Abstract
Primary cilia are microtubule-based organelles that are important for signaling and sensing in eukaryotic cells. Unlike the thoroughly studied motile cilia, the three-dimensional architecture and molecular composition of primary cilia are largely unexplored. Yet, studying these aspects is necessary to understand how primary cilia function in health and disease. We developed an enabling method for investigating the structure of primary cilia isolated from MDCK-II cells at molecular resolution by cryo-electron tomography. We show that the textbook ‘9 + 0’ arrangement of microtubule doublets is only present at the primary cilium base. A few microns out, the architecture changes into an unstructured bundle of EB1-decorated microtubules and actin filaments, putting an end to a long debate on the presence or absence of actin filaments in primary cilia. Our work provides a plethora of insights into the molecular structure of primary cilia and offers a methodological framework to study these important organelles.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$189.00 per year
only $15.75 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
References
Badano, J. L., Mitsuma, N., Beales, P. L. & Katsanis, N. The ciliopathies: an emerging class of human genetic disorders. Annu. Rev. Genomics Hum. Genet. 7, 125–148 (2006).
Fliegauf, M., Benzing, T. & Omran, H. When cilia go bad: cilia defects and ciliopathies. Nat. Rev. Mol. Cell Biol. 8, 880–893 (2007).
Waters, A. M. & Beales, P. L. Ciliopathies: an expanding disease spectrum. Pediatr. Nephrol. 26, 1039–1056 (2011).
Afzelius, B. A. A human syndrome caused by immotile cilia. Science 193, 317–319 (1976).
Mitchison, H. M. & Valente, E. M. Motile and non-motile cilia in human pathology: from function to phenotypes. J. Pathol. 241, 294–309 (2017).
Satir, P., Heuser, T. & Sale, W. S. A structural basis for how motile cilia beat. Bioscience 64, 1073–1083 (2014).
Harris, E. H., Stern, D. B. & Witman, G. B. The Chlamydomonas Sourcebook 2nd edn (Academic Press, 2009).
Pigino, G. et al. Comparative structural analysis of eukaryotic flagella and cilia from Chlamydomonas, Tetrahymena, and sea urchins. J. Struct. Biol. 178, 199–206 (2012).
Bui, K. H., Sakakibara, H., Movassagh, T., Oiwa, K. & Ishikawa, T. Asymmetry of inner dynein arms and inter-doublet links in Chlamydomonas flagella. J. Cell Biol. 186, 437–446 (2009).
Jordan, M. A., Diener, D. R., Stepanek, L. & Pigino, G. The cryo-EM structure of intraflagellar transport trains reveals how dynein is inactivated to ensure unidirectional anterograde movement in cilia. Nat. Cell Biol. 20, 1250–1255 (2018).
Nicastro, D. et al. The molecular architecture of axonemes revealed by cryoelectron tomography. Science 313, 944–948 (2006).
Witman. G. B. in Methods in Enzymology, Vol. 134 (ed. Vallee, R. B.) 280–290 (Academic Press, 1986).
Ma, M. et al. Structure of the decorated ciliary doublet microtubule. Cell 179, 909–922.e12 (2019).
Lin, J. & Nicastro, D. Asymmetric distribution and spatial switching of dynein activity generates ciliary motility. Science 360, eaar1968 (2018).
Pigino, G. & Ishikawa, T. Axonemal radial spokes: 3D structure, function and assembly. Bioarchitecture 2, 50–58 (2012).
Oda, T., Yanagisawa, H., Kamiya, R. & Kikkawa, M. A molecular ruler determines the repeat length in eukaryotic cilia and flagella. Science 346, 857–860 (2014).
Ishikawa, H., Thompson, J., Yates, J. R. III. & Marshall, W. F. Proteomic analysis of mammalian primary cilia. Curr. Biol. 22, 414–419 (2012).
Mick, D. U. et al. Proteomics of primary cilia by proximity labeling. Dev. Cell 35, 497–512 (2015).
Ott, C. & Lippincott-Schwartz, J. Visualization of live primary cilia dynamics using fluorescence microscopy. Curr. Protoc. Cell Biol. 57, 4.26.1–4.26.22 (2012).
Sun, S., Fisher, R. L., Bowser, S. S., Pentecost, B. T. & Sui, H. Three-dimensional architecture of epithelial primary cilia. Proc. Natl Acad. Sci. USA 116, 9370–9379 (2019).
Gluenz, E. et al. Beyond 9+0: noncanonical axoneme structures characterize sensory cilia from protists to humans. FASEB J. 24, 3117–3121 (2010).
Doroquez, D. B., Berciu, C., Anderson, J. R., Sengupta, P. & Nicastro, D. A high-resolution morphological and ultrastructural map of anterior sensory cilia and glia in Caenorhabditis elegans. Elife 3, e01948 (2014).
Ishikawa, H. & Marshall, W. F. in Methods in Enzymology Vol. 525 (ed. Marshall, W. F.) 311–325 (Academic Press, 2013).
Huang, B., Masyuk, T. & LaRusso, N. in Methods in Cell Biology Vol. 94 (ed. Sloboda, R. D.) 103–115 (Academic Press, 2009).
Bujakowska, K. M. & Liu, Q. & Pierce, E. A. Photoreceptor cilia and retinal ciliopathies. Cold Spring Harb. Perspect. Biol. 9, a028274 (2017).
Yuan, S., Zhao, L., Brueckner, M. & Sun, Z. Intraciliary calcium oscillations initiate vertebrate left-right asymmetry. Curr. Biol. 25, 556–567 (2015).
Essner, J. J., Amack, J. D., Nyholm, M. K., Harris, E. B. & Yost, H. J. Kupffer’s vesicle is a ciliated organ of asymmetry in the zebrafish embryo that initiates left-right development of the brain, heart and gut. Development 132, 1247–1260 (2005).
Wheway, G., Nazlamova, L. & Hancock, J. T. Signaling through the primary cilium. Front. Cell Dev. Biol. https://doi.org/10.3389/fcell.2018.00008 (2018).
Anvarian, Z., Mykytyn, K., Mukhopadhyay, S., Pedersen, L. B. & Christensen, S. T. Cellular signalling by primary cilia in development, organ function and disease. Nat. Rev. Nephrol. 15, 199–219 (2019).
Goetz, J. G. et al. Endothelial cilia mediate low flow sensing during zebrafish vascular development. Cell Rep. 6, 799–808 (2014).
Praetorius, H. A. The primary cilium as sensor of fluid flow: new building blocks to the model. A review in the theme: cell signaling: proteins, pathways and mechanisms. Am. J. Physiol. Cell Physiol. 308, C198–C208 (2015).
Yoder, B. K. Role of primary cilia in the pathogenesis of polycystic kidney disease. J. Am. Soc. Nephrol. 18, 1381–1388 (2007).
Kathem, S. H., Mohieldin, A. M. & Nauli, S. M. The roles of primary cilia in polycystic kidney disease. AIMS Mol. Sci. 1, 27–46 (2014).
Stepanek, L. & Pigino, G. Microtubule doublets are double-track railways for intraflagellar transport trains. Science 352, 721–724 (2016).
Kozminsky, K. G., Johnson, K. A., Forscher, P. & Rosenbaum, J. L. A motility in the eukaryotic flagellum unrelated to flagellar beating. Proc. Natl Acad. Sci. USA 90, 5519–5523 (1993).
Ishikawa, H. & Marshall, W. F. Efficient live fluorescence imaging of intraflagellar transport in mammalian primary cilia. Methods Cell Biol. 127, 189–201 (2015).
Sui, H. & Downing, K. H. Molecular architecture of axonemal microtubule doublets revealed by cryo-electron tomography. Nature 442, 475–478 (2006).
Song, K. et al. In situ structure determination at nanometer resolution using TYGRESS. Nat. Methods 17, 201–208 (2020).
von Loeffelholz, O. et al. Nucleotide- and Mal3-dependent changes in fission yeast microtubules suggest a structural plasticity view of dynamics. Nat. Commun. 8, 2110 (2017).
Fisch, C. & Dupuis-Williams, P. Ultrastructure of cilia and flagella—back to the future! Biol. Cell 103, 249–270 (2011).
Hess, R. A. Small tubules, surprising discoveries: from efferent ductules in the turkey to the discovery that estrogen receptor alpha is essential for fertility in the male. Anim. Reprod. 1, 7–23 (2015).
Nguyen, A. M., Young, Y.-N. & Jacobs, C. R. The primary cilium is a self-adaptable, integrating nexus for mechanical stimuli and cellular signaling. Biol. Open 4, 1733–1738 (2015).
Owa, M. et al. Inner lumen proteins stabilize doublet microtubules in cilia and flagella. Nat. Commun. 10, 1143 (2019).
Garvalov, B. K. et al. Luminal particles within cellular microtubules. J. Cell Biol. 174, 759–765 (2006).
Coombes, C. et al. Mechanism of microtubule lumen entry for the α-tubulin acetyltransferase enzyme αTAT1. Proc. Natl Acad. Sci. USA 113, E7176–E7184 (2016).
Boehlke, C. et al. Differential role of Rab proteins in ciliary trafficking: Rab23 regulates Smoothened levels. J. Cell Sci. 123, 1460–1467 (2010).
Schrøder, J. M. et al. EB1 and EB3 promote cilia biogenesis by several centrosome-related mechanisms. J. Cell Sci. 124, 2539–2551 (2011).
Pedersen, L. B., Geimer, S., Sloboda, R. D. & Rosenbaum, J. L. The microtubule plus end-tracking protein EB1 is localized to the flagellar tip and basal bodies in Chlamydomonas reinhardtii. Curr. Biol. 13, 1969–1974 (2003).
Roth, D., Fitton, B. P., Chmel, N. P., Wasiluk, N. & Straube, A. Spatial positioning of EB family proteins at microtubule tips involves distinct nucleotide-dependent binding properties. J. Cell Sci. 132, jcs219550 (2018).
Leterrier, C. et al. End-binding proteins EB3 and EB1 link microtubules to ankyrin G in the axon initial segment. Proc. Natl Acad. Sci. USA 108, 8826–8831 (2011).
Schrøder, J. M., Schneider, L., Christensen, S. T. & Pedersen, L. B. EB1 is required for primary cilia assembly in fibroblasts. Curr. Biol. 17, 1134–1139 (2007).
Lopez, B. J. & Valentine, M. T. Mechanical effects of EB1 on microtubules depend on GTP hydrolysis state and presence of paclitaxel. Cytoskeleton (Hoboken) 71, 530–541 (2014).
Zhang, R., LaFrance, B. & Nogales, E. Separating the effects of nucleotide and EB binding on microtubule structure. Proc. Natl Acad. Sci. USA 115, E6191–E6200 (2018).
Stroud, M. J. et al. GAS2-like proteins mediate communication between microtubules and actin through interactions with end-binding proteins. J. Cell Sci. 127, 2672–2682 (2014).
Nazgiewicz, A., Atherton, P. & Ballestrem, C. GAS2-like 1 coordinates cell division through its association with end-binding proteins. Sci. Rep. 9, 5805 (2019).
Slep, K. C. et al. Structural determinants for EB1-mediated recruitment of APC and spectraplakins to the microtubule plus end. J. Cell Biol. 168, 587–598 (2005).
Lee, S., Tan, H. Y., Geneva, I. I., Kruglov, A. & Calvert, P. D. Actin filaments partition primary cilia membranes into distinct fluid corrals. J. Cell Biol. 217, 2831–2849 (2018).
Copeland, S. J., McRae, A., Guarguaglini, G., Trinkle-Mulcahy, L. & Copeland, J. W. Actin-dependent regulation of cilia length by the inverted formin FHDC1. Mol. Biol. Cell 29, 1611–1627 (2018).
Kohli, P. et al. The ciliary membrane-associated proteome reveals actin-binding proteins as key components of cilia. EMBO Rep. 18, 1521–1535 (2017).
Phua, S. C. et al. Dynamic remodeling of membrane composition drives cell cycle through primary cilia excision. Cell 168, 264–279.e15 (2017).
Zuo, X. et al. Primary cilia and the exocyst are linked to urinary extracellular vesicle production and content. J. Biol. Chem. 294, 19099–19110 (2019).
Nager, A. R. et al. An actin network dispatches ciliary GPCRs into extracellular vesicles to modulate signaling. Cell 168, 252–263.e14 (2017).
Mirvis, M., Stearns, T. & James Nelson, W. Cilium structure, assembly, and disassembly regulated by the cytoskeleton. Biochem. J. 475, 2329–2353 (2018).
Maraspini, R., Wang, C.-H. & Honigmann, A. Optimization of 2D and 3D cell culture to study membrane organization with STED microscopy. J. Phys. D Appl. Phys. 53, 014001 (2020).
Sowa, M. E., Bennett, E. J., Gygi, S. P. & Harper, J. W. Defining the human deubiquitinating enzyme interaction landscape. Cell 138, 389–403 (2009).
Shaner, N. C. et al. A bright monomeric green fluorescent protein derived from Branchiostoma lanceolatum. Nat. Methods 10, 407–409 (2013).
Rogowski, M., Scholz, D. & Geimer, S. in Methods in Enzymology Vol. 524 (ed. Marshall, W. F.) 243–263 (Academic Press, 2013).
Cardona, A. et al. TrakEM2 software for neural circuit reconstruction. PLoS ONE 7, e38011 (2012).
Mastronarde, D. N. Automated electron microscope tomography using robust prediction of specimen movements. J. Struct. Biol. 152, 36–51 (2005).
Kremer, J. R., Mastronade, D. N. & McIntosh, J. R computer visualization of three-dimensional image data using IMOD. J. Struct. Biol. 116, 71–76 (1996).
Schindelin, J. et al. Fiji: an open source platform for biological-image analysis. Nat. Methods 9, 676–682 (2012).
Li, X. et al. Electron counting and beam-induced motion correction enable near-atomic-resolution single-particle cryo-EM. Nat. Methods 10, 584–590 (2013).
Xiong, Q., Morphew, M. K., Schwartz, C. L., Hoenger, A. H. & Mastronarde, D. N. CTF determination and correction for low dose tomographic tilt series. J. Struct. Biol. 168, 378–387 (2009).
Buchholz, T.-O., Jordan, M., Pigino, G. & Jug, F. Cryo-CARE: content-aware image restoration for cryo-transmission electron microscopy data. Preprint at arXiv https://arxiv.org/abs/1810.05420 (2018).
Buchholz, T.-O. et al. Content-aware image restoration for electron microscopy. Methods Cell Biol. 152, 277–289 (2019).
Heumann, J. M., Hoenger, A. & Mastronarde, D. N. Clustering and variance maps for cryo-electron tomography using wedge-masked differences. J. Struct. Biol. 175, 288–299 (2011).
Pettersen, E. F. et al. UCSF Chimera—a visualization system for exploratory research and analysis. J. Comput. Chem. 25, 1605–1612 (2004).
Acknowledgements
We thank the Electron Microscopy Facility (in particular T. Fürstenhaupt, W. Leng, M. Wilsch-Bräuninger) and the Light Microscope Facility from the Services and Facilities of the Max Planck Institute of Molecular Cell Biology and Genetics (Dresden) for their support. We thank H. Rägel and C. Martin-Lemaitre for their tips on the MDCK-II cell culture, N. Walker for the Imaris tutorial and T.-O. Buchholz for denoising the cryo-ET data. We thank P. Tomancak, F. Jug, D. Diener and J. Brugues for the fruitful discussions and suggestions on the manuscript. We thank O. Gonzales for IT support. We thank the Light Microscopy Core Facility, IMG CAS (Prague) for their support with the confocal imaging. This work was supported by the Max Planck Society and by the European Research Council under the European Union’s Horizon 2020 research and innovation program (grant no. 819826) to G.P. Work in V.V.’s laboratory was supported by the Czech Science Foundation (project no. 20-23165J).
Author information
Authors and Affiliations
Contributions
P.K. developed the cryo-peel off method, prepared the samples for FM and EM imaging, acquired and reconstructed room temperature and cryo-tomograms, contributed to the FM data acquisition, analyzed the EM and FM data, prepared the figures, interpreted the results and contributed to writing and revising the manuscript. G.A.V. prepared the samples and contributed to data acquisition of the room temperature tomography, analyzed the cryo-EM data with StA and tomogram segmentation, analyzed the EM and FM data, prepared the figures, interpreted the results and contributed to writing and revising the manuscript. N.T. analyzed the cryo-ET data to average the microtubule singlets, contributed to the preparation of the supplementary figures, contributed to the interpretation of the data and contributed to writing and revising the manuscript. R.M. prepared the samples for FM, contributed to the FM data acquisition and contributed to creating the figures. P.G. and V.V. generated the MDCK-II cells stably expressing mNeonGreen-tagged EB1 and imaged them using confocal microscopy. A.H. contributed to the FM experimental design, provided access to research equipment, contributed to data interpretation and revised the manuscript. G.P. conceived and supervised the project, contributed to the experimental design, data analysis and results interpretation, contributed to writing the manuscript and creating the figures, provided access to crucial research components and provided funding.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Peer review information Peer reviewer reports are available. Inês Chen was the primary editor on this article and managed its editorial process and peer review in collaboration with the rest of the editorial team.
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Extended data
Extended Data Fig. 1 Assessment of structural measurements performed on averaged data of microtubule singlets from MDCKII primary cilia.
a, Fourier Shell Correlation (FSC) curve of the average electron density map from MDCKII microtubule singlets depicting the resolution associated with typical criteria (FSC = 0.5 and 0.143). b, Power spectrum of the singlet microtubule average showing tubulin monomer and dimer repeats.
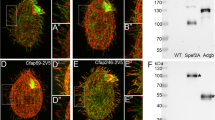
Extended Data Fig. 2 Immunofluorescence microscopy of EB1 in primary cilia.
A1-3 Immunofluorescence staining showing EB1 (green) and acetylated tubulin (magenta) along the axoneme of peeled-off cilia. B1-3 and C1-3 EB1 (green) is also present along cell-attached cilia of wildtype cells and of cells stably expressing mNeonGreen-tagged EB1 (mNG-EB1). In B, cilia were stained with an antibody against EB1 (green) while in C, EB1 was imaged directly through the fluorescence signal of the mNG tag (mNG-EB1, green). Magenta, acetylated tubulin; Green, EB1; (-), cilium base.
Extended Data Fig. 3 Vesicles were found in the vicinity of ciliary membranes and ciliary filaments.
(a-g), Longitudinal cryo-tomographic slices through peeled-off MDCKII primary cilia showing the presence of vesicles (V) in the proximity of the cilium, often tethered to the ciliary membrane by thin connections (C). (b-g). Half of the identified vesicles were found along regions of the cilium that contained filaments (l). Magenta arrowheads indicate examples of filaments located in the vicinity of vesicles (a,d-j). (h-j), Vesicles were found associated with the ciliary membrane, and in the proximity of filaments, also in tomograms from fixed and plastic embedded cell-attached cilia. k, Number of cryo-tomograms containing membrane and filament associated vesicles. l, Quantification of vesicles with diverse interactions with ciliary membrane and filaments in cryo-tomograms. C, vesicle−membrane connections; MT, microtubule; M, membrane, V, vesicle.
Supplementary information
Supplementary Information
Supplementary Tables 1–3.
Supplementary Video 1
Proximodistal tomographic sections through resin-embedded MDCK-II primary cilium from the ciliary base towards the ciliary shaft depicting the early migration of a doublet towards the center of the axoneme.
Supplementary Video 2
Proximodistal sections through a resin-embedded MDCK-II primary cilium depicting the rotation of the inner junction of a microtubule doublet around the ciliary central axis, measured across a portion of the proximal end of the doublet region.
Supplementary Video 3
Proximodistal cryo-tomographic slices through a MDCK-II primary cilium depicting the location of each microtubule singlet seam (colored dots).
Supplementary Video 4
Proximodistal cryo-tomographic slices through a MDCK-II primary cilium showing the termination of a microtubule singlet and the presence of two IFT-B polymers.
Supplementary Video 5
Longitudinal cryo-tomographic slices through a MDCK-II primary cilium depicting the presence of two IFT-B polymers.
Supplementary Video 6
Longitudinal slices through a cryo-CARE-denoised tomogram depicting the presence of EB1 singlet decoration and F-actin within the primary cilium of MDCK-II cells.
Supplementary Video 7
Confocal microscopy of MDCK-II cells stably expressing mNeonGreen-tagged EB1. EB1 signal is visible in the cilium and in the cytoplasm (EB1 comets). In the cilium, the mNG-EB1 signal is stronger at the base and progressively decreases towards the tip, probably because of the reduced number of microtubules towards the ciliary tip.
Supplementary Video 8
Fitting of a deposited structure of F-actin (EMD-6448) (gray surface) in the subtomogram-averaged model of F-actin from the primary cilium (magenta mesh).
Supplementary Data 1
Measurements of ciliary length under different experimental conditions: cilia attached to cells imaged by confocal immunofluorescence microscopy, peeled-off cilia on glass slides imaged by immunofluorescence microscopy and peeled-off cilia imaged by cryo-EM.
Rights and permissions
About this article
Cite this article
Kiesel, P., Alvarez Viar, G., Tsoy, N. et al. The molecular structure of mammalian primary cilia revealed by cryo-electron tomography. Nat Struct Mol Biol 27, 1115–1124 (2020). https://doi.org/10.1038/s41594-020-0507-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41594-020-0507-4
This article is cited by
-
Regulation of ciliary homeostasis by intraflagellar transport-independent kinesins
Cell Death & Disease (2024)
-
Transport and barrier mechanisms that regulate ciliary compartmentalization and ciliopathies
Nature Reviews Nephrology (2024)
-
Emerging mechanistic understanding of cilia function in cellular signalling
Nature Reviews Molecular Cell Biology (2024)
-
Primary cilia as dynamic and diverse signalling hubs in development and disease
Nature Reviews Genetics (2023)
-
FAP106 is an interaction hub for assembling microtubule inner proteins at the cilium inner junction
Nature Communications (2023)