Abstract
The endosomal sorting complex required for transport (ESCRT) is a highly conserved protein machinery that drives a divers set of physiological and pathological membrane remodeling processes. However, the structural basis of ESCRT-III polymers stabilizing, constricting and cleaving negatively curved membranes is yet unknown. Here we present cryo-EM structures of membrane-coated CHMP2A–CHMP3 filaments from Homo sapiens of two different diameters at 3.3 and 3.6 Å resolution. The structures reveal helical filaments assembled by CHMP2A–CHMP3 heterodimers in the open ESCRT-III conformation, which generates a partially positive charged membrane interaction surface, positions short N-terminal motifs for membrane interaction and the C-terminal VPS4 target sequence toward the tube interior. Inter-filament interactions are electrostatic, which may facilitate filament sliding upon VPS4-mediated polymer remodeling. Fluorescence microscopy as well as high-speed atomic force microscopy imaging corroborate that VPS4 can constrict and cleave CHMP2A–CHMP3 membrane tubes. We therefore conclude that CHMP2A–CHMP3–VPS4 act as a minimal membrane fission machinery.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$189.00 per year
only $15.75 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
Data availability
Cryo-EM maps and models were deposited to the Protein Data Bank (PDB) and Electron Microscopy Data Bank (EMDB) with the following codes: membrane-bound CHMP2A–CHMP3, 430 Å diameter (PDB ID 7ZCG, EMD-14630) and membrane-bound CHMP2A–CHMP3, 410 Å diameter (PDB ID 7ZCH, EMD-14631). Raw gels are provided as source data. Source data are provided with this paper.
References
Henne, W. M., Stenmark, H. & Emr, S. D. Molecular mechanisms of the membrane sculpting ESCRT pathway. Cold Spring Harb. Perspect. Biol. 5, a016766 (2013).
Votteler, J. & Sundquist, W. I. Virus budding and the ESCRT pathway. Cell Host Microbe 14, 232–241 (2013).
Allison, R. et al. An ESCRT-spastin interaction promotes fission of recycling tubules from the endosome. J. Cell Biol. 202, 527–543 (2013).
Loncle, N., Agromayor, M., Martin-Serrano, J. & Williams, D. W. An ESCRT module is required for neuron pruning. Sci. Rep. 5, 8461 (2015).
Olmos, Y. & Carlton, J. G. The ESCRT machinery: new roles at new holes. Curr. Opin. Cell Biol. 38, 1–11 (2016).
Scourfield, E. J. & Martin-Serrano, J. Growing functions of the ESCRT machinery in cell biology and viral replication. Biochem. Soc. Trans. 45, 613–634 (2017).
Sadoul, R. et al. The role of ESCRT during development and functioning of the nervous system. Semin. Cell Dev. Biol. 74, 40–49 (2018).
Mast, F. D. et al. ESCRT-III is required for scissioning new peroxisomes from the endoplasmic reticulum. J. Cell Biol. 217, 2087–2102 (2018).
Zhen, Y., Radulovic, M., Vietri, M. & Stenmark, H. Sealing holes in cellular membranes. EMBO J. 40, e106922 (2021).
McCullough, J., Frost, A. & Sundquist, W. I. Structures, functions, and dynamics of ESCRT-III/Vps4 membrane remodeling and fission complexes. Annu. Rev. Cell Dev. Biol. 34, 85–109 (2018).
Bertin, A. et al. Human ESCRT-III polymers assemble on positively curved membranes and induce helical membrane tube formation. Nat. Commun. 11, 2663 (2020).
Moser von Filseck, J. et al. Anisotropic ESCRT-III architecture governs helical membrane tube formation. Nat. Commun. 11, 1516 (2020).
Caillat, C., Maity, S., Miguet, N., Roos, W. H. & Weissenhorn, W. The role of VPS4 in ESCRT-III polymer remodeling. Biochem. Soc. Trans. 47, 441–448 (2019).
Liu, J. et al. Bacterial Vipp1 and PspA are members of the ancient ESCRT-III membrane-remodeling superfamily. Cell 184, 3660–3673 (2021).
Junglas, B. et al. PspA adopts an ESCRT-III-like fold and remodels bacterial membranes. Cell 184, 3674–3688 (2021).
Pfitzner, A. K., Moser von Filseck, J. & Roux, A. Principles of membrane remodeling by dynamic ESCRT-III polymers. Trends Cell Biol. 31, 856–868 (2021).
Nguyen, H. C. et al. Membrane constriction and thinning by sequential ESCRT-III polymerization. Nat. Struct. Mol. Biol. 27, 392–399 (2020).
Remec Pavlin, M. & Hurley, J. H. The ESCRTs—converging on mechanism. J. Cell Sci. 133, jcs240333 (2020).
Harker-Kirschneck, L. et al. Physical mechanisms of ESCRT-III-driven cell division. Proc. Natl Acad. Sci. USA 119, e2107763119 (2022).
Babst, M., Katzmann, D. J., Estepa-Sabal, E. J., Meerloo, T. & Emr, S. D. ESCRT-III: an endosome-associated heterooligomeric protein complex required for MVB sorting. Dev. Cell 3, 271–282 (2002).
Morita, E. et al. ESCRT-III protein requirements for HIV-1 budding. Cell Host Microbe 9, 235–242 (2011).
Teis, D., Saksena, S. & Emr, S. D. Ordered assembly of the ESCRT-III complex on endosomes is required to sequester cargo during MVB formation. Dev. Cell 15, 578–589 (2008).
Saksena, S., Wahlman, J., Teis, D., Johnson, A. E. & Emr, S. D. Functional reconstitution of ESCRT-III assembly and disassembly. Cell 136, 97–109 (2009).
Zamborlini, A. et al. Release of autoinhibition converts ESCRT-III components into potent inhibitors of HIV-1 budding. Proc. Natl Acad. Sci. USA 103, 19140–19145 (2006).
Rheinemann, L. et al. RetroCHMP3 blocks budding of enveloped viruses without blocking cytokinesis. Cell 184, 5419–5431 (2021).
Effantin, G. et al. ESCRT-III CHMP2A and CHMP3 form variable helical polymers in vitro and act synergistically during HIV-1 budding. Cell Microbiol 15, 213–226 (2013).
Schoneberg, J. et al. ATP-dependent force generation and membrane scission by ESCRT-III and Vps4. Science 362, 1423–1428 (2018).
Muziol, T. et al. Structural basis for budding by the ESCRT-III factor CHMP3. Dev. Cell 10, 821–830 (2006).
Bajorek, M. et al. Structural basis for ESCRT-III protein autoinhibition. Nat. Struct. Mol. Biol. 16, 754–762 (2009).
Xiao, J. et al. Structural basis of Ist1 function and Ist1-Did2 interaction in the multivesicular body pathway and cytokinesis. Mol. Biol. Cell 20, 3514–3524 (2009).
Im, Y. J., Wollert, T., Boura, E. & Hurley, J. H. Structure and function of the ESCRT-II-III interface in multivesicular body biogenesis. Dev. Cell 17, 234–243 (2009).
Pineda-Molina, E. et al. The crystal structure of the C-terminal domain of Vps28 reveals a conserved surface required for Vps20 recruitment. Traffic 7, 1007–1016 (2006).
McCullough, J., Fisher, R. D., Whitby, F. G., Sundquist, W. I. & Hill, C. P. ALIX-CHMP4 interactions in the human ESCRT pathway. Proc. Natl Acad. Sci. USA 105, 7687–7691 (2008).
Tang, S. et al. ESCRT-III activation by parallel action of ESCRT-I/II and ESCRT-0/Bro1 during MVB biogenesis. eLife 5, e15507 (2016).
Shim, S., Kimpler, L. A. & Hanson, P. I. Structure/function analysis of four core ESCRT-III proteins reveals common regulatory role for extreme C-terminal domain. Traffic 8, 1068–1079 (2007).
Lata, S. et al. Structural basis for autoinhibition of ESCRT-III CHMP3. J. Mol. Biol. 378, 818–827 (2008).
McCullough, J. et al. Structure and membrane remodeling activity of ESCRT-III helical polymers. Science 350, 1548–1551 (2015).
Cada, A. K. et al. Friction-driven membrane scission by the human ESCRT-III proteins CHMP1B and IST1. Proc. Natl Acad. Sci. USA 119, e2204536119 (2022).
Tang, S. et al. Structural basis for activation, assembly and membrane binding of ESCRT-III Snf7 filaments. eLife 4, e12548 (2015).
McMillan, B. J. et al. Electrostatic interactions between elongated monomers drive filamentation of Drosophila shrub, a metazoan ESCRT-III protein. Cell Rep. 16, 1211–1217 (2016).
Pires, R. et al. A crescent-shaped ALIX dimer targets ESCRT-III CHMP4 filaments. Structure 17, 843–856 (2009).
Chiaruttini, N. et al. Relaxation of loaded ESCRT-III spiral springs drives membrane deformation. Cell 163, 866–879 (2015).
Mierzwa, B. E. et al. Dynamic subunit turnover in ESCRT-III assemblies is regulated by Vps4 to mediate membrane remodelling during cytokinesis. Nat. Cell Biol. 19, 787–798 (2017).
Lee, I. H., Kai, H., Carlson, L. A., Groves, J. T. & Hurley, J. H. Negative membrane curvature catalyzes nucleation of endosomal sorting complex required for transport (ESCRT)-III assembly. Proc. Natl Acad. Sci. USA 112, 15892–15897 (2015).
Hanson, P. I., Roth, R., Lin, Y. & Heuser, J. E. Plasma membrane deformation by circular arrays of ESCRT-III protein filaments. J. Cell Biol. 180, 389–402 (2008).
Cashikar, A. G. et al. Structure of cellular ESCRT-III spirals and their relationship to HIV budding. eLife 3, e02184 (2014).
Gupta, T. K. et al. Structural basis for VIPP1 oligomerization and maintenance of thylakoid membrane integrity. Cell 184, 3643–3659 (2021).
Lata, S. et al. Helical structures of ESCRT-III are disassembled by VPS4. Science 321, 1354–1357 (2008).
Moriscot, C. et al. Crenarchaeal CdvA forms double-helical filaments containing DNA and interacts with ESCRT-III-like CdvB. PLoS ONE 6, e21921 (2011).
Dobro, M. J. et al. Electron cryotomography of ESCRT assemblies and dividing Sulfolobus cells suggests that spiraling filaments are involved in membrane scission. Mol. Biol. Cell 24, 2319–2327 (2013).
Henne, W. M., Buchkovich, N. J., Zhao, Y. & Emr, S. D. The endosomal sorting complex ESCRT-II mediates the assembly and architecture of ESCRT-III helices. Cell 151, 356–371 (2012).
Bodon, G. et al. Charged multivesicular body protein 2B (CHMP2B) of the endosomal sorting complex required for transport-III (ESCRT-III) polymerizes into helical structures deforming the plasma membrane. J. Biol. Chem. 286, 40276–40286 (2011).
Guizetti, J. et al. Cortical constriction during abscission involves helices of ESCRT-III-dependent filaments. Science 331, 1616–1620 (2011).
Sherman, S. et al. Resolving new ultrastructural features of cytokinetic abscission with soft-X-ray cryo-tomography. Sci. Rep. 6, 27629 (2016).
Goliand, I. et al. Resolving ESCRT-III spirals at the intercellular bridge of dividing cells using 3D STORM. Cell Rep. 24, 1756–1764 (2018).
Maity, S. et al. VPS4 triggers constriction and cleavage of ESCRT-III helical filaments. Sci. Adv. 5, eaau7198 (2019).
Kieffer, C. et al. Two distinct modes of ESCRT-III recognition are required for VPS4 functions in lysosomal protein targeting and HIV-1 budding. Dev. Cell 15, 62–73 (2008).
Adell, M. A. et al. Coordinated binding of Vps4 to ESCRT-III drives membrane neck constriction during MVB vesicle formation. J. Cell Biol. 205, 33–49 (2014).
Adell, M. A. Y. et al. Recruitment dynamics of ESCRT-III and Vps4 to endosomes and implications for reverse membrane budding. eLife 6, e31652 (2017).
He, S. & Scheres, S. H. W. Helical reconstruction in RELION. J. Struct. Biol. 198, 163–176 (2017).
Huber, S. T., Mostafavi, S., Mortensen, S. A. & Sachse, C. Structure and assembly of ESCRT-III helical Vps24 filaments. Sci. Adv. 6, eaba4897 (2020).
Buchkovich, N. J., Henne, W. M., Tang, S. & Emr, S. D. Essential N-terminal insertion motif anchors the ESCRT-III filament during MVB vesicle formation. Dev. Cell 27, 201–214 (2013).
Vietri, M., Radulovic, M. & Stenmark, H. The many functions of ESCRTs. Nat. Rev. Mol. Cell Biol. 21, 25–42 (2020).
Prescher, J. et al. Super-resolution imaging of ESCRT-proteins at HIV-1 assembly sites. PLoS Pathog. 11, e1004677 (2015).
Johnson, D. S., Bleck, M. & Simon, S. M. Timing of ESCRT-III protein recruitment and membrane scission during HIV-1 assembly. eLife 7, e36221 (2018).
Pfitzner, A. K. et al. An ESCRT-III polymerization sequence drives membrane deformation and fission. Cell 182, 1140–1155 (2020).
Banjade, S., Tang, S., Shah, Y. H. & Emr, S. D. Electrostatic lateral interactions drive ESCRT-III heteropolymer assembly. eLife 8, e46207 (2019).
Whitley, P. et al. Identification of mammalian Vps24p as an effector of phosphatidylinositol 3,5-bisphosphate-dependent endosome compartmentalization. J. Biol. Chem. 278, 38786–38795 (2003).
Lin, Y., Kimpler, L. A., Naismith, T. V., Lauer, J. M. & Hanson, P. I. Interaction of the mammalian endosomal sorting complex required for transport (ESCRT) III protein hSnf7-1 with itself, membranes, and the AAA+ ATPase SKD1. J. Biol. Chem. 280, 12799–12809 (2005).
De Franceschi, N. et al. The ESCRT protein CHMP2B acts as a diffusion barrier on reconstituted membrane necks. J. Cell Sci. 132, jcs217968 (2018).
Banjade, S., Shah, Y. H., Tang, S. & Emr, S. D. Design principles of the ESCRT-III Vps24-Vps2 module. eLife 10, e67709 (2021).
Caillat, C. et al. Asymmetric ring structure of Vps4 required for ESCRT-III disassembly. Nat. Commun. 6, 8781 (2015).
Fabrikant, G. et al. Computational model of membrane fission catalyzed by ESCRT-III. PLoS Comput. Biol. 5, e1000575 (2009).
Caspi, Y. & Dekker, C. Dividing the archaeal way: the ancient Cdv cell-division machinery. Front. Microbiol. 9, 174 (2018).
Ithurbide, S., Gribaldo, S., Albers, S. V. & Pende, N. Spotlight on FtsZ-based cell division in Archaea. Trends Microbiol. 30, 665–678 (2022).
Maity, S. et al. Caught in the act: mechanistic insight into supramolecular polymerization-driven self-replication from real-time visualization. J. Am. Chem. Soc. 142, 13709–13717 (2020).
Keya, J. J. et al. High-resolution imaging of a single gliding protofilament of tubulins by HS-AFM. Sci. Rep. 7, 6166 (2017).
Maity, S. et al. High-speed atomic force microscopy reveals a three-state elevator mechanism in the citrate transporter CitS. Proc. Natl Acad. Sci. USA 119, e2113927119 (2022).
Jouvenet, N., Bieniasz, P. D. & Simon, S. M. Imaging the biogenesis of individual HIV-1 virions in live cells. Nature 454, 236–240 (2008).
Kandiah, E. et al. CM01: a facility for cryo-electron microscopy at the European Synchrotron. Acta Crystallogr. D. Struct. Biol. 75, 528–535 (2019).
Zheng, S. Q. et al. MotionCor2: anisotropic correction of beam-induced motion for improved cryo-electron microscopy. Nat. Methods 14, 331–332 (2017).
Rohou, A. & Grigorieff, N. CTFFIND4: fast and accurate defocus estimation from electron micrographs. J. Struct. Biol. 192, 216–221 (2015).
Tang, G. et al. EMAN2: an extensible image processing suite for electron microscopy. J. Struct. Biol. 157, 38–46 (2007).
Scheres, S. H. RELION: implementation of a Bayesian approach to cryo-EM structure determination. J. Struct. Biol. 180, 519–530 (2012).
Zivanov, J. et al. New tools for automated high-resolution cryo-EM structure determination in RELION-3. eLife 7, e42166 (2018).
Desfosses, A., Ciuffa, R., Gutsche, I. & Sachse, C. SPRING—an image processing package for single-particle based helical reconstruction from electron cryomicrographs. J. Struct. Biol. 185, 15–26 (2014).
Rosenthal, P. B. & Henderson, R. Optimal determination of particle orientation, absolute hand, and contrast loss in single-particle electron cryomicroscopy. J. Mol. Biol. 333, 721–745 (2003).
Pettersen, E. F. et al. UCSF Chimera–a visualization system for exploratory research and analysis. J. Comput. Chem. 25, 1605–1612 (2004).
Waterhouse, A. et al. SWISS-MODEL: homology modelling of protein structures and complexes. Nucleic Acids Res. 46, W296–W303 (2018).
Emsley, P., Lohkamp, B., Scott, W. G. & Cowtan, K. Features and development of Coot. Acta Crystallogr. D. Biol. Crystallogr. 66, 486–501 (2010).
Adams, P. D. et al. PHENIX: a comprehensive Python-based system for macromolecular structure solution. Acta Crystallogr. D. Biol. Crystallogr. 66, 213–221 (2010).
Williams, C. J. et al. MolProbity: more and better reference data for improved all-atom structure validation. Protein Sci. 27, 293–315 (2018).
Afonine, P. V. et al. Real-space refinement in PHENIX for cryo-EM and crystallography. Acta Crystallogr. D. Struct. Biol. 74, 531–544 (2018).
Goddard, T. D. et al. UCSF ChimeraX: meeting modern challenges in visualization and analysis. Protein Sci. 27, 14–25 (2018).
Goujon, M. et al. A new bioinformatics analysis tools framework at EMBL-EBI. Nucleic Acids Res. 38, W695–W699 (2010).
Robert, X. & Gouet, P. Deciphering key features in protein structures with the new ENDscript server. Nucleic Acids Res. 42, W320–W324 (2014).
Acknowledgements
This research was funded by the ANR (grant nos. ANR-14-CE09-0003-01 and ANR-19-CE11-0002-02 to W.W.). W.W. acknowledges support from the Institut Universitaire de France and access to the platforms of the Grenoble Instruct-ERIC center (IBS and ISBG; grant no. UAR 3518 CNRS-CEA-UGA-EMBL) within the Grenoble Partnership for Structural Biology, with support from FRISBI (grant no. ANR-10-INBS-05-02) and GRAL, a project of the University Grenoble Alpes graduate school (Ecoles Universitaires de Recherche) CBH-EUR-GS (grant no. ANR-17-EURE-0003). The IBS electron microscope facility is supported by the Auvergne-Rhône-Alpes Region, the Fondation pour la Recherche Medicale, the FEDER/ERDF fund (European Regional Development Fund) and the GIS-IBiSA (Infrastructures en Biologie, Sante et Agronomie). We acknowledge the provision of in-house experimental time from the CM01 facility at the European Synchrotron Radiation Facility and we thank L. Estrozi for extensive discussion and help with helical image analysis. We further thank the HIV Reagent Program, Division of AIDS, NIAID, NIH for providing the Anti-Human Immunodeficiency Virus 1 (HIV-1) p24 Monoclonal (183-H12-5C), ARP-3537, contributed by B. Chesebro and K. Wehrly.
Author information
Authors and Affiliations
Contributions
W.W. conceived the study, designed experiments, interpreted experiments, supervised and received funding for the study. P.B. supervised and received funding for the study. K.A. performed all cryo-EM data analyses. N.D.F. established the membrane-coating protocol. D.G. prepared wild-type and mutant CHMP2A–CHMP3 polymers for all analyses. G. Sulbaran performed negative staining EM analyses. C.B. and J.-P.K. performed fluorescence microscopy imaging and H.W. mutant analyses. S.M. performed AFM analyses and W.H.R. supervised AFM analyses. G.E. and G. Schoehn collected cryo-EM data and A.D. supervised all aspects of cryo-EM data analyses and structure solution. W.W. wrote the paper with input from all authors.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Structural & Molecular Biology thanks Aurélien Roux and the other, anonymous, reviewer(s) for their contribution to the peer review of this work. Peer reviewer reports are available. Primary Handling Editor: Carolina Perdigoto, in collaboration with the Nature Structural & Molecular Biology team.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Extended data
Extended Data Fig. 1 Cryo-EM data processing of CHMP2A-CHMP3 membrane-coated tubes and helical symmetry analyses.
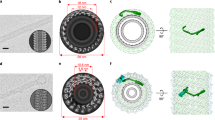
(a) Representative cryo-electron micrograph of CHMP2A-CHMP3 membrane tubes; arrow pointing to the lipid bilayer. Scale bar, 50 nm. (b) Selected 2D class averages of manually picked datasets; tube diameter range from 380 to 490 Å as indicated. (c), (d) Helical symmetry determination and representation of the elementary and biological helices for the 430 Å and 410 Å diameter tubes. Left panel, the sum of the 2D power spectra of segments corresponding to one class-average show in both cases a maximum on or near the meridian corresponding to the pitch (Bessel order n = 1 for C1 helix; n = 2 for C2 helix). The left half of the sum of the power spectra show the calculated position from helixplorer of the two first maxima of each Bessel function. The right half of the sum of the power spectra highlight for each symmetry the layer line corresponding to the pitch and the layer line spacing corresponding to the repeat distance. Middle panel, six asymmetric units of the elementary helix are represented; CHMP2A-CHMP3 dimers; in red, symmetry-related protomers in light red. Right panel, side view of the elementary helix with turns (red, yellow, green, blue and aqua) indicated with a black dashed line. The gray dashed line follows one turn of the biological helix. The central grey line (in c) highlights the C2 symmetry axis. Symmetry parameters of both the elementary and biological helices are indicated. (e, f) Central slices looking down the helical axis. The red arrows in the right zoom-in images indicate the density of the N-terminal region prone to insert into the lipid bilayer. (g) Representative of a cryoEM image showing protein-free vesicles, generated during the membrane coating protocol. 257 particles were picked and the diameter determined from 2-D class averages. (h) Average membrane thickness of protein-free vesicle compared to the membrane coated onto CHMP2A-CHMP3 polymers was determined from 30 measurements in each group from one preparation. The boxes show the lower quartile (25th percentile), the median, and the upper quartile (75th percentile). The smallest and largest values are indicated by the small horizontal bars at the end of the whiskers. The statistical significance was assessed using two-tailed t test.
Extended Data Fig. 2 Cryo-EM image processing workflow and structure determination.
Basic image processing strategy used for helical 3D reconstruction and refinement of 430 and 410 Å diameter tubes is shown. Helical filaments were segmented and classified based on the tube diameter. Segment subsets were subjected to symmetry determination (https://rico.ibs.fr/helixplorer) and initial 3D model generation in SPRING, followed by symmetry refinement and final 3D structure refinement in RELION. A complete description of the processing workflow is provided in ‘Materials and Methods’ section.
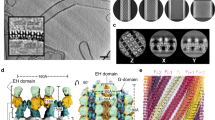
Extended Data Fig. 3 Fourier shell correlation (FSC) curves, local resolution maps and atomic model fitting.
(a) FSC curves for the 430 Å (black) and 410 Å (red) diameter tube maps, with the resolutions at the FSC cut-off of 0.143 are indicated. Model versus map FSC curves, with the resolutions at the FSC cut-off of 0.5 are indicated for the 430 Å (blue) and 410 Å (pink) diameter tube maps. Local resolution estimates are mapped onto the 430 Å (b), and 410 Å (c) diameter tube cryo-EM density maps and the color keys (right) highlight the local resolution values in Å. (d) The refined atomic model of CHMP2A-CHMP3 dimer was fit into the corresponding cryo-EM density map of the 430 Å diameter tube. The inset (below) represents the zoomed-in view of the fitted model, indicating CHMP2A and CHMP3 helices and the corresponding map.
Extended Data Fig. 4 Structure-based mutagenesis of CHMP2A-CHMP3 heterodimer formation and polymerization in vitro.
(a) Close-up views of the pairs of residues (black) mutated to cysteine to induce the formation of disulfide-linked CHMP2A (light blue) - CHMP3 (pink) heterodimers upon polymerization. (b) Cysteine cross-linking of the CHMP2A-CHMP3 heterodimer. Mutant CHMP2A_D57C was incubated with CHMP3_S75C and CHMP2A_N18C with CHMP3_V110C to induce polymerization as reported for wild-type CHMP2A and CHMP3. SDS-PAGE analysis showing that both CHMP2A_D57C-CHMP3_S75C and CHMP2A_N18C-CHMP3_V110C formed disulfide-linked dimers under non-reducing SDS PAGE conditions. (c) Negative staining electron micrographs showing regular tube formation for CHMP2A_N18C-CHMP3_V110C (right), while CHMP2A_D57C-CHMP3_S75C (left) produced only shorter tubes. Scale bar, 100 nm. (d) Close-up views of the CHMP3 interface residues A96, A82 and M89E tested for heterodimer formation and polymerization. (e) Negative staining electron micrographs of CHMP2A-CHMP3 wild-type and mutants (CHMP3_A96E, CHMP3_A82E, CHMP3-A82E_A96E and CHMP3_M89E). Scale bar, 200 nm. Experiments shown in b, c and e have been repeated three times.
Extended Data Fig. 5 ESCRT-III sequence alignment.
(a) Sequence alignment of CHMP3 (AF219226), S. cerevisiae Vps24 (QHB09957), S. cerevisiae Vps2 (P36108.2) and CHMP2A (NM_198426.3). Secondary structure elements are shown for CHMP3 above the sequence and for CHMP2A below the sequence alignment. Blue triangles indicate basic residues of CHMP3 (above) and CHMP2A (below) exposed at the membrane binding interface. Blue rectangles show basic residues and red squares conserved acidic residues exposed at the interface between filaments. (b) Sequence alignment of S. cerevisiae Snf7 (Z73197.1) and its secondary structure (pdb 5FD9), CHMP4A (NM_014169.5), CHMP4B (NM_176812.5) CHMP4C (NM_152284), CHMP5 (NM_016410.6) and CHMP6 (NM_024591.5). Conserved acidic residues implicated in inter-filament interactions in the CHMP2A-CHMP3 polymer are indicated as red squares.
Extended Data Fig. 6 Structure-based mutagenesis of CHMP2A-CHMP3 polymer formation.
(a) Negative staining electron micrograph showing regular tube formation by CHMP2A-CHMP3_K112A, K119A, K132A, K136A polymerization. Scale bar, 200 nm. (b) The substitution of four basic residues in CHMP3(1–150)-GFP (4KA: K112A, K119A, K132A, K136A) does not diminish its dominant-negative effect on HIV-1 budding. Western blot analyses of Gag released from Gag expressing cells as Gag-VLPs (upper panel) and detection of Gag in total cell extracts (lower panel): lane 1, control transfected with pcDNA; lane 2, Gag expression; lane 3, Gag and GFP-VPS4A E228Q expression; lane 4, Gag and CHMP3(1-150)-GFP expression; lane 5, Gag and CHMP3(1-150)-4KA-GFP (4KA) expression. (c) Representative fluorescence images of HeLa cells transfected with Gag/mCherry-Gag and CHMP3(1-150)-GFP or 4KA (CHMP3(1-150)4KA-GFP). Cellular distribution of wild-type and mutant 4KA indicates predominantly plasma membrane and intracellular localization as well as co-localization with mCherry-Gag. Scale bar, 10 µm. (d) Negative staining electron micrographs showing no polymer formation of CHMP3 mutants (upper left panel, close-up of a ribbon diagram illustrating the interface residues) R24A, K25A, R28A, R32A (upper middle panel) and R24E, K25E, R28E, R32E (upper right panel) with CHMP2A. (Lower panel) CHMP2A mutants (lower left panel, close-up of a ribbon diagram illustrating the interface residues) R16A, R20A, R24A, R31A (lower middle panel) and R16E, R20E, R24E, R31E (lower right panel) did not polymerize with CHMP3 in vitro. Scale bar, 200 nm. Experiments shown in a and d have been repeated two times and experiments shown in b and c one times.
Extended Data Fig. 7 High ionic strength unwinds the CHMP2A-CHMP3 filaments.
(a) Negative staining electron micrographs showing CHMP2A-CHMP3 wild-type polymers after treatment with 1 M NaCl (b, c) and 1 M KCl (d). (e) Close-up of cryo-EM images shows unwinding of ~20 nm wide filaments corresponding to the six-start helix observed in the structure. Single and multi-stranded unwound filaments are indicated by arrows. Experiments shown in a-e have been repeated three times.
Extended Data Fig. 8 Incorporation of VPS4B and ATP into CHMP2A-CHMP3 membrane tubes induces their disassembly.
(a) SDS-PAGE analyses of purified CHMP2A-CHMP3 polymers; lane 1, CHMP2A-CHMP3 polymers cleaved with TEV and coated with a lipid bilayer; lane 2 CHMP2A-CHMP3 polymers, TEV cleaved and incorporation of VPS4B prior to lipid bilayer coating. Negative staining electron micrographs of CHMP2A-CHMP3-VPS4B membrane-coated polymers before (b) and after (c) incubation with ATP and Mg2+. Red arrows point to membrane vesicles resulting from tube cleavage. Scale bar, 200 nm. Experiments have been repeated three times.
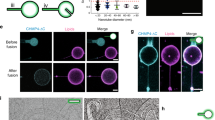
Extended Data Fig. 9 Imaging of VPS4B and ATP induced cleavage of CHMP2A-CHMP3 membrane coated tubes.
(a) A CHMP2A-CHMP3-caged ATP membrane coated tube was activated at 365 nm (365 nm 30%, 100 ms at each time point) to uncage ATP and imaged over 282 s, which indicated that uncaging did not change the tube structure (snapshots from Supplementary Video 2). (b) The kymograph of the tube (yellow line) shows that the tube stays intact over the imaging time. (c) Imaging of a CHMP2A-CHMP3-VPS4B-caged ATP membrane-coated tube following ATP uncaging (365 nm, 30%, 100 ms at each time point) reveals cleavage of the tube at several sites over the imaging time (snapshots from Supplementary Video 5). Scale Bar, 1 µm. (d) The kymograph of the tube (yellow line) indicates tube cleavage (arrows) over the imaging time. Scale bars are 1 μm.
Extended Data Fig. 10 Height distribution of CHMP2A-CHMP3 tubes with and without membrane coating.
(a) AFM image of CHMP2A-CHMP3 tubes without membrane. (b) AFM image of CHMP2A-CHMP3 tubes coated with membrane. (c) Cross-section of AFM images of CHMP2A-CHMP3 in panel a (blue dotted line) and panel b (red dotted line). (d) Height histogram of CHMP2A-CHMP3 tubes with (red, n = 84) and without (blue, n = 98) membrane coating with respect to the surface. (e) Snapshots HS-AFM images (from Supplementary Video 7) of membrane-coated tubes loaded with 10 mM caged ATP initially taken without and later with UV irradiation. The UV was turned on from 700 s onwards. No VPS4B is present. Scale bar, 200 nm.
Supplementary information
Supplementary Video 1
Fluorescence microscopy imaging of membrane-coated tubes containing caged ATP (example 1). A CHMP2A–CHMP3-caged ATP membrane-coated tube was activated at 365 nm for 10 s to uncage ATP and imaged over 291 s.
Supplementary Video 2
Fluorescence microscopy imaging of membrane-coated tubes containing caged ATP (example 2). Another dataset of CHMP2A–CHMP3-caged ATP membrane-coated tube activated at 365 nm for 100 ms at each time point to uncage ATP and imaged over 300 s.
Supplementary Video 3
Fluorescence microscopy imaging of membrane-coated tubes containing VPS4B. A CHMP2A–CHMP3–VPS4B membrane-coated tube was activated at 365 nm for 10 s to uncage ATP and imaged over 251 s.
Supplementary Video 4
Fluorescence microscopy imaging of membrane-coated tubes containing VPS4 and caged ATP (example 1). Imaging of a CHMP2A–CHMP3–VPS4B-caged ATP membrane-coated tube following ATP uncaging (365 nm, 10 s). This demonstrates tube fission followed by a shrinking event from both sides.
Supplementary Video 5
Fluorescence microscopy imaging of membrane-coated tubes containing VPS4B-caged ATP and caged ATP (example 2). Another dataset of CHMP2A–CHMP3–VPS4B-caged ATP membrane-coated tube following ATP uncaging (365 nm, 100 ms at each time point) reveals cleavage of the tube at several sites over the imaging time.
Supplementary Video 6
Fluorescence microscopy imaging of membrane-coated tubes containing VPS4B and caged ATP (example 3). Imaging of a CHMP2A–CHMP3–VPS4B-caged ATP membrane-coated tube following ATP uncaging (365 nm) showing at 36 s a shrinking event from the end of a tube and at 51 s a cleavage of the tube.
Supplementary Video 7
HS-AFM imaging of membrane-coated tubes with caged ATP, without VPS4B, UV on 700 s. HS-AFM video of membrane-coated CHMP2A–CHMP3 tubes loaded with 10 mM caged ATP, taken before and after UV (365 nm) irradiation. Imaging time 5 s per frame.
Supplementary Video 8
HS-AFM imaging of membrane-coated tubes with VPS4B and caged ATP, UV off. HS-AFM video of membrane-coated CHMP2A–CHMP3 tubes loaded with 5 µM VPS4B and 10 mM caged ATP, taken without UV irradiation. Imaging time 3 s per frame.
Supplementary Video 9
HS-AFM imaging of membrane-coated tubes with VPS4B and caged ATP, UV on. HS-AFM video of membrane-coated CHMP2A–CHMP3 tubes loaded with 5 µM VPS4B and 10 mM caged ATP, taken with 365 nm UV irradiation. UV was switched on <5 s before the start of the imaging (corresponds to 0 s). Imaging time 2 s per frame.
Source data
Source Data Fig. 1
Data.
Source Data Extended Data Fig. 4
Unprocessed SDS–PAGE.
Source Data Extended Data Fig. 6
Unprocessed western blot, upper panel (VLPs) Extended Data Fig. 6b.
Source Data Extended Data Fig. 6
Unprocessed western blot, lower panel (cells) Extended Data Fig. 6b.
Source Data Extended Data Fig. 8
Unprocessed SDS–PAGE.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Azad, K., Guilligay, D., Boscheron, C. et al. Structural basis of CHMP2A–CHMP3 ESCRT-III polymer assembly and membrane cleavage. Nat Struct Mol Biol 30, 81–90 (2023). https://doi.org/10.1038/s41594-022-00867-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41594-022-00867-8