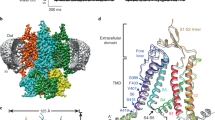
Abstract
Cyclic nucleotide-gated ion channels are crucial in many physiological processes such as vision and pacemaking in the heart. SthK is a prokaryotic homolog with high sequence and structure similarities to hyperpolarization-activated and cyclic nucleotide-modulated and cyclic nucleotide-gated channels, especially at the level of the cyclic nucleotide binding domains (CNBDs). Functional measurements showed that cyclic adenosine monophosphate (cAMP) is a channel activator while cyclic guanosine monophosphate (cGMP) barely leads to pore opening. Here, using atomic force microscopy single-molecule force spectroscopy and force probe molecular dynamics simulations, we unravel quantitatively and at the atomic level how CNBDs discriminate between cyclic nucleotides. We find that cAMP binds to the SthK CNBD slightly stronger than cGMP and accesses a deep-bound state that a cGMP-bound CNBD cannot reach. We propose that the deep binding of cAMP is the discriminatory state that is essential for cAMP-dependent channel activation.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$189.00 per year
only $15.75 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
Data availability
The source data files contain all data (force distribution histograms, H-bond distributions) necessary to interpret, verify and extend the presented work. In the absence of dedicated data repositories for raw data AFM force curves and MDS trajectories, and in light of the instructions needed to open these files in proprietary software (in the case of the AFM force curves) and the additional information (parameters and conditions) needed to understand and use the data, raw data AFM force curves and MDS trajectories can be received from S.S. (sis2019@med.cornell.edu) and H.G. (hgrubmu@gwdg.de), respectively, upon reasonable request. Source data are provided with this paper.
References
Kaupp, U. B. & Seifert, R. Cyclic nucleotide-gated ion channels. Physiol. Rev. 82, 769–824 (2002).
Craven, K. B. & Zagotta, W. N. CNG and HCN channels: two peas, one pod. Annu. Rev. Physiol. 68, 375–401 (2006).
Robinson, R. B. & Siegelbaum, S. A. Hyperpolarization-activated cation currents: from molecules to physiological function. Annu. Rev. Physiol. 65, 453–480 (2003).
Biel, M., Wahl-Schott, C., Michalakis, S. & Zong, X. Hyperpolarization-activated cation channels: from genes to function. Physiol. Rev. 89, 847–885 (2009).
James, Z. M. & Zagotta, W. N. Structural insights into the mechanisms of CNBD channel function. J. Gen. Physiol. 150, 225–244 (2017).
Yu, F. H., Yarov-Yarovoy, V., Gutman, G. A. & Catterall, W. A. Overview of molecular relationships in the voltage-gated ion channel superfamily. Pharmacol. Rev. 57, 387–395 (2005).
Rheinberger, J., Gao, X., Schmidpeter, P. A. M. & Nimigean, C. M. Ligand discrimination and gating in cyclic nucleotide-gated ion channels from apo and partial agonist-bound cryo-EM structures. eLife 7, e39775 (2018).
Lee, C.-H. & MacKinnon, R. Structures of the human HCN1 hyperpolarization-activated channel. Cell 168, 111–120.e111 (2017).
Saponaro, A. et al. Gating movements and ion permeation in HCN4 pacemaker channels. Mol. Cell 81, 2929–2943.e2926 (2021).
Li, M. et al. Structure of a eukaryotic cyclic-nucleotide-gated channel. Nature 542, 60–65 (2017).
Schmidpeter, P. A. M., Gao, X., Uphadyay, V., Rheinberger, J. & Nimigean, C. M. Ligand binding and activation properties of the purified bacterial cyclic nucleotide–gated channel SthK. J. Gen. Physiol. 150, 821–834 (2018).
Marchesi, A. et al. An iris diaphragm mechanism to gate a cyclic nucleotide-gated ion channel. Nat. Commun. 9, 3978 (2018).
Kesters, D. et al. Structure of the SthK carboxy-terminal region reveals a gating mechanism for cyclic nucleotide-modulated Ion channels. PLoS ONE 10, e0116369 (2015).
Florin, E. L., Moy, V. T. & Gaub, H. E. Adhesion forces between individual ligand-receptor pairs. Science 264, 415 (1994).
Thess, A. et al. Specific orientation and two-dimensional crystallization of the proteasome at metal-chelating lipid interfaces. J. Biol. Chem. 277, 36321–36328 (2002).
Nye, J. A. & Groves, J. T. Kinetic control of histidine-tagged protein surface density on supported lipid bilayers. Langmuir 24, 4145–4149 (2008).
Lolicato, M. et al. Tetramerization dynamics of C-terminal domain underlies isoform-specific cAMP gating in hyperpolarization-activated cyclic nucleotide-gated channels. J. Biol. Chem. 286, 44811–44820 (2011).
Zagotta, W. N. et al. Structural basis for modulation and agonist specificity of HCN pacemaker channels. Nature 425, 200–205 (2003).
Lolicato, M. et al. Cyclic dinucleotides bind the C-linker of HCN4 to control channel cAMP responsiveness. Nat. Chem. Biol. 10, 457–462 (2014).
Ebner, A. et al. A new, simple method for linking of antibodies to atomic force microscopy tips. Bioconjug. Chem. 18, 1176–1184 (2007).
Baumgartner, W. et al. Cadherin interaction probed by atomic force microscopy. Proc. Natl Acad. Sci. USA 97, 4005–4010 (2000).
Evans, E. & Ritchie, K. Dynamic strength of molecular adhesion bonds. Biophys. J. 72, 1541–1555 (1997).
Huang, J. et al. The kinetics of two-dimensional TCR and pMHC interactions determine T-cell responsiveness. Nature 464, 932–936 (2010).
Lo Giudice, C., Zhang, H., Wu, B. & Alsteens, D. Mechanochemical activation of class-B G-protein-coupled receptor upon peptide–ligand binding. Nano Lett. 20, 5575–5582 (2020).
Zhu, R. et al. Allosterically linked binding sites in serotonin transporter revealed by single molecule force spectroscopy. Front. Mol. Biosci. 7, 99 (2020).
Williams, P. M. Analytical descriptions of dynamic force spectroscopy: behaviour of multiple connections. Anal. Chim. Acta 479, 107–115 (2003).
Rankl, C. et al. Multiple receptors involved in human rhinovirus attachment to live cells. Proc. Natl Acad. Sci. USA 105, 17778–17783 (2008).
Sulchek, T. A. et al. Dynamic force spectroscopy of parallel individual Mucin1–antibody bonds. Proc. Natl Acad. Sci. USA 102, 16638–16643 (2005).
White, D. S. et al. cAMP binding to closed pacemaker ion channels is non-cooperative. Nature 595, 606–610 (2021).
Schmidpeter, P. A. M., Rheinberger, J. & Nimigean, C. M. Prolyl isomerization controls activation kinetics of a cyclic nucleotide-gated ion channel. Nat. Commun. 11, 6401 (2020).
Dudko, O. K., Hummer, G. & Szabo, A. Intrinsic rates and activation free energies from single-molecule pulling experiments. Phys. Rev. Lett. 96, 108101 (2006).
Aman, T. K., Gordon, S. E. & Zagotta, W. N. Regulation of CNGA1 channel gating by interactions with the membrane. J. Biol. Chem. 291, 9939–9947 (2016).
Brady, J. D. et al. Interplay between PIP3 and calmodulin regulation of olfactory cyclic nucleotide-gated channels. Proc. Natl Acad. Sci. USA 103, 15635–15640 (2006).
Asakawa, H., Yoshioka, S., Nishimura, K.-I. & Fukuma, T. Spatial distribution of lipid headgroups and water molecules at membrane/water interfaces visualized by three-dimensional scanning force microscopy. ACS Nano 6, 9013–9020 (2012).
Koehler, M. et al. Control of ligand-binding specificity using photocleavable linkers in AFM force spectroscopy. Nano Lett. 20, 4038–4042 (2020).
Miyagi, A. & Scheuring, S. Automated force controller for amplitude modulation atomic force microscopy. Rev. Sci. Instrum. 87, 053705 (2016).
Miyagi, A. & Scheuring, S. A novel phase-shift-based amplitude detector for a high-speed atomic force microscope. Rev. Sci. Instrum. 89, 083704 (2018).
Sali, A. & Blundell, T. L. Comparative protein modelling by satisfaction of spatial restraints. J. Mol. Biol. 234, 779–815 (1993).
Wang, J., Wolf, R. M., Caldwell, J. W., Kollman, P. A. & Case, D. A. Development and testing of a general amber force field. J. Comput. Chem. 25, 1157–1174 (2004).
Wang, J., Wang, W., Kollman, P. A. & Case, D. A. Automatic atom type and bond type perception in molecular mechanical calculations. J. Mol. Graph Model 25, 247–260 (2006).
Bayly, C. I. et al. A well-behaved electrostatic potential based method using charge restraints for deriving atomic charges: the RESP model. J. Phys. Chem. 97, 10269–10280 (1993).
Frisch, M. J. et al. Gaussian v.3, Revision C.02 (Gaussian, Inc., 2004).
Roothaan, C. C. J. New developments in molecular orbital theory. Rev. Mod. Phys. 23, 69–89 (1951).
Rassolov, V. A., Pople, J. A., Ratner, M. A. & Windus, T. L. 6-31G* basis set for atoms K through Zn. J. Chem. Phys. 109, 1223–1229 (1998).
Mobley, D. L., Chodera, J. D. & Dill, K. A. On the use of orientational restraints and symmetry corrections in alchemical free energy calculations. J. Chem. Phys. 125, 084902–084902 (2006).
Sousa da Silva, A. W. & Vranken, W. F. ACPYPE—AnteChamber PYthon Parser interfacE. BMC Res. Notes 5, 367 (2012).
Larsson, P., Kneiszl, R. C. & Marklund, E. G. MkVsites: a tool for creating GROMACS virtual sites parameters to increase performance in all-atom molecular dynamics simulations. J. Comput. Chem. 41, 1564–1569 (2020).
Jorgensen, W. L., Chandrasekhar, J., Madura, J. D., Impey, R. W. & Klein, M. L. Comparison of simple potential functions for simulating liquid water. J. Chem. Phys. 79, 926–935 (1983).
Abraham, M. J. et al. GROMACS: high performance molecular simulations through multi-level parallelism from laptops to supercomputers. SoftwareX 1–2, 19–25 (2015).
Verlet, L. Computer ‘experiments’ on classical fluids. I. Thermodynamical properties of Lennard-Jones molecules. Phys. Rev. 159, 98–103 (1967).
Lindorff-Larsen, K. et al. Improved side-chain torsion potentials for the Amber ff99SB protein force field. Proteins 78, 1950–1958 (2010).
Darden, T., York, D. & Pedersen, L. Particle mesh Ewald: an N⋅log(N) method for Ewald sums in large systems. J. Chem. Phys. 98, 10089–10092 (1993).
Hess, B., Bekker, H., Berendsen, H. J. C. & Fraaije, J. G. E. M. LINCS: a linear constraint solver for molecular simulations. J. Comput. Chem. 18, 1463–1472 (1997).
Vriend, G. WHAT IF: a molecular modeling and drug design program. J. Mol. Graph 8, 52–56 (1990).
Bussi, G., Donadio, D. & Parrinello, M. Canonical sampling through velocity rescaling. J. Chem. Phys. 126, 014101 (2007).
Berendsen, H. J. C., Postma, J. P. M., Gunsteren, W. F. V., DiNola, A. & Haak, J. R. Molecular dynamics with coupling to an external bath. J. Chem. Phys. 81, 3684–3690 (1984).
Espinosa, E. & Molins, E. Retrieving interaction potentials from the topology of the electron density distribution: the case of hydrogen bonds. J. Chem. Phys. 113, 5686–5694 (2000).
Evans, E. Probing the relation between force—lifetime—and chemistry in single molecular bonds. Annu. Rev. Biophys. Biomol. Struct. 30, 105–128 (2001).
Acknowledgements
We thank M. Rangl for initial experiments. Work in the Scheuring laboratory was supported by grants from the National Institute of Health, National Center for Complementary and Integrative Health DP1AT010874 and National Institute of Neurological Disorders and Stroke R01NS110790, and by the Kavli Institute at Cornell. Work in the Nimigean laboratory was supported by the National Institute of Health (GM124451 to C.M.N.) and the American Heart Association (18POST33960309 to P.A.M.S.). Work in the Grubmüller laboratory was funded by the Max Planck Society and by the German Science Foundation (DFG), Excellence Strategy Grant MBExC 2067/1-390729940; computer time was provided by the Max Planck Computing and Data Facility. A.C.V. was additionally supported by the European Union’s Horizon 2020 Framework Programme for Research and Innovation, Specific Grant Agreement No. 945539 (Human Brain Project SGA3).
Author information
Authors and Affiliations
Contributions
Y.P., C.M.N. and S.S. designed the experiments. P.A.M.S. expressed and purified the protein. Y.P. performed HS-AFM and AFM-SMFS experiments. Y.P. did the HS-AFM and AFM-SMFS data analysis. E.P., A.C.V. and H.G. performed and analyzed force probe MDS. Y.P., E.P., H.G. and S.S. wrote the paper. All authors edited the paper.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Structural & Molecular Biology thanks Baron Chanda and the other, anonymous, reviewer(s) for their contribution to the peer review of this work. Peer reviewer reports are available. Primary Handling Editors: Florian Ullrich and Katarzyna Ciazynska, in collaboration with the Nature Structural & Molecular Biology team.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Extended data
Extended Data Fig. 1 cNs-CNBD binding studies using microscale thermophoresis (MST).
a) and c) Microscale thermophoresis (MST) traces of cAMP-CNBD (a) and cGMP-CNBD (c) interactions, respectively. b) Binding curve of labeled CNBD with cAMP. Two binding phases were detected. The first had a KD of 0.4 ± 0.5 µM, and the second had a KD of 1.6 ± 1.1 µM. d) Binding curve of labeled CNBD with cGMP. The KD value of cGMP-CNBD was 3.3 ± 1.8 µM. The concentration of labeled His6-C-linker-CNBD was kept constant (50 nM), and the concentration of cNs was varied from 0.00305 µM to 100 µM.
Extended Data Fig. 2 Distributions of rupture forces of cAMP-CNBD or cGMP-CNBD bonds under various conditions.
a) Force distribution of cAMP-CNBD unbinding following 0.02 s bond formation at varying pulling velocity (top right in each graph). b) Force distribution of cAMP-CNBD unbinding following 1.00 s bond formation at varying pulling velocities (top right in each graph). The force distributions are fitted with bimodal Gaussian fits to extract the most probable rupture forces for binding state 1 (first peak) and binding state 2 (second peak), which are then used for Bell–Evans model fitting. c) Force distribution of cGMP-CNBD unbinding following 0.02 s bond formation at varying pulling velocities (top right in each graph). d) Force distribution of cGMP-CNBD unbinding following 1.00 s bond formation at varying pulling velocities (top right in each graph). e) and f) Distributions of rupture forces of cGMP-CNBD (e) and cAMP-CNBD (f) unbinding following different bond formation times and at 0.4 µm/s pulling velocity). Bimodal Gaussian fits are used to extract the number of events for each bond state, which is then used to calculate the probability of occurrence of binding state 2.
Extended Data Fig. 3 Deviations of rupture force distributions.
Theoretical, simulation and experimental unbinding force distributions (normalized by loading rates) of cAMP (left) and cGMP (right). The experimental unbinding force distributions are the distributions after 0.02 s bond formation time.
Extended Data Fig. 4 Number of bond ruptures as a function of bond-formation time for cAMP-CNBD (left) and cGMP-CNBD (right).
With increasing cN contact time, the frequency of unsuccessful (0 bond) force-distance cycles decreases, while the number of successful (1 bond) force-distance cycles increases. Concomitantly, a fraction of force-distance cycles reported multiple (2 or 3) binding events.
Extended Data Fig. 5 Markovian sequence analysis for the force spectroscopy experiments of cAMP-CNBD at 1 s contact time.
Markovian model fitting for 1 (gray line) and 2 (dashed line) bonds. koff and xβ values were derived from the Bell–Evans model fit to the canonical binding mode (see Fig. 5b). The most probable rupture force (Gaussian peak) and error (full width at half maximum of the Gaussian peak) at each loading rate was determined through Gaussian fitting of the corresponding histogram (total data points for all histograms, N = 1898, Extended Data Fig. 2b).
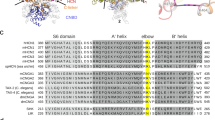
Extended Data Fig. 6 Comparison of SthK with CNG and HCN channels, with a focus on their cN binding pockets.
Top: cNs binding pocket of SthK (left, gray) and CNGA1 (right, orange) showing that the residues Y and F are swapped in CNGA1 channels compared with SthK. Bottom: Sequence alignment of SthK with CNG and HCN channels showing that Y357 and F365 identified by MDS to be crucial in cN discrimination in SthK are not conserved, whereas M369 is a conservative mutation and A370 is a semi-conservative mutation. cAMP interacts with Y357, M369 and A370, and cGMP interacts with F365.
Supplementary information
Source data
Source Data Fig. 1
Statistical source data.
Source Data Fig. 2
Statistical source data.
Source Data Fig. 3
Statistical source data.
Source Data Fig. 4
Statistical source data.
Source Data Fig. 5
Statistical source data.
Source Data Extended Data Fig. 1
Statistical source data.
Source Data Extended Data Fig. 2
Statistical source data.
Source Data Extended Data Fig. 3
Statistical source data.
Source Data Extended Data Fig. 4
Statistical source data.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Pan, Y., Pohjolainen, E., Schmidpeter, P.A.M. et al. Discrimination between cyclic nucleotides in a cyclic nucleotide-gated ion channel. Nat Struct Mol Biol 30, 512–520 (2023). https://doi.org/10.1038/s41594-023-00955-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41594-023-00955-3