibitory brain networks. Glia 67, 1842–1851 (2019).
Haller, C. et al. Floxed allele for conditional inactivation of the GABAB(1) gene. Genesis 40, 125–130 (2004).
Deacon, R. M. J., Penny, C. & Rawlins, J. N. P. Effects of medial prefrontal cortex cytotoxic lesions in mice. Behav. Brain Res. 139, 139–155 (2003).
Petrides, M. & Pandya, D. N. Comparative cytoarchitectonic analysis of the human and the macaque ventrolateral prefrontal cortex and corticocortical connection patterns in the monkey. Eur. J. Neurosci. 16, 291–310 (2002).
Yang, Y. & Mailman, R. B. Strategic neuronal encoding in medial prefrontal cortex of spatial working memory in the T-maze. Behav. Brain Res. 343, 50–60 (2018).
Buzsáki, G. & Wang, X.-J. Mechanisms of gamma oscillations. Annu. Rev. Neurosci. 35, 203–225 (2012).
Sakatani, S. et al. Neural-activity-dependent release of S100B from astrocytes enhances kainate-induced gmma oscillations in vivo. J. Neurosci. 28, 10928–10936 (2008).
Lee, H. S. et al. Astrocytes contribute to gamma oscillations and recognition memory. Proc. Natl Acad. Sci. USA 111, E3343–E3352 (2014).
Sohal, V. S., Zhang, F., Yizhar, O. & Deisseroth, K. Parvalbumin neurons and gamma rhythms enhance cortical circuit performance. Nature 459, 698–702 (2009).
Lisman, J. E. & Jensen, O. The theta-gamma neural code. Neuron 77, 1002–1016 (2013).
Devilbiss, D. M., Jenison, R. L. & Berridge, C. W. Stress-induced impairment of a working memory task: role of spiking rate and spiking history predicted discharge. PLoS Comput. Biol. 8, e1002681 (2012).
Vijayraghavan, S., Wang, M., Birnbaum, S. G., Williams, G. V. & Arnsten, A. F. T. Inverted-U dopamine D1 receptor actions on prefrontal neurons engaged in working memory. Nat. Neurosci. 10, 376–384 (2007).
Srinivasan, R. et al. Ca2+ signaling in astrocytes from Ip3r2−/− mice in brain slices and during startle responses in vivo. Nat. Neurosci. 18, 708–717 (2015).
Mederos, S. et al. Melanopsin for precise optogenetic activation of astrocyte–neuron networks. Glia 67, 915–934 (2019).
Panatier, A. et al. Astrocytes are endogenous regulators of basal transmission at central synapses. Cell 146, 785–798 (2011).
MacVicar, B. A., Tse, F. W. Y., Crichton, S. A. & Kettenmann, H. GABA-activated Cl− channels in astrocytes of hippocampal slices. J. Neurosci. 9, 3577–3583 (1989).
Sun, H. & Neugebauer, V. mGluR1, but not mGluR5, activates feed-forward inhibition in the medial prefrontal cortex to impair decision making. J. Neurophysiol. 106, 960–973 (2011).
Kamigaki, T. & Dan, Y. Delay activity of specific prefrontal interneuron subtypes modulates memory-guided behavior. Nat. Neurosci. 20, 854–863 (2017).
Yizhar, O. et al. Neocortical excitation/inhibition balance in information processing and social dysfunction. Nature 477, 171–178 (2011).
Carus-Cadavieco, M. et al. Gamma oscillations organize top-down signalling to hypothalamus and enable food seeking. Nature 542, 232–236 (2017).
Kim, J. G. et al. Leptin signaling in astrocytes regulates hypothalamic neuronal circuits and feeding. Nat. Neurosci. 17, 908–910 (2014).
Oe, Y. et al. Distinct temporal integration of noradrenaline signaling by astrocytic second messengers during vigilance. Nat. Commun. 11, 471 (2020).
Steckler, T. et al. Metabotropic glutamate receptor 1 blockade impairs acquisition and retention in a spatial water maze task. Behav. Brain Res. 164, 52–60 (2005).
Liu, D. et al. Medial prefrontal activity during delay period contributes to learning of a working memory task. Science 346, 458–463 (2014).
Tremblay, R., Lee, S. & Rudy, B. GABAergic interneurons in the neocortex: from cellular properties to circuits. Neuron 91, 260–292 (2016).
Gonzalez-Roca, E. et al. Accurate expression profiling of very small cell populations. PLoS ONE 5, e14418 (2010).
Bender, F., Korotkova, T. & Ponomarenko, A. Optogenetic entrainment of hippocampal theta oscillations in behaving mice. J. Vis. Exp. https://doi.org/10.3791/57349 (2018).
Vandecasteele, M. et al. Large-scale recording of neurons by movable silicon probes in behaving rodents. J. Vis. Exp. https://doi.org/10.3791/3568 (2012).
Bender, F. et al. Theta oscillations regulate the speed of locomotion via a hippocampus to lateral septum pathway. Nat. Commun. 6, 8521 (2015).
Herrera, C. G. et al. Hypothalamic feedforward inhibition of thalamocortical network controls arousal and consciousness. Nat. Neurosci. 19, 290–298 (2016).
Hazan, L., Zugaro, M. & Buzsáki, G. Klusters, NeuroScope, NDManager: a free software suite for neurophysiological data processing and visualization. J. Neurosci. Methods 155, 207–216 (2006).
Schmitzer-Torbert, N. & Redish, A. D. Neuronal activity in the rodent dorsal striatum in sequential navigation: separation of spatial and reward responses on the multiple T task. J. Neurophysiol. 91, 2259–2272 (2004).
Wulff, P. et al. Hippocampal theta rhythm and its coupling with gamma oscillations require fast inhibition onto parvalbumin-positive interneurons. Proc. Natl Acad. Sci. USA 106, 3561–3566 (2009).
Tort, A. B. L., Komorowski, R., Eichenbaum, H. & Kopell, N. Measuring phase-amplitude coupling between neuronal oscillations of different frequencies. J. Neurophysiol. 104, 1195–1210 (2010).
Buzsáki, G. & Mizuseki, K. The log-dynamic brain: how skewed distributions affect network operations. Nat. Rev. Neurosci. 15, 264–278 (2014).
Valero, M., Averkin, R. G., Fernandez-Lamo, I., Cid, E. & Tamas, G. Mechanisms for selective single-cell reactivation during offline sharp-wave ripples and their distortion by fast ripples. Neuron 94, 1234–1247.e7 (2017).
Rolls, E. T. & Treves, A. The neuronal encoding of information in the brain. Prog. Neurobiol. 95, 448–490 (2011).
Quian Quiroga, R. & Panzeri, S. Extracting information from neuronal populations: information theory and decoding approaches. Nat. Rev. Neurosci. 10, 173–185 (2009).
Csicsvari, J., Jamieson, B., Wise, K. D. & Buzsáki, G. Mechanisms of gamma oscillations in the hippocampus of the behaving rat. Neuron 37, 311–322 (2003).
Toth, L. A. & Gardiner, T. W. Food and water restriction protocols: physiological and behavioral considerations. Contemp. Top. Lab. Anim. Sci. 39, 9–17 (2000).
Klavir, O., Prigge, M., Sarel, A., Paz, R. & Yizhar, O. Manipulating fear associations via optogenetic modulation of amygdala inputs to prefrontal cortex. Nat. Neurosci. 20, 836–844 (2017).
Jain, A. K. & Dubes, R. C. Algorithms for Clustering Data (Prentice Hall, 1988).
Paxinos, G. & Franklin, K. The Mouse Brain in Stereotaxic Coordinates, Compact 3rd edn (Academic Press, 2008).
Reyes, A., Lee, D., Graziani, C. & Tzeferacos, P. A new class of high-order methods for fluid dynamics simulations using Gaussian process modeling: one-dimensional case. J. Sci. Comput. 76, 443–480 (2018).
Poskanzer, K. E. & Yuste, R. Astrocytes regulate cortical state switching in vivo. Proc. Natl Acad. Sci. USA 113, E2675–E2684 (2016).
Fernández de Sevilla, D., Cabezas, C., Oshima de Prada, A. N., Sánchez-Jiménez, A. & Buño, W. Selective muscarinic regulation of functional glutamatergic Schaffer collateral synapses in rat CA1 pyramidal neurons. J. Physiol. 545, 51–63 (2002).
Faber, D. S. & Korn, H. Applicability of the coefficient of variation method for analyzing synaptic plasticity. Biophys. J. 60, 1288–1294 (1991).
Acknowledgements
We thank the T. Korotkova Lab, C. González-Arias and C. Heikenfeld for technical support; the Molecular and Cellular Biology Unit from the Cajal Institute for technical assistance; B. Bettler, A. Araque, M. Navarrete, E. D. Martín and A. Fernández-Ruiz for critical review of the manuscript; and Buzsáki Lab members for valuable suggestions. This work was supported by the MCIU/AEI/FEDER, UE (BES-2014-067594) to S.M.; a European Molecular Biology Organization (EMBO) postdoctoral fellowship (EMBO ALTF 1161-2017) and a Human Frontiers Science Program (HFSP) postdoctoral fellowship (LT0000717/2018) to M.V.; the Deutsche Forschungsgemeinschaft (DFG: SPP1665, 1799/1-2 and Heisenberg Program 1799/2-1 and 1799/3-1) to A.P.; the MCIU/AEI/FEDER, UE (BFU2016-75107-P), and MCIU/AEI/FEDER, UE (PID2019-106579RB-I00), 202020E073 from the CSIC, and the Cajal Blue Brain Project (C080020-09; the Spanish partner of the Blue Brain Project initiative from École Polytechnique Fédérale de Lausanne (EPFL), Switzerland) to G.P.
Author information
Authors and Affiliations
Contributions
S.M. and G.P. conceptualized the study. S.M., G.P. and A.P. designed the in vivo experiments. S.M. performed the ex vivo and in vivo experiments and analyzed the data. A.P. supervised the in vivo experiments. M.V. and A.P. supervised and contributed to the in vivo data analyses. J.E. contributed to the in vivo data analyses. S.M. and G.P. designed the ex vivo experiments. S.M. and C.S.-P. acquired the confocal images and performed imaging data processing. S.M. and G.P. interpreted the data. All the authors discussed the data. S.M. and G.P. wrote the paper with input from all the authors. G.P. provided resources and acquired funding.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Peer review information Nature Neuroscience thanks Inbal Goshen, Dmitri Rusakov, and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Extended data
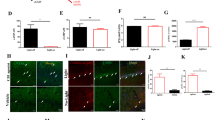
Extended Data Fig. 1 Downregulation of GABABR1s specifically in mPFC astrocytes using a viral strategy.
(a) Scheme showing mPFC region and viral injections of AAV2.5-GFAP-Cre-mCherry (shaded area in red) in GABAB flox−/− and GABAB flox+/+ mice, the Control and GFAP/PFCΔGb mice, respectively. (b) Histological verification of mCherry-Cre-recombinase expression (red) in astrocytes (S100 labeling, green) and not in neurons (NeuN labeling, blue). Scale bar, 20 μm. (c) Colocalization of Cre positive cells with S100-labeled astrocytes and NeuN-labeled neurons. Note the selective expression in astrocytes confirming the specificity of GABABR1 deletion in astrocytes from mPFC (n = 210 Cre-positive cells from 6 slices, 2 mice; P <0.001; z-test two-sided). (d) Representative confocal images showing astrocyte S100 labeling and GABABR1 puncta in Control and GFAP/PFCΔGb mice (astrocytes in S100 image were outlined by dotted lines and superimposed in GABABR1 image). Scale bar, 5 μm. (e) Average data of GABABR1 fluorescence intensity (129 astrocytes from 6 slices, 2 control mice; 151 astrocytes from 6 slices, 2 GFAP/PFCΔGb mice; P <0.001; One-way ANOVA two-sided), and GABAbR1 puncta in Control and GFAP/PFCΔGb mice (43 astrocytes from 2 slices, 2 control mice; 42 astrocytes from 2 slices, 2 GFAP/PFCΔGb mice; P <0.001; One-way ANOVA two-sided). (f) PCR of genomic DNA isolated from cortical slices of Control (GABAB flox−/−) and GFAP/PFCΔGb (GABAB flox+/+) mice showing that slices from GABAB flox+/+ were positive for the targeted DNA sequence (shorter allele of 237 bp; KO sequence; upper image), as a consequence of the cre recombinase action within astrocytes (n = 2 slices from 2 mice for each condition). (g) Specific qPCR for mPFC astrocytes revealed downregulation of Gabbr1 and Gabbr2 gene expression in GFAP/PFCΔGb mice (n = 20 cells/mouse; 3 mice for each condition (See Source Data file). (h) Scheme showing mPFC transfected with AVV2.5-GFAP-cytoGCaMP6f to monitor Ca2+ dynamics in astrocytes in control and after local application of baclofen (2 mM). (i) Confocal images showing cyto-GCaMP6f expressing cells and S100-labeling astrocytes and NeuN-labeling neurons in mPFC. Scale bars, 200 μm and 20 μm (inset), respectively. (j) Colocalization of cyto-GCaMP6f positive cells with astrocytic (S100) and neuronal (NeuN) markers (90 cyto-GCaMP6f positive cells from 6 slices, 3 mice; P <0.001; z-test two-sided). (k) Representative Ca2+ signals evoked by local application of baclofen (2 mM, 5 s) in astrocytes from control mice before and after bath application of CGP55845 (5 μM) and GFAP/PFCΔGb mice. Note that astrocytes from GFAP/PFCΔGb mice did not respond to baclofen, but they did respond to ATP (1 mM) indicating their ability to increase the intracellular Ca2+ signaling in response to other transmitters. (l) Summary graph showing astrocyte Ca2+ event frequency and event amplitude under different experimental conditions (P <0.001; One Way ANOVA two-sided; see Source Data file). (m) Scheme showing the local stimulation of neuronal GABABRs with baclofen, and the representative evoked currents in GFAP/PFCΔGb mice before and after blockage of these receptors with CGP55845 (5 μM). Note the presence of functional GABABRs in PC neurons from GFAP/PFCΔGb mice (n = 8 from 2 mice; P <0.001; One Way ANOVA two-sided). *** P <0.001 (see Source Data file). Box plots indicate median and whiskers indicate the 25th and 75th percentiles, respectively.
Extended Data Fig. 2 Astrocytic downregulation of GABABR1s altered behavioral performance.
(a) Histological verification of bilateral cre expression (red) in astrocytes in mPFC. Scale bar, 200 μm. (b) Correlation between cre-transfected area and T-maze performance. Dots represented the number of incorrect trials at day 4 for individual GFAP/PFCΔGb mice (linear fitting curve, black line). (c) BW plots for behavioral sessions in T-maze after training (recall sessions, post day 6) for Control (black) and GFAP/PFCΔGb (purple) (n = 10 recall sessions; n = 4 mice for each group; P <0.001; One Way ANOVA two-sided). (d, e) Anxiety levels measured for control and GFAP/PFCΔGb mice. Note the lower anxiety levels showed by mice with downregulated GABABRs specifically in astrocytes. (d) Representative traces for Control and GFAP/PFCΔGb mice in an open field test. BW plot showing the increased exploration index for GFAP/PFCΔGb mice (n = 4 mice for each group; P = 0.044; One Way ANOVA two-sided), while average of running speed during exploration was similar in both mice. (e) Scheme and activity traces in an elevated plus maze (EPM) for Control and GFAP/PFCΔGb mice. Note that GFAP/PFCΔGb mice had more entries into the open arms than Control. Right, EPM exploration index (n = 4 for each condition; P = 0.037; One Way ANOVA two-sided). (f) Scheme and activity traces of object in place (OIP) test for Control and GFAP/PFCΔGb mice. BW plot for exploration index showing a reduced working memory performance in GFAP/PFCΔGb mice (n = 5 for each condition; P = 0.019; One Way ANOVA two-sided). Right, BW plot for the exploration time in the arena for both mice. * P <0.05; *** P <0.001 (see Source Data file). Box plots indicate median and whiskers indicate the 25th and 75th percentiles, respectively.
Extended Data Fig. 3 In vivo electrophysiological analysis of control and GFAP/PFCΔGb mice during resting and active behavioral responses.
(a) Schematic drawing indicating last location of middle shank for BL32 movable silicon probes in recorded mice (post hoc reconstruction of positions; n = 4 mice for each group), and stereomicroscope image showing the position of the probe in mPFC from a control mouse. Scale bar, 1 mm. (b) BW plot of mean speed during correct and incorrect trials in T-maze for both animal groups. (c) Relative LFP spectral density power (0–120 Hz) during T-maze resting periods (shaded areas, SEM), and BW plot of LFP values for the different frequency bands (n = 4 for each group). (d) BW plots of the average time mice were moving, and mean speed during moving periods (active exploring, AE) for both control (black) and GFAP/PFCΔGb (purple). (e) Representative comodulogram of frequency-frequency for a control animal. (f) The magnitude of the modulation of gamma amplitude for both control (black) and GFAP/PFCΔGb (purple) mice across theta cycles. Au, theta amplitude scaled by theta/delta power ratio. Data are shown as mean ± SEM (shaded areas). (g) Left, BW plots for the ratio of theta/delta amplitude epochs in correct trials of control and GFAP/PFCΔGb mice. Right, BW plots of mean duration of detected epochs (see Source Data file). Box plots indicate median and whiskers indicate the 25th and 75th percentiles, respectively.
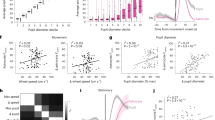
Extended Data Fig. 4 Firing rate of excitatory and inhibitory units during T-maze in Control and GFAP/PFCΔGb mice.
(a) Representative waveforms for putative excitatory units (EU, blue) and inhibitory units (IU, orange) and corresponding auto-correlograms. (b), (c) BW plots showing information content analysis for EU (b) and IU (c). Note the reduced magnitude of information content index for GFAP/PFCΔGb mice. (d), (e) Left, histograms of normalized CV probability distribution (control black line, GFAP/PFCΔGb purple area). Right, percentage of EU (d) and IU (e) displaying and enhanced activity (open boxes), decreased activity (filled boxes) or no changes in firing rate (dashed boxes) based on CV values during correct trials (>1.5 deviation from median population). Note the reduced magnitude of open boxed in GFAP/PFCΔGb mice for excitatory units indicating a minor population of cells increasing their firing rate after entering the DZ (values for Z-test values indicated in panel d, e). (f), (g) Left, Histograms showing the diversity of firing rates across the neuronal populations, as reflected in a lognormal distribution. GFAP/PFCΔGb mice (purple histogram) showed right-shifted distributions towards higher values of firing rate for both EU (f) and IU (g). Insets, BW plots for the mean firing rate of all units along the trials (EU, n = 490 for Control; n = 396 for GFAP/PFCΔGb; 4 mice for each group; P < 0.001; One Way ANOVA two-sided; IU, n = 110 for Control; n = 109 for GFAP/PFCΔGb; P = 0.021; KW test). Right, Mean Z-score of the firing rate for EU and IU populations, respectively, including correct and incorrect trials for Control and GFAP/PFCΔGb mice. Data are shown as mean ± SEM (shaded areas). Note the disruption of firing pattern during T-maze performance in GFAP/PFCΔGb mice for both EU (P = 0.01; One Way ANOVA two-sided) and IU (P = 0.016; One Way ANOVA two-sided). * P <0.05; *** P <0.001 (see Source Data file). Data shown are mean±SEM. Box plots indicate median and whiskers indicate the 25th and 75th percentiles, respectively.
Extended Data Fig. 5 Ca2+ signaling evoked by PV interneuron in mPFC slices.
(a) Schematic drawing of an mPFC slice indicating the targeted area with AAV2.5-GFAP-cyto-GCaMP6f (green shaded area) in PV-tdTomato mice, and the depolarization protocol used to stimulate PV interneurons (120 ms @ 5 Hz, 30 s; firing rate per depolarization pulse ~ 50 Hz), mimicking theta-gamma cycles; PV cells (red) and surrounded by astrocytes (green). (b) Confocal images of NeuN labeling (blue), cyto-GCaMP6f (green) and PV-tdTomato with S100 labeling (red and white, respectively) from a, showing the selective expression of GCaMP6f in astrocytes. Scale bar, 20 μm. (c) Representative fluorescence images of cyto-GCaMP6f labeling astrocytes before (pre stim) and after (post stim) PV depolarization. Scale bar, 15 μm. (d) Representative Ca2+ traces showing an enhanced Ca2+ signaling from surrounding astrocytes by PV stimulation (control), and after perfusion of CGP55845 (5 μm) (PV stimulation denoted by red line). (e) Summary graph showing astrocyte Ca2+ event frequency and amplitude before (light color bars) and after the PV stimulation (dark color bars) in control, after bath application of CGP55845, and Ip3r2-/- mice. *** P <0.001. One-Way ANOVA two-sided (see Source Data file). Data shown are mean±SEM. Box plots indicate median and whiskers indicate the 25th and 75th percentiles, respectively.
Extended Data Fig. 6 PV-driven IPSCs potentiation onto PCs relies on presynaptic mechanisms.
(a) Relative changes of IPSC amplitude over time (red arrow denotes PV stimulation), showing different outcomes after PV depolarization. Right, BW plot showing changes in IPSC amplitude after <2 min PV stimulation for both groups (P = 0.030; One-way ANOVA two-sided). Inset, Pie plot representing the percentage of PCs showing changes (79%, gray) vs no changes (21%, blue) in IPSCs amplitude. Note the preponderance of PC responses showing IPSCs potentiation. (b) Relative changes of the coefficient of variation (CV) after <2 min PV depolarization for PCs showing IPSCs potentiation (n = 16), suggesting a presynaptic locus for IPSC modulation. (c) Representative traces of a connected PV-PC pair (current clamp recordings for PV interneuron, voltage clamp recordings for PCs) showing unitary synaptic responses onto PCs. Note the increase of unitary IPSCs responses after the PV stimulation protocol. Right, Relative changes of synaptic parameters elicited by the PV stimulation protocol: success rate (P = 0.022), synaptic efficacy (P = 0.008) and synaptic potentiation (P = 0.360; KW test). * P <0.05; ** P <0.01 (see Source Data file). Data shown are mean±SEM. Box plots indicate median and whiskers indicate the 25th and 75th percentiles, respectively.
Extended Data Fig. 7 PV-ChR2 stimulation enhances inhibitory synaptic transmission in PCs from mPFC slices.
(a) Confocal images showing PV cells expressing ChR2 after viral transfection with AAV2.5-Ef1a-DIO-hChR2(H134R)-mcherry: NeuN (blue), ChR2 (red) and astrocytic marker S100 (green). Scale bar, 10 μm. (b) Top, Scheme of experimental approach stimulating PV-expressing ChR2 interneurons with blue light while recording PCs from layer 2/3. Bottom, Representative IPSCs traces from PCs before and after PV-ChR2 stimulation (light stim 30 s) in control and in Ip3r2-/–PV-ChR2 mice (average traces from 20 consecutive responses). Note the absence of synaptic modulation in Ip3r2-/–PV-ChR2 mice. (c) Relative changes of IPSC amplitude over time after PV-ChR2 activation (blue arrow denotes light stimulation, 30 s) for control, PV-ChR2 mice, and in absence of Ca2+ signaling in astrocytes, Ip3r2-/–PV-ChR2 mice. Inset, Pie plot representing the percentage of PCs showing changes (73%, gray) vs no changes (27%, blue) in IPSCs amplitude after PV-ChR stimulation. Note the similar values found for IPSCs potentiation after single PV cell depolarization (Extended Data Fig. 6a), and after stimulation of the PV interneuron population with ChR2, confirming the consequences of selective PV activation and astrocytic recruitment for the inhibitory synaptic plasticity at mPFC. (d) BW plot showing changes in IPSC amplitude after 10 min PV-ChR2 stimulation for control (n =15; P < 0.001), Ip3r2-/–PV-ChR2 (n =10; P < 0.001), and control + CGP 55845 (n =11; P = 0.019) and LY367385 (n =7; P = 0.042; One-way ANOVA two-sided). (e) Time course of relative IPSC changes right after PV-single depolarization (n = 16), PV-ChR2 (n = 10) and melanopsin-astrocytes (n = 10). (f) BW plot showing changes in IPSC amplitude after 30 and 60 s post stimulation. Note the faster onset of IPSC potentiation for PV-single depolarization and PV-ChR2 compared with melanopsin-activated astrocytes. * P <0.05; *** P <0.001 One-way ANOVA two-sided (see Source Data file). Data shown are mean±SEM. Box plots indicate median and whiskers indicate the 25th and 75th percentiles, respectively.
Extended Data Fig. 8 Melanopsin stimulated astrocytes in mPFC enhance cognitive performance.
(a) Scheme showing optical fiber implantation in mPFC and image of optical fiber tract in a melanopsin-mcherry expressing mouse denoted by a dotted line. Scale bar, 100 μm. (b) BW plot for behavioral sessions in the T-maze after training (recall sessions, post day 6) for vector (gray) and melanopsin (coral green) (n = 36 total recall sessions for vector, and n= 28 total recall sessions for melanopsin; n = 4 mice for each group; P <0.001; One Way ANOVA). (c) BW plot showing control (n = 15 mice and mcherry reporter (vector; n = 7 mice; stimulated with blue light) transfected mice for the different sessions of training. One-way ANOVA performed for each group between control (black) and vector (grey), and for each group vs first two sessions (1-20 trials). No differences between animal groups were found (see Source Data file). (d) Scheme and activity traces for OIP test for vector (black) and melanopsin-expressing astrocytes (coral green) mice. (e) BW plot for exploration index showing an enhanced working memory performance in melanopsin-expressing astrocytes mice (n ≥ 6 for each condition; vector vs melanopsin P = 0.022; One Way ANOVA two-sided). Right, BW plot for the exploration time in the arena of different mice. * P <0.05; *** P <0.001. Data shown are mean±SEM. Box plots indicate median and whiskers indicate the 25th and 75th percentiles, respectively.
Extended Data Fig. 9 In vivo electrophysiological analysis of mice expressing vector and melanopsin-infected astrocytes during active behavioral responses.
(a) Schematic drawing indicating last location of middle shank for BL32 movable silicon probes in recorded mice (post hoc reconstruction of positions; n = 4 mice for each group). (b) Relative LFP spectral density power (0–120 Hz) during T-maze for vector (gray) and melanopsin-injected (coral green) mice. Data are shown as mean ± SEM (shaded areas). (c) BW plot of mean speed during correct and incorrect trials in T-maze for both animal groups. (d) Left, BW plots for the ratio of theta/delta amplitude epochs in correct trials of vector and melanopsin-infected animals. Right, BW plots of mean duration of detected epochs. (e) The magnitude of the modulation of gamma amplitude for both vector and melanopsin mice across theta cycles. Au, theta amplitude scaled by theta/delta power ratio. Data are shown as mean ± SEM (shaded areas). (f) BW plots for the mean firing rate of all units during resting states (EU, n = 541 for vector; n = 397 for melanopsin; 4 mice for each group; P = 0.649; KW test; IU, n = 121 for vector; n = 67 for melanopsin; P = 0.305; KW test). Note that during inter-trial periods and no-light stimulation, melanopsin-infected astrocytes did not alter neuronal firing rate. (g) BW plot showing information content analysis for EU. (h) Left, histogram of normalized CV probability distribution (vector in black, melanopsin in coral green area). Right, percentage of EU displaying and enhanced activity (open boxes), decreased activity (filled boxes) or no changes in firing rate (dashed boxes) based on CV values during correct trials (>1.5 deviation from median population). Note the reduced magnitude for filled boxes in melanopsin mice indicating the reduction of the population that decreased its firing after entering the DZ (values for Z-test values indicated in panel). (i) BW plot showing information content analysis for IU. (j) Similar to h for IU populations (n = 121 for vector, n = 101 for melanopsin; P = 0.041; KW test). Note that melanopsin injected mice (coral green histogram) showed right-shifted distributions towards higher values of firing rate for IU. Right, Mean Z-score of the firing rate for IU including correct and incorrect trials for vector and melanopsin mice (P = 0.001; One-Way ANOVA two-sided). Data are shown as mean ± SEM (shaded areas). (k) Histograms showing the diversity of firing rates across the EU populations, as reflected in a lognormal distribution. Insets, BW plots for the mean firing rate of all EU along the T-maze trials (n = 496 for vector; n = 425 for melanopsin; 4 mice for each group; P = 0.505; KW test). Right, Mean Z-score of the firing rate for EU including correct and incorrect trials in T-maze for vector and melanopsin mice (P = 0.734; One Way ANOVA two-sided). (l) Similar to k for IU populations. * P <0.05; ** P <0.01 two-sided tests (see Source Data file). Data shown are mean±SEM. Box plots indicate median and whiskers indicate the 25th and 75th percentiles, respectively.
Extended Data Fig. 10 mGluR1 activation contributes to cognitive performance.
(a) Left, scheme of local injection of LY367385 (100 μM) into mPFC to selectively block mGluR1 receptors. Right, stereoscopic image showing bilateral cannula positions for drug delivery into the PFC. Scale bar, 500 μm. (b) Top, representative traces of behavioral responses in T-maze at day 4 for wildtype mice treated with saline and LY367385 (100 μM) to selectively block mGluR1 receptors. Bottom, temporal profile of learning task. Data are shown as mean ± SEM (shaded areas). (c) BW plot showing the average performance for control (n = 15), saline-treated mice (n = 4), and LY367385-treated mice (n = 4) during training. (d) Contingency table for data statistics shown in c. Heat map denote significant levels; Two Way ANOVA two-sided (see Source Data file). (e) Confocal images of GAD67 labeling (blue), mDlx-GFP (green), s100 (gray) and Cre-mCherry (red), showing the selective expression of mDlx-GFP in GABAergic cells and Cre-mCherry in astrocytes in GFAP/PFCΔGb mice. Scale bar, 25 μm. Data shown are mean±SEM. Box plots indicate median and whiskers indicate the 25th and 75th percentiles, respectively. (f) Scheme summarizing data from this study highlighting the role of astrocytes in goal-directed actions.
Supplementary information
Source data
Source Data Fig. 1
Statistical source data.
Source Data Fig. 2
Statistical source data.
Source Data Fig. 3
Statistical source data.
Source Data Fig. 4
Statistical source data.
Source Data Fig. 5
Statistical source data.
Source Data Fig. 6
Statistical source data.
Source Data Extended Data Fig. 1
Statistical source data.
Source Data Extended Data Fig. 1
Uncropped gels.
Source Data Extended Data Fig. 2
Statistical source data.
Source Data Extended Data Fig. 3
Statistical source data.
Source Data Extended Data Fig. 4
Statistical source data.
Source Data Extended Data Fig. 5
Statistical source data.
Source Data Extended Data Fig. 6
Statistical source data.
Source Data Extended Data Fig. 7
Statistical source data.
Source Data Extended Data Fig. 8
Statistical source data.
Source Data Extended Data Fig. 9
Statistical source data.
Source Data Extended Data Fig. 10
Statistical source data.
Rights and permissions
About this article
Cite this article
Mederos, S., Sánchez-Puelles, C., Esparza, J. et al. GABAergic signaling to astrocytes in the prefrontal cortex sustains goal-directed behaviors. Nat Neurosci 24, 82–92 (2021). https://doi.org/10.1038/s41593-020-00752-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41593-020-00752-x
This article is cited by
-
Astrocyte Activation in the ACC Contributes to Comorbid Anxiety in Chronic Inflammatory Pain and Involves in The Excitation-Inhibition Imbalance
Molecular Neurobiology (2024)
-
Astrocytic chloride is brain state dependent and modulates inhibitory neurotransmission in mice
Nature Communications (2023)
-
Astrocyte regulation of synaptic signaling in psychiatric disorders
Neuropsychopharmacology (2023)
-
Dysfunctional serotonergic neuron-astrocyte signaling in depressive-like states
Molecular Psychiatry (2023)
-
The neurobiology of insulin-like growth factor I: From neuroprotection to modulation of brain states
Molecular Psychiatry (2023)