Abstract
Cotranslational protein targeting is a conserved process for membrane protein biogenesis. In Escherichia coli, the essential ATPase SecA was found to cotranslationally target a subset of nascent membrane proteins to the SecYEG translocase at the plasma membrane. The molecular mechanism of this pathway remains unclear. Here we use biochemical and cryoelectron microscopy analyses to show that the amino-terminal amphipathic helix of SecA and the ribosomal protein uL23 form a composite binding site for the transmembrane domain (TMD) on the nascent protein. This binding mode further enables recognition of charged residues flanking the nascent TMD and thus explains the specificity of SecA recognition. Finally, we show that membrane-embedded SecYEG promotes handover of the translating ribosome from SecA to the translocase via a concerted mechanism. Our work provides a molecular description of the SecA-mediated cotranslational targeting pathway and demonstrates an unprecedented role of the ribosome in shielding nascent TMDs.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$189.00 per year
only $15.75 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout







Similar content being viewed by others
Data Availability
Cryo-EM maps are deposited in the electron microscopy databank (EMDB) with accession codes EMD-10073 (RNCRodZ–SecA) and EMD-10074 (SecA, local refinement), and model coordinates are deposited in the worldwide PDB with accession code PDB 6S0K. Other data are available from corresponding authors upon reasonable request.
References
Shao, S. & Hegde, R. S. Target selection during protein quality control. Trends Biochem. Sci. 41, 124–137 (2015).
Brandman, O. & Hegde, R. S. Ribosome-associated protein quality control. Nat. Struct. Mol. Biol. 23, 7–15 (2016).
Zhang, X. & Shan, S. O. Fidelity of cotranslational protein targeting by the signal recognition particle. Annu. Rev. Biophys. 43, 381–408 (2014).
Cymer, F., von Heijne, G. & White, S. H. Mechanisms of integral membrane protein insertion and folding. J. Mol. Biol. 427, 999–1022 (2015).
Elvekrog, M. M. & Walter, P. Dynamics of co-translational protein targeting. Curr. Opin. Chem. Biol. 29, 79–86 (2015).
Nyathi, Y., Wilkinson, B. M. & Pool, M. R. Co-translational targeting and translocation of proteins to the endoplasmic reticulum. Biochim. Biophys. Acta 1833, 2392–2402 (2013).
Zhang, X., Rashid, R., Wang, K. & Shan, S. O. Sequential checkpoints govern substrate selection during cotranslational protein targeting. Science 328, 757–760 (2010).
Chartron, J. W., Hunt, K. C. & Frydman, J. Cotranslational signal-independent SRP preloading during membrane targeting. Nature 536, 224–228 (2016).
Schibich, D. et al. Global profiling of SRP interaction with nascent polypeptides. Nature 536, 219–223 (2016).
Jomaa, A., Boehringer, D., Leibundgut, M. & Ban, N. Structures of the E. coli translating ribosome with SRP and its receptor and with the translocon. Nat. Commun. 7, 10471 (2016).
Jomaa, A. et al. Structure of the quaternary complex between SRP, SR, and translocon bound to the translating ribosome. Nat. Commun. 8, 15470 (2017).
Aviram, N. et al. The SND proteins constitute an alternative targeting route to the endoplasmic reticulum. Nature 540, 134–138 (2016).
Ast, T., Cohen, G. & Schuldiner, M. A network of cytosolic factors targets SRP-independent proteins to the endoplasmic reticulum. Cell 152, 1134–1145 (2013).
Chitwood, P. J., Juszkiewicz, S., Guna, A., Shao, S. & Hegde, R. S. EMC is required to initiate accurate membrane protein topogenesis. Cell 175, 1507–1519.e16 (2018).
Guna, A., Volkmar, N., Christianson, J. C. & Hegde, R. S. The ER membrane protein complex is a transmembrane domain insertase. Science 359, 470–473 (2018).
Shurtleff, M. J. et al. The ER membrane protein complex interacts cotranslationally to enable biogenesis of multipass membrane proteins. eLife 7, 1–23 (2018).
Huber, D. et al. SecA interacts with ribosomes in order to facilitate posttranslational translocation in bacteria. Mol. Cell. 41, 343–353 (2011).
Wang, S., Yang, C. I. & Shan, S. O. SecA mediates cotranslational targeting and translocation of an inner membrane protein. J. Cell Biol. 216, 3639–3653 (2017).
Singh, R. et al. Cryo-electron microscopic structure of SecA bound to the 70S ribosome. J. Biol. Chem. 289, 7190–7199 (2014).
Huber, D. et al. SecA cotranslationally interacts with nascent substrate proteins in vivo. J. Bacteriol. 199, e00622–16 (2016).
Rawat, S., Zhu, L., Lindner, E., Dalbey, R. E. & White, S. H. SecA drives transmembrane insertion of RodZ, an unusual single-span membrane protein. J. Mol. Biol. 427, 1023–1037 (2015).
Hartl, F. U., Lecker, S., Schiebel, E., Hundrick, J. P. & Wickner, W. The binding cascade of SecB to SecA to SecY/E mediates preprotein targeting to the E. coli plasma membrane. Cell 63, 269–279 (1990).
Kimura, E., Akita, M., Matsuyaman, S. & Mizushima, S. Determination of a region in SecA that interacts with presecretory proteins in Escherichia coli. J. Biol. Chem. 266, 6600–6606 (1991).
Gelis, I. et al. Structural basis for signal-sequence recognition by the translocase motor SecA as determined by NMR. Cell 131, 756–769 (2007).
Chatzi, K. E. et al. Preprotein mature domains contain translocase targeting signals that are essential for secretion. J. Cell Biol. 216, 1357–1369 (2017).
Zimmer, J., Nam, Y. & Rapoport, T. A. Structure of a complex of the ATPase SecA and the protein-translocation channel. Nature 455, 936–943 (2008).
Bauer, B. W. & Rapoport, T. A. Mapping polypeptide interactions of the SecA ATPase during translocation. Proc. Natl Acad. Sci. USA 106, 20800–20805 (2009).
Frauenfeld, J. et al. Cryo-EM structure of the ribosome-SecYE complex in the membrane environment. Nat. Struct. Mol. Biol. 18, 614–621 (2011).
Park, E. et al. Structure of the SecY channel during initiation of protein translocation. Nature 506, 102–106 (2014).
Wu, Z. C., De Keyzer, J., Kedrov, A. & Driessen, A. J. M. Competitive binding of the SecA ATPase and ribosomes to the SecYEG translocon. J. Biol. Chem. 287, 7885–7895 (2012).
Nakatogawa, H. & Ito, K. The ribosomal exit tunnel functions as a discriminating gate. Cell 108, 629–636 (2002).
Koch, S. et al. Lipids activate SecA for high affinity binding to the SecYEG complex. J. Biol. Chem. 291, 22534–22543 (2016).
Erlandson, K. J. et al. A role for the two-helix finger of the SecA ATPase in protein translocation. Nature 455, 984–987 (2008).
Or, E., Navon, A. & Rapoport, T. Dissociation of the dimeric SecA ATPase during protein translocation across the bacterial membrane. EMBO J. 21, 4470–4479 (2002).
Osborne, A. R., Clemons, W. M. Jr. & Rapoport, T. A. Monomer closed PPXD; dimer open PPXD. Proc. Natl Acad. Sci. USA 101, 10937–10942 (2004).
Sharma, V. et al. Crystal structure of Mycobacterium tuberculosis SecA, a preprotein translocating ATPase. Proc. Natl Acad. Sci. USA 100, 2243–2248 (2003).
Li, L. et al. Crystal structure of a substrate-engaged SecY protein-translocation channel. Nature 531, 395–399 (2016).
Knorr, A. G. et al. Ribosome-NatA architecture reveals that rRNA expansion segments coordinate N-terminal acetylation. Nat. Struct. Mol. Biol. 26, 35–39 (2019).
Ferbitz, L. et al. Trigger factor in complex with the ribosome forms a molecular cradle for nascent proteins. Nature 431, 590–596 (2004).
Akopian, D., Shen, K., Zhang, X. & Shan, S. O. Signal recognition particle: an essential protein-targeting machine. Annu. Rev. Biochem 82, 693–721 (2013).
Ariosa, A., Lee, J. H., Wang, S., Saraogi, I. & Shan, S. Regulation by a chaperone improves substrate selectivity during cotranslational protein targeting. Proc. Natl Acad. Sci. USA 112, E3169–E3178 (2014).
Ranjan, A., Mercier, E., Bhatt, A. & Wintermeyer, W. Signal recognition particle prevents N-terminal processing of bacterial membrane proteins. Nat. Commun. 8, 15562 (2017).
Saraogi, I., Akopian, D. & Shan, S. O. Regulation of cargo recognition, commitment, and unloading drives cotranslational protein targeting. J. Cell Biol. 205, 693–706 (2014).
Devaraneni, P. K. et al. Stepwise insertion and inversion of a type II signal anchor sequence in the ribosome-Sec61 translocon complex. Cell 146, 134–147 (2011).
Traxler, B. & Murphy, C. Insertion of the polytopic membrane protein MalF is dependent on the bacterial secretion machinery. J. Biol. Chem. 271, 12394–12400 (1996).
Sääf, A., Andersson, H., Gafvelin, G. & von Heijne, G. SecA-dependence of the translocation of a large periplasmic loop in the Escherichia coli MalF inner membrane protein is a function of sequence context. Mol. Membr. Biol. 12, 209–215 (1995).
Gebert, J. F., Overhoff, B., Manson, M. D. & Boos, W. The Tsr chemosensory transducer of Escherichia coli assembles into the cytoplasmic membrane via a SecA-dependent process. J. Biol. Chem. 263, 16652–16660 (1988).
Yu, D., Wowor, A. J., Cole, J. L. & Kendall, D. A. Defining the Escherichia coli SecA dimer interface residues through in vivo site-specific photo-cross-linking. J. Bacteriol. 195, 2817–2825 (2013).
Akopian, D., Dalal, K., Shen, K., Duong, F. & Shan, S. O. SecYEG activates GTPases to drive the completion of cotranslational protein targeting. J. Cell Biol. 200, 397–405 (2013).
Saraogi, I., Zhang, D., Chandrasekaran, S. & Shan, S. O. Site-specific fluorescent labeling of nascent proteins on the translating ribosome. J. Am. Chem. Soc. 133, 14936–14939 (2011).
Calhoun, K. A. & Swartz, J. R. Total amino acid stabilization during cell-free protein synthesis reactions. J. Biotechnol. 123, 193–203 (2006).
Datsenko, K. A. & Wanner, B. L. One-step inactivation of chromosomal genes in Escherichia coli K-12 using PCR products. Proc. Natl Acad. Sci. USA 97, 6040–6045 (2000).
Zheng, S. Q. et al. MotionCor2: anisotropic correction of beam-induced motion for improved cryo-electron microscopy. Nat. Methods 14, 331–332 (2017).
Zhang, K. Gctf: Real-time CTF determination and correction. J. Struct. Biol. 193, 1–12 (2016).
Ludtke, S. J., Baldwin, P. R. & Chiu, W. EMAN: semiautomated software for high-resolution single-particle reconstructions. J. Struct. Biol. 128, 82–97 (1999).
Zivanov, J. et al. New tools for automated high-resolution cryo-EM structure determination in RELION-3. eLife 7, e42166 (2018).
Punjani, A., Rubinstein, J. L., Fleet, D. J. & Brubaker, M. A. cryoSPARC: algorithms for rapid unsupervised cryo-EM structure determination. Nat. Methods 14, 290–296 (2017).
Afonine, P. V. et al. New tools for the analysis and validation of cryo-EM maps and atomic models. Acta Crystallogr. D. Struct. Biol. 74, 814–840 (2018).
Pettersen, E. F. et al. UCSF Chimera—a visualization system for exploratory research and analysis. J. Comput. Chem. 25, 1605–1612 (2004).
Emsley, P. & Cowtan, K. Coot: model-building tools for molecular graphics. Acta Crystallogr. D. Biol. Crystallogr. 60, 2126–2132 (2004).
Afonine, P. V. et al. Real-space refinement in PHENIX for cryo-EM and crystallography. Acta Crystallogr. D. Struct. Biol. 74, 531–544 (2018).
Blanchette, C. D. et al. Quantifying size distributions of nanolipoprotein particles with single-particle analysis and molecular dynamic simulations. J. Lipid Res. 49, 1420–1430 (2008).
Acknowledgements
We thank A. McDowall and H. Wang for assistance with negative stain electron microscopy data collection, J. Rothman for sharing plasmid ApoE422k, D. Boehringer and A. Scaiola for the support with EM data collection and processing, M. Leibundgut and M. Saurer for the support with model building and members of the Shan and Ban groups for discussions and comments on the manuscript. We also thank T. Miller, M. Zimmer and F. Huber for helpful discussions. Cryo-EM data was collected at the Scientific Center for Optical and Electron Microscopy at the ETH Zurich (ScopeM). We gratefully acknowledge the support of NVIDIA Corporation for the Titan Xp GPU used in this research through a GPU Grant program awarded to A.J.; M.J. was supported by the internal research grant of the ETH to N.B. (ETH-40 16-2). This work was supported by National Institutes of Health grant GM107368A and the Gordon and Betty Moore Foundation through grant GBMF2939 to S.S. and by the Swiss National Science Foundation (SNSF) (grant number 310030B_163478), National Center of Excellence in Research (NCCR) RNA & Disease Program of the SNSF (grant number 51NF40_141735) to N.B.
Author information
Authors and Affiliations
Contributions
S.W. and S.S. conceived the project. S.W. performed most of the biochemical experiments and analyzed data. A.J. acquired cryo-electron microscopy data and performed reconstructions and model building. M.J. performed initial sample preparation for cryo-EM analysis. C.Y. measured the association rate constant of SecA binding to RNCRodZ. N.B. and S.S. supervised the structural and biochemical experiments, respectively. All authors interpreted the data and contributed to the final versions of the manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Peer review information Ines Chen was the primary editor on this article and managed its editorial process and peer review in collaboration with the rest of the editorial team.
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Integrated supplementary information
Supplementary Figure 1 Controls and additional data to map the interaction of SecA with hydrophobic sequences on nascent protein in the co- versus post-translational mode.
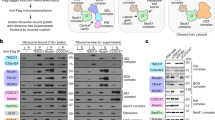
a,b, The activity of SecA variants were tested by assaying the translocation of proOmpA, a model post-translational SecA substrate (a), and RodZ, a model co-translational SecA substrate (b). ProOmpA and RodZ was in vitro translated using the PURE system supplemented with 1 μM SecA variants and urea-washed inverted membrane vesicles as described previously18. Successful insertion into the membrane was detected by protection against proteinase K (Prot.K) digestion. In the left panel of a, lane 1 shows the reaction of mutant SecA with deletion on residues 1–11, and lanes 2–14 show the data with single cysteine variants of SecA. The right panel of a shows the comparison of representative single cysteine mutants of SecA relative to WT SecA. The data in each gel are from side-by-side experiments. c, Scheme of the composition of the nascent chains on stalled RNCs used in this study. MreB-binding domain (MBD, residues 1–103) was removed from the RodZ nascent chain. Arrest peptide is from SecM residues 133–170. The positions of engineered cysteines at residues 111, 115 and 146 are indicated. 1A9L nascent chain was constructed by replacing the TMD of RodZ with 1A9L (ref. 10) followed by the mature region (residues 16–50) of PhoA. RNC, ribosome-nascent chain complex. d–f, The Mreb-binding domain (MBD; residues 1–103) of RodZ is not essential for SecA-dependent co-translational translocation in a coupled in vitro translation-translocation assay. As previously described18, RodZ or RodZΔMBD was translated using the PURE system supplemented with the indicated concentrations of SecA (d) or SRP (e) and urea-washed, inverted membrane vesicles. Successful insertion into the membrane was detected by protection against proteinase K (Prot.K) digestion. The reactions in d also contained 3.8 μM TF, 400 nM SRP, 1 μM FtsY. The reactions in e also contained 3.8 μM TF, 50 nM SecA, and a fivefold excess of FtsY over SRP. f, Summary of the insertion efficiency of RodZ or RodZΔMBD from the data in d and e. Insertion efficiency was calculated by dividing the amount of proteinase K-resistant protein by the total amount of protein, normalized by the number of methionines before and after Prot.K digestion. g, Crosslinking of SecA to C111 in the RodZ nascent chain depends on engineered cysteine on SecA. All lanes contain the BMH crosslinker. Wild-type (WT) SecA contains four cysteines (residue 98, 885, 887, 896), none of which crosslinked to RodZ nascent chain. In C98S, the cysteine at residue 98 was mutated to serine. In ΔZFD, the non-essential C-terminus of SecA containing three cysteines were removed. All the other crosslinking experiments in this work contained the C98S mutation and ΔZFD deletion for clean interpretation of results. Asterisks indicate crosslinked products detected by the anti-strep and anti-T7 antibodies. h, Crosslinking of SecA(C12) to C111 in the RodZ nascent chain depends on the crosslinker, SecA and RNCRodZ. Asterisks indicate crosslinked products detected by the anti-strep and anti-T7 antibodies. i, Crosslinking of SecA (C193) to C111 in SUMO-RodZ depends on the crosslinker, SecA and SUMO-RodZ. Asterisks indicate crosslinked products detected by the anti-SUMO and anti-T7 antibodies. j, Engineered single cysteines at the indicated positions of SecA were tested for crosslinking to RodZ (C111) on RNC by BMH or BMOE. Asterisks indicate crosslinked products that are detected by the anti-strep and anti-T7 antibodies.
Supplementary Figure 2 Additional data to map the interaction surface of SecA with nascent chains on RNC and with post-translational substrates.
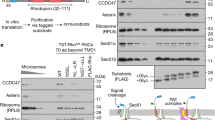
a, FRET experiments to monitor the proximity between Cm (blue star)-labeled RNCRodZ or RNCphoA and BDP (green star) labeled at indicated positions on SecA. Cm was incorporated at residue 111 immediately upstream of the RodZ TMD (magenta) or residue 4 upstream of the phoA signal sequence (magenta). Top left panel, scheme of the FRET-based binding assay. Lower left panel, SecA residues for acceptor labeling are mapped onto the structure of SecA from this work. b, Representative equilibrium titrations showing the binding of SecABDP to Cm-labeled RNCRodZ. Reactions used 20 nM RNCCm (donor) and indicated concentrations of SecABDP (acceptor). All titrations saturated above 20 nM SecA, indicating tight binding of all the fluorescently labeled SecA variants. The data for individual SecA variants are colored as in the lower panel of a. c, Summary of FRET efficiency in the complexes formed between the indicated SecA variants and RNCRodZ or RNCPhoA. FRET efficiency was calculated at 500 nM SecABDP according to Equation 1 in Supplementary Note 1. The data for individual SecA positions are colored as in the lower panel of a. All values represent mean ± s.d., with n = 2 or 3 independent experiments. d,e, Engineered single cysteines at indicated positions on SecA were tested for crosslinking by BMH to the RodZ TMD (residues 104-133) or the phoA signal sequence (residues 1-21) fused to the C-terminus of SUMO (SMT3 residues 1-101). The cysteines on RodZ and PhoA are at the same locations as in Fig. 1a,b, respectively. Crosslinking reactions used 8.3 μM SUMO fusion proteins and 1 μM SecA. Asterisks denote crosslinked products detected by both the anti-SUMO and anti-T7 antibodies. f,g, Crosslinking efficiency from the data in d and e, respectively, are summarized in the structural model of SecA from this work. Crosslinking efficiencies (normalized) were relative to the crosslinked product formed by SecA (C193), based on western-blots against SUMO and strep-tag. Residues are colored based on crosslinking efficiency as indicated. h, Characterization of samples for cryo-EM. RNCs were tested for crosslinking between the indicated cysteines on the nascent chain and SecA (C12). RNC6KR_1A9L contained a model signal sequence 1A9L in place of the RodZ-TMD preceded by six consecutive basic residues derived from residues 104–109 of RodZ. Single bands were observed for both the tRNA-linked nascent chain and crosslinked products with SecA, probably due to the removal of polysomes during preparation of the samples for cryo-EM. Asterisks denote major crosslinked products detected by anti-strep antibody. Crosslinking efficiency was quantified from the ratio of the intensity of crosslinked nascent chain relative to the total intensity of bands containing the nascent chain.
Supplementary Figure 3 Image classification and refinement of the structure of the RNCRodZ–SecA complex.
An initial 2D classification was performed on 4-fold binned particles with pixel size of 5.56 Å per pixel (box size 80 × 80 pixels) to remove bad particles. The selected particle images were then subjected to 3D refinement in RELION3 to obtain an initial map of the ribosome. Using a circular mask applied at the ribosome tunnel region, a 3D focused classification without alignment was performed. This approach yielded a 3D class with a density corresponding to SecA. The remaining classes contained either no or weak density at the exit tunnel region and were discarded. A second round of focused 3D classification on two-fold binned images (160 × 160 pixels) yielded a class with an improved EM density of SecA. A final round of focused 3D classification by adjusting tau values in RELION3 (T = 10), which yielded a 3D class with resolved secondary structure elements in the EM density of SecA. The selected particle images in this 3D class were subjected to a 3D refinement using full-size images without binning (320 × 320 pixels) in RELION3, which yielded a map with an overall resolution of 3.3 Å, and was further improved to 3.1 Å when refined in cryoSPARC (Map1). To improve the local resolution of SecA, a focused 3D refinement scheme was used by first shifting the center of the box from the ribosome to SecA and re-extracting the new particle coordinates using the re-centering option in RELION3 (box size 120 × 120 pixels). Local searches along with a mask around the SecA density were then applied, which resulted in a map of SecA resolved to 5.7 Å resolution (Map2).
Supplementary Figure 4 Local resolution and validation of the cryo-EM maps.
a, Local resolution of Map1 obtained from the 3D refinement (left), and close-up of the contact points and the resolved density for the RodZ TMD at a similar resolution as the overall resolution (right). b, Comparison of the local resolution plot for the EM density corresponding to SecA using the global refinement approach (left) and the focused refinement approach (right). Corresponding color keys are shown on the right side of each map. c, Fourier Shell Correlation (FSC) plots for Map1 (3.1 Å) and Map2 (5.7 Å) using the gold-standard FSC criteria cutoff (FSC = 0.143) using independent two half maps as implemented in RELION3 and cryoSPARC. Map1 versus obtained model plot shown in red and depicts a similar resolution 3.3 Å using cutoff (FSC = 0.5) as that of the cryo-EM Map1.
Supplementary Figure 5 Overview of the RodZ TMD binding pocket and the ribosome tunnel region.
a, Close-up of the N-terminal amphipathic helix of SecA in the RNCRodZ–SecA complex with an overlay of the EM-density. Color scheme is the same as in Fig. 3. The EM density was filtered based on the local resolution. b,c, Local EM-densities outlining regions of RodZ TMD interactions with SecA, uL23 and uL29 are shown with fitted atomic models. EM-densities are low-pass filtered to 3.5 Å resolution for clarity. d, Hydrophobicity gradient of the TMD binding pocket formed by SecA and uL23. Hydrophobic gradient shows hydrophobic amino acids in red and non-hydrophobic residues to white, applied from script “color_h” in pymol. Orange and green dashed lines outline the surfaces from SecA and uL23,respectively. e, A cross-section of the ribosome tunnel region with the EM-density of the RodZ nascent chain colored in magenta. The asterisk indicates the position of the CAA end of the P-site tRNA. EM-densities of the RodZ nascent chain was filtered to 4.5 Å resolution for clarity. f, Distance between C12 of SecA and C111/C146 on the RodZ nascent chain. C12 and C111 are shown in sticks, and the hypothetical location of C146 is shown in sphere. Residues 134–160 of nascent chain are not resolved and are shown as a dashed line.
Supplementary Figure 6 Characterization of the samples for fluorescence measurements of RNCRodZ transfer from SecA to SecYEG.
a, SecA surfaces contacting RNC (blue), anionic phospholipid (green) and SecYEG (grey). Blue and grey highlight SecA residues within 6 Å of RNC (this work) and SecYEG (PDB ID 5EUL), respectively. Green highlights residues 1–20 of SecA that mediate its lipid binding32. b, Coomassie-blue stained gel showing reconstituted ApoE422k nanodisc (Nd) with and without SecYEG. ApoE422k contains two thrombin cleavage sites at the N-terminus, and the observed minor band (asterisk, ~5% of total) may represent incompletely cleaved ApoE422k. c, Quantification of SecYEG-Nd by Coomassie-blue stained gel. Lanes 1–4 are purified ApoE422k at known concentrations. Lanes 6-8 are reconstituted SecYEG-Nd at different dilutions. Lane 5 shows the mixture of SecYEG:ApoE422k:lipid (molar ratio = 0.1:1:91) before removing detergent (see Methods). The intensity of bands with purified ApoE422k was used to generate a standard curve from which we calculated the concentration of ApoE422k in the nanodisc. The concentration of nanodisc was determined to be 136.5 μM by dividing the concentration of ApoE422k in nanodisc by 8 (see Methods). The concentration of SecYEG in nanodisc was 75.4 μM, determined as with ApoE422k using purified SecYEG to construct a standard curve. These values indicate that there is 0.55 copy of SecYEG per copy of nanodisc on average. The observed minor band (asterisk, ~5% of total) may represent incompletely cleaved ApoE422k. d,e, Negative stain electron microscopy images of the empty (d) and SecYEG (e) nanodiscs. Scale bar, 50 nm. f, Summary of the steady-state fluorescence intensity of the Cm dye on RNCRodZ under the indicated reactions. RNCRodZ 91aa, 131aa, and 171aa contains RodZ residues 104–160, 104–200, and 104–240, respectively. Nd, nanodisc. c.p.s, counts per second.
Supplementary Figure 7 Supporting information for the kinetic simulations, and additional data for chase experiments of the RNCRodZ–SecA complex.
a, Measurement of the association rate constant of the SecA–RNCRodZ complex. 10 nM Cm-labeled RNCRodZ was mixed with indicated concentrations of BDP-labeled SecA, and the fluorescence change was fit to Equation 3 in Supplementary Note 1 to extract the observed association rate constant (kobsd). The value of kobsd was plotted against SecA concentration and fit to Equation 7 in Supplementary Note 1 to determine k-1. b, Equilibrium measurement of the transfer reaction. 10 nM at indicated chain lengths was pre-incubated with 30 nM BDP-labeled SecA. Increasing amounts of SecYEG-Nd were then added to the preformed complex, and the increase in Cm fluorescence due to the loss of FRET was monitored. The data were fit to Equation 8 in Supplementary Note 1 and gave a K1/2 value of 45 ± 18 nM, 29 ± 5.4 nM, and 13 ± 2.1 nM for RNCRodZ at nascent chain lengths of 91, 131, and 171aa, respectively. c–f, Changes in the rate constants of RNCRodZ–SecYEG association (k2) and dissociation (k-2) (the Kd value for RNCRodZ–SecYEG was held constant) do not affect the kinetics behavior for both the passive (c,d) and active (e,f) models. g, Reaction scheme (left) and simulation (right) of the experiments to measure the dissociation rate constant (k1) of SecA from RNCRodZ. A preformed complex of Cm (blue star)-labeled RNCRodZ with BDP-labeled SecA was chased with excess unlabeled SecA to initiate complex dissociation, and the loss of FRET was monitored in real time. h,i, Representative time courses for measurement of k1 at nascent chain lengths of 91aa (h) and 131aa (i). The data were fit to Equation 3. All traces are the average of 6–8 measurements. j,k, Representative fluorescence time traces for chase of SecA-bound RNCRodZ complex with SecYEG-Nd (j) or empty nanodisc (k) at a nascent chain length of 131aa. Reactions were carried out and analyzed as in Supplementary Fig. 6c,d, and the obtained rate constants are summarized in Fig. 6f. Note that the time traces are biphasic, and control experiment indicated that the slow phase was due to dye bleaching (see Methods).
Supplementary Figure 8 Comparison of the structure from this work with previous structures.
a–c, Comparisons of SecA (a; this work), TF (b; PDB ID: 1W26 and 1W28) and SRP (c; PDB ID: 5GAF) bound to RNC. d, Overlay of SecA and SRP on the ribosome. The NG domain of the SRP protein Ffh was removed due to steric clash with SecA. The arrow indicates the difference in the position of the signal sequence versus TMD on the ribosome in the presence of SRP versus SecA. The following coloring scheme is used. TF, salmon; Ffh, cyan; SRP RNA, dark orange; signal sequence and TMD, magenta. e–g, Comparison of the structure of the RNC–SecA complex (g; this work) with the RNC–SecYEG (e; PDB ID: 3J46) and SecA–SecYEG (f; PDB ID: 5EUL) structures. The color scheme is: SecA, orange; SecYEG, blue; uL23, green; H59, red; RodZ TMD, magenta. h, The structures of SecA- (PDB ID 5EUL) and RNC-bound SecYEG (PDB ID: 3J46) were overlaid to show the steric clash between SecA and RNC on SecYEG.
Supplementary information
Supplementary Information
Supplementary Figures 1–8 and Supplementary Notes 1 and 2
Rights and permissions
About this article
Cite this article
Wang, S., Jomaa, A., Jaskolowski, M. et al. The molecular mechanism of cotranslational membrane protein recognition and targeting by SecA. Nat Struct Mol Biol 26, 919–929 (2019). https://doi.org/10.1038/s41594-019-0297-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41594-019-0297-8
This article is cited by
-
The translating bacterial ribosome at 1.55 Å resolution generated by cryo-EM imaging services
Nature Communications (2023)
-
The mechanisms of integral membrane protein biogenesis
Nature Reviews Molecular Cell Biology (2022)
-
Ribosome profiling reveals multiple roles of SecA in cotranslational protein export
Nature Communications (2022)
-
An intrinsically disordered nascent protein interacts with specific regions of the ribosomal surface near the exit tunnel
Communications Biology (2021)