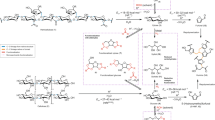
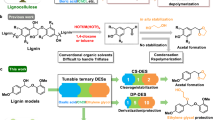
Abstract
Lignin is one of the most promising sources of renewable aromatic hydrocarbons. Current methods for its extraction from lignocellulosic biomass—which include the kraft, sulfite, and organosolv processes—result in the rapid formation of carbon–carbon bonds, leading to a condensed lignin that cannot be effectively depolymerized into its constituent monomers. Treatment of lignocellulosic biomass with aldehydes during lignin extraction generates an aldehyde-stabilized lignin that is uncondensed and can be converted into its monomers at near-theoretical yields. Here, we outline an efficient, reproducible, and scalable process for extracting and purifying this aldehyde-stabilized lignin as a solid, which can easily be re-dissolved in an organic solvent. Upon exposure to hydrogenolysis conditions, this material provides near-theoretical yields of aromatic monomers (~40–50% of the Klason lignin for a typical hardwood). Cellulose and hemicellulose are also efficiently fractionated. This protocol requires 6–7 h for the extraction of the stabilized lignin and a basic proficiency in synthetic chemistry.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
Data availability
The exemplary data that were produced in support of the described procedures are available from the corresponding author upon reasonable request.
Change history
23 December 2022
A Correction to this paper has been published: https://doi.org/10.1038/s41596-022-00760-0
References
BP. BP Statistical Review of World Energy. https://www.bp.com/content/dam/bp/business-sites/en/global/corporate/pdfs/energy-economics/statistical-review/bp-stats-review-2018-full-report.pdf (2018).
Cox, P. M., Betts, R. A., Jones, C. D., Spall, S. A. & Totterdell, I. J. Acceleration of global warming due to carbon-cycle feedbacks in a coupled climate model. Nature 408, 184–187 (2000).
Sabine, C. L. et al. The oceanic sink for anthropogenic CO2. Science 305, 367–371 (2004).
Shuai, L. & Luterbacher, J. Organic solvent effects in biomass conversion reactions. ChemSusChem 9, 133–155 (2016).
Van den Bosch, S. et al. Catalytic strategies towards lignin-derived chemicals. Top. Curr. Chem. (Cham). 376, 36 (2018).
Isikgor, F. H. & Becer, C. R. Lignocellulosic biomass: a sustainable platform for the production of bio-based chemicals and polymers. Polym. Chem. 6, 4497–4559 (2015).
Kaparaju, P., Serrano, M., Thomsen, A. B., Kongjan, P. & Angelidaki, I. Bioethanol, biohydrogen and biogas production from wheat straw in a biorefinery concept. Bioresour. Technol. 100, 2562–2568 (2009).
Banerjee, A., Dick, G. R., Yoshino, T. & Kanan, M. W. Carbon dioxide utilization via carbonate-promoted C–H carboxylation. Nature 531, 215–219 (2016).
Dick, G. R., Frankhouser, A. D., Banerjee, A. & Kanan, M. W. A scalable carboxylation route to furan-2,5-dicarboxylic acid. Green Chem 19, 2966–2972 (2017).
Zakzeski, J., Bruijnincx, P. C. A., Jongerius, A. L. & Weckhuysen, B. M. The catalytic valorization of lignin for the production of renewable chemicals. Chem. Rev. 110, 3552–3599 (2010).
Alvira, P., Tomás-Pejó, E., Ballesteros, M. & Negro, M. J. Pretreatment technologies for an efficient bioethanol production process based on enzymatic hydrolysis: a review. Bioresour. Technol. 101, 4851–4861 (2010).
Ragnar, M. et al. Pulp. in Ullmann’s Encyclopedia of Industrial Chemistry (ed. Elvers, B. et al.) 1–92 (Wiley-VCH, Weinheim, Germany, 2014).
Luterbacher, J. S., Martin Alonso, D. & Dumesic, J. A. Targeted chemical upgrading of lignocellulosic biomass to platform molecules. Green Chem. 16, 4816–4838 (2014).
Tadesse, H. & Luque, R. Advances on biomass pretreatment using ionic liquids: an overview. Energy Environ. Sci. 4, 3913–3929 (2011).
Luterbacher, J. S. et al. Nonenzymatic sugar production from biomass using biomass-derived γ-valerolactone. Science 343, 277–280 (2014).
Shuai, L., Questell-Santiago, Y. M. & Luterbacher, J. S. A mild biomass pretreatment using γ-valerolactone for concentrated sugar production. Green Chem. 18, 937–943 (2016).
Ragauskas, A. J. et al. Lignin valorization: improving lignin processing in the biorefinery. Science 344, 1246843–1246843 (2014).
Sturgeon, M. R. et al. A mechanistic investigation of acid-catalyzed cleavage of aryl-ether linkages: Implications for lignin depolymerization in acidic environments. ACS Sustain. Chem. Eng. 2, 472–485 (2014).
Van den Bosch, S. et al. Integrating lignin valorization and bio-ethanol production: on the role of Ni-Al 2 O 3 catalyst pellets during lignin-first fractionation. Green Chem. 19, 3313–3326 (2017).
Xu, C., Arancon, R. A. D., Labidi, J. & Luque, R. Lignin depolymerisation strategies: towards valuable chemicals and fuels. Chem. Soc. Rev. 43, 7485–7500 (2014).
Roberts, V. M. et al. Towards quantitative catalytic lignin depolymerization. Chem. Eur. J. 17, 5939–5948 (2011).
Lai, C. et al. Lignin alkylation enhances enzymatic hydrolysis of lignocellulosic biomass. Energy Fuels 31, 12317–12326 (2017).
Shuai, L. et al. Formaldehyde stabilization facilitates lignin monomer production during biomass depolymerization. Science 354, 329–333 (2016).
Lan, W., Amiri, M. T., Hunston, C. M. & Luterbacher, J. S. Protection group effects during α,γ-diol lignin stabilization promote high-selectivity monomer production. Angew. Chem. Int. Ed. 57, 1356–1360 (2018).
Sluiter, J. & Sluiter, A. Summative Mass Closure: Laboratory Analytical Procedure (LAP) Review and Integration. https://www.nrel.gov/docs/gen/fy11/48087.pdf (National Renewable Energy Laboratory, 2011).
Galkin, M. V. & Samec, J. S. M. Selective route to 2-propenyl aryls directly from wood by a tandem Organosolv and palladium-catalysed transfer hydrogenolysis. ChemSusChem 7, 2154–2158 (2014).
Yan, N. et al. Selective degradation of wood lignin over noble‐metal catalysts in a two‐step process. ChemSusChem 1, 626–629 (2008).
Parsell, T. et al. A synergistic biorefinery based on catalytic conversion of lignin prior to cellulose starting from lignocellulosic biomass. Green Chem. 17, 1492–1499 (2015).
Van den Bosch, S. et al. Reductive lignocellulose fractionation into soluble lignin-derived phenolic monomers and dimers and processable carbohydrate pulps. Energy Environ. Sci. 8, 1748–1763 (2015).
Phongpreecha, T. et al. Predicting lignin depolymerization yields from quantifiable properties using fractionated biorefinery lignins. Green Chem. 19, 5131–5143 (2017).
Schutyser, W. et al. Chemicals from lignin: an interplay of lignocellulose fractionation, depolymerisation, and upgrading. Chem. Soc. Rev. 47, 852–908 (2018).
Van Den Bosch, S. et al. Tuning the lignin oil OH-content with Ru and Pd catalysts during lignin hydrogenolysis on birch wood. Chem. Commun. 51, 13158–13161 (2015).
Van Den Bosch, S. et al. Reductive lignocellulose fractionation into soluble lignin-derived phenolic monomers and dimers and processable carbohydrate pulps. Energy Environ. Sci. 8, 1748–1763 (2015).
Pepper, J. M. & Lee, Y. W. Lignin and related compounds. I. A comparative study of catalysts for lignin hydrogenolysis. Can. J. Chem. 47, 723–727 (1969).
Chang, H., Cowling, E. B. & Brown, W. Comparative studies on cellulolytic enzyme lignin and milled wood lignin of sweetgum and spruce. Holzforschung 29, 153–159 (1975).
Maekawa, E., Ichizawa, T. & Koshijima, T. An evaluation of the acid-soluble lignin determination in analyses of lignin by the sulfuric acid method. J. Wood Chem. Technol. 9, 549–567 (1989).
Kaar, W. E. & Brink, D. L. Simplified analysis of acid soluble lignin. J. Wood Chem. Technol. 11, 465–477 (1991).
Yuan, G., Qi, C., Wu, W. & Jiang, H. Recent advances in organic synthesis with CO2 as C1 synthon. Curr. Opin. Green Sustain. Chem. 3, 22–27 (2017).
Acknowledgements
This work was supported by the European Research Council (ERC) under the European Union’s Horizon 2020 research and innovation program (starting grant: CATACOAT, no. 758653), the Swiss National Science Foundation through grant PYAPP2_154281, and the École Polytechnique Fédérale de Lausanne. This work was also accomplished within the framework of the Swiss Competence Center for Bioenergy Research (SCCER-BIOSWEET). We thank L. Menin and D. Ortiz of the SSMI mass spectrometry facility at EPFL for their assistance. We thank W. Lan for helpful discussions during the preparation of the manuscript, especially for the structural assignments of the lignin NMRs.
Author information
Authors and Affiliations
Contributions
M.T.A. and G.R.D. developed and performed the aldehyde-based fractionations, cellulose hydrolyses, and lignin hydrogenolyses. M.T.A., G.R.D., and Y.M.Q.-S. performed the cellulose compositional analyses. G.R.D. performed the biomass compositional analyses. The project was conceived of by M.T.A., G.R.D., and J.S.L. and supervised by J.S.L. All authors participated in the preparation of the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare competing interests. J.S.L. is an inventor on a European patent application (EP16165180.7) that was submitted by EPFL and covers methods for producing lignin monomers from biomass during biomass depolymerization.
Additional information
Journal peer review information: Nature Protocols thanks Robert Brown and other (anonymous) reviewer(s) for their contribution to the peer review of this work.
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Related links
Key references using this protocol
Shuai, L. et al. Science. 354, 329–333 (2016): http://science.sciencemag.org/content/354/6310/329
Lan, W. et al. Angew. Chem. Int. Ed. 57, 1356–1360 (2018): https://onlinelibrary.wiley.com/doi/abs/10.1002/anie.201710838
Integrated supplementary information
Supplementary Figure 1 Data for the unextracted and extracted propionaldehyde-stabilized lignins compared with the direct hydrogenolyses of the feedstock biomass.
These two charts compare the monomer yields from the hydrogenolysis of the raw biomass (Direct Hydrogenolysis), propionaldehyde-stabilized lignin derived from unextracted wood, and propionaldehyde-stabilized lignin derived from extracted wood for two biomass sources: 2018 Birch and 2018 Beech. The direct hydrogenolysis represents the highest possible yield (% (wt/wt)) of monomers for these biomass sources and was performed on biomass that had not been extracted or dried. The difference in yields between the extracted and unextracted propionaldehyde protected lignins is approximately 1% on a Klason Lignin Weighted Basis. Each data point represents one experiment.
Supplementary Figure 2 HSQCs in DMSO-d6 of the formaldehyde-stabilized lignins.
(a) 2018 birch wood and (b) 2018 beech wood.
Supplementary Figure 3 HSQCs in DMSO-d6 of the propionaldehyde-stabilized lignins.
(a) 2018 birch wood and (b) 2018 beech wood.
Supplementary Figure 4
1H-NMR of diformylxylose in CDCl3.
Supplementary Figure 5
13C-NMR of diformylxylose in CDCl3.
Supplementary Figure 6
HSQC of diformylxylose in CDCl3.
Supplementary Figure 7
1H-NMR in CDCl3 of diformylxylose isolated from the 2018 birch wood using the formaldehyde fractionation protocol.
Supplementary Figure 8
1H-NMR in CDCl3 of diformylxylose isolated from the 2018 beech wood using the formaldehyde fractionation protocol.
Supplementary Figure 9
1H-NMR of (2R,3aR,3bS,5R,7aR,8aR)-2,5-diethyltetrahydro-7H-[1,3]dioxolo[4′,5′:4,5]furo[3,2-d][1,3]dioxine (dipropylxylose) in CDCl3.
Supplementary Figure 10
13C-NMR of (2R,3aR,3bS,5R,7aR,8aR)-2,5-diethyltetrahydro-7H-[1,3]dioxolo[4′,5′:4,5]furo[3,2-d][1,3]dioxine (dipropylxylose) in CDCl3.
Supplementary Figure 11
HSQC of (2R,3aR,3bS,5R,7aR,8aR)-2,5-diethyltetrahydro-7H-[1,3]dioxolo[4′,5′:4,5]furo[3,2-d][1,3]dioxine (dipropylxylose) in CDCl3.
Supplementary Figure 12
1H-NMR of (2S,3aR,3bS,5R,7aR,8aR)-2,5-diethyltetrahydro-7H-[1,3]dioxolo[4′,5′:4,5]furo[3,2-d][1,3]dioxine.dioxine (dipropylxylose) in CDCl3.
Supplementary Figure 13
13C-NMR of (2S,3aR,3bS,5R,7aR,8aR)-2,5-diethyltetrahydro-7H-[1,3]dioxolo[4′,5′:4,5]furo[3,2-d][1,3]dioxine.dioxine (dipropylxylose) in CDCl3.
Supplementary Figure 14
HSQC of purified (2S,3aR,3bS,5R,7aR,8aR)-2,5-diethyltetrahydro-7H-[1,3]dioxolo[4′,5′:4,5]furo[3,2-d][1,3]dioxine.dioxine (dipropylxylose) in CDCl3.
Supplementary Figure 15
1H-NMR in CDCl3 of dipropylxylose (mixture of isomers) isolated from the 2018 birch wood using the propionaldehyde fractionation protocol.
Supplementary Figure 16
1H-NMR in CDCl3 of dipropylxylose (mixture of isomers) isolated from the 2018 beech wood using the propionaldehyde fractionation protocol.
Supplementary information
Supplementary Text and Figures
Supplementary Figures 1–16
Rights and permissions
About this article
Cite this article
Talebi Amiri, M., Dick, G.R., Questell-Santiago, Y.M. et al. Fractionation of lignocellulosic biomass to produce uncondensed aldehyde-stabilized lignin. Nat Protoc 14, 921–954 (2019). https://doi.org/10.1038/s41596-018-0121-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41596-018-0121-7
This article is cited by
-
Carbon–carbon bond cleavage for a lignin refinery
Nature Chemical Engineering (2024)
-
Valorization of Boehmeria nivea stalk towards multipurpose fractionation: furfural, pulp, and phenolic monomers
Biotechnology for Biofuels and Bioproducts (2023)
-
One-Step Acid Bleachable Pretreatment with a Recyclable Acid Hydrotrope and Chlorate for Biomass Valorization
BioEnergy Research (2023)
-
Lignocellulose pyrolysis by-products as an underestimated source of chemicals: separation and characterisation
Biomass Conversion and Biorefinery (2023)
-
Skeletal Ni electrode-catalyzed C-O cleavage of diaryl ethers entails direct elimination via benzyne intermediates
Nature Communications (2022)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.