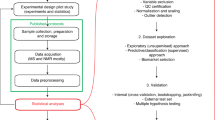
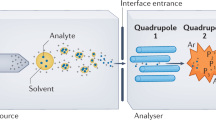
Abstract
A standardized data workflow is described for large-scale serum metabolomic studies using multisegment injection-capillary electrophoresis-mass spectrometry. Multiplexed separations increase throughput (<4 min/sample) for quantitative determination of 66 polar/ionic metabolites in serum filtrates consistently detected (coefficient of variance (CV) <30%) with high frequency (>75%) from a multi-ethnic cohort of pregnant women (n = 1,004). We outline a validated protocol implemented in four batches over a 7-month period that includes details on preventive maintenance, sample workup, data preprocessing and metabolite authentication. We achieve stringent quality control (QC) and robust batch correction of long-term signal drift with good mutual agreement for a wide range of metabolites, including serum glucose as compared to a clinical chemistry analyzer (mean bias = 11%, n = 668). Control charts for a recovery standard (mean CV = 12%, n = 2,412) and serum metabolites in QC samples (median CV = 13%, n = 202) demonstrate acceptable intermediate precision with a median intraclass coefficient of 0.87. We also report reference intervals for 53 serum metabolites from a diverse population of women in their second trimester of pregnancy.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout









Similar content being viewed by others
Data availability
Full datasets generated and/or analyzed in the current study are not publicly available since participants in the FAMILY, CHILD, START and ABC cohorts did not consent to public sharing of their data at the time of recruitment. Datasets can be obatined from the corresponding author on reasonable request.
Software availability
Our protocol used vendor-specific software (Agilent Technologies Inc.) for processing raw data generated by (Q)TOF mass analyzers. We are developing customized open-access software for processing MSI-CE-MS data in future work.
Change history
17 May 2021
A Correction to this paper has been published: https://doi.org/10.1038/s41596-021-00569-3
References
Viant, M. R., Kurland, I. J., Jones, M. R. & Dunn, W. B. How close are we to complete annotation of metabolomes? Curr. Opin. Chem. Biol. 36, 64–69 (2017).
Johnson, C. H., Ivanisevic, J. & Siuzdak, G. Metabolomics: beyond biomarkers and towards mechanisms. Nat. Rev. Mol. Cell Biol. 17, 451–459 (2016).
Wishart, D. S. Emerging applications of metabolomics in drug discovery and precision medicine. Nat. Rev. Drug Discov. 15, 473–484 (2016).
Zhou, W. et al. Longitudinal multi-omics of host–microbe dynamics in prediabetes. Nature 569, 663–671 (2019).
Brennan, L. & Hu, F. B. Metabolomics-based dietary biomarkers in nutritional epidemiology—current status and future opportunities. Mol. Nutr. Food Res. 63, 1–5 (2019).
Scalbert, A. et al. Narrative review the food metabolome: a window over dietary exposure. Am. J. Clin. Nutr. 99, 1286–1308 (2014).
Psychogios, N. et al. The human serum metabolome. PLoS ONE 6, e16957 (2011).
Kuehnbaum, N. L. & Britz-McKibbin, P. New advances in separation science for metabolomics: resolving chemical diversity in a post-genomic era. Chem. Rev. 113, 2437–2468 (2013).
Tzoulaki, I., Ebbels, T. M. D., Valdes, A., Elliott, P. & Ioannidis, J. P. A. Design and analysis of metabolomics studies in epidemiologic research: a primer on-omic technologies. Am. J. Epidemiol. 180, 129–139 (2014).
Dunn, W. B. et al. Procedures for large-scale metabolic profiling of serum and plasma using gas chromatography and liquid chromatography coupled to mass spectrometry. Nat. Protoc. 6, 1060–1083 (2011).
Würtz, P. et al. Metabolic signatures of birthweight in 18 288 adolescents and adults. Int. J. Epidemiol. 45, 1539–1550 (2016).
Playdon, M. C. et al. Metabolomics analytics workflow for epidemiological research: Perspectives from the consortium of metabolomics studies (COMETS). Metabolites 9, 145 (2019).
Kuehnbaum, N. L., Kormendi, A. & Britz-McKibbin, P. Multisegment injection-capillary electrophoresis-mass spectrometry: a high-throughput platform for metabolomics with high data fidelity. Anal. Chem. 85, 10664–10669 (2013).
Nori de Macedo, A. et al. The sweat metabolome of screen-positive cystic fibrosis infants: Revealing mechanisms beyond impaired chloride transport. ACS Cent. Sci. 3, 904–913 (2017).
DiBattista, A. et al. Metabolic signatures of cystic fibrosis identified in dried blood spots for newborn screening without carrier identification. J. Proteome Res. 18, 841–854 (2019).
Saoi, M. et al. Characterization of the human skeletal muscle metabolome for elucidating the mechanisms of bicarbonate ingestion on strenuous interval exercise. Anal. Chem. 91, 4709–4718 (2019).
Souza, R. T. et al. Metabolomics applied to maternal and perinatal health: a review of new frontiers with a translation potential. Clinics 74, 1–12 (2019).
Kadakia, R. et al. Maternal metabolites during pregnancy are associated with newborn outcomes and hyperinsulinaemia across ancestries. Diabetologia 62, 473–484 (2019).
Blaženović, I., Kind, T., Ji, J. & Fiehn, O. Software tools and approaches for compound identification of LC-MS/MS data in metabolomics. Metabolites 8, 31 (2018).
Southam, A. D., Weber, R. J. M., Engel, J., Jones, M. R. & Viant, M. R. A complete workflow for high-resolution spectral-stitching nanoelectrospray direct-infusion mass-spectrometry-based metabolomics and lipidomics. Nat. Protoc. 12, 255–273 (2017).
Fuhrer, T. & Zamboni, N. High-throughput discovery metabolomics. Curr. Opin. Biotechnol. 31, 73–78 (2015).
Levy, A. J. et al. Recent progress in metabolomics using ion mobility-mass spectrometry. TrAC Trends Anal. Chem 116, 274–281 (2019).
Würtz, P. et al. Quantitative serum nuclear magnetic resonance metabolomics in large-scale epidemiology: a primer on -omic technologies. Am. J. Epidemiol. 186, 1084–1096 (2017).
Wishart, D. S. NMR metabolomics: a look ahead. J. Magn. Reson. 306, 155–161 (2019).
Yu, B. et al. The consortium of metabolomics studies (COMETS): metabolomics in 47 prospective cohort studies. Am. J. Epidemiol. 188, 991–1012 (2019).
Miggiels, P., Wouters, B., van Westen, G. J. P., Dubbelman, A.-C. & Hankemeier, T. Novel technologies for metabolomics: more for less. Trends Anal. Chem. 120, 115323 (2019).
Büscher, J. M., Czernik, D., Ewald, J. C., Sauer, U. & Zamboni, N. Cross-platform comparison of methods for quantitative metabolomics of primary metabolism. Anal. Chem. 81, 2135–2143 (2009).
Soga, T. et al. Metabolomic profiling of anionic metabolites by capillary electrophoresis mass spectrometry. Anal. Chem. 81, 6165–6174 (2009).
Boizard, F. et al. A capillary electrophoresis coupled to mass spectrometry pipeline for long term comparable assessment of the urinary metabolome. Sci. Rep. 6, 1–17 (2016).
Harada, S. et al. Metabolomic profiling reveals novel biomarkers of alcohol intake and alcohol-induced liver injury in community-dwelling men. Environ. Health Prev. Med. 21, 18–26 (2016).
Fukai, K. et al. Metabolic profiling of total physical activity and sedentary behavior in community dwelling men. PLoS ONE 11, 1–14 (2016).
Belczacka, I. et al. Urinary CE-MS peptide marker pattern for detection of solid tumors. Sci. Rep. 8, 1–11 (2018).
Wild, J., Shanmuganathan, M., Hayashi, M., Potter, M. & Britz-McKibbin, P. Metabolomics for improved treatment monitoring of phenylketonuria: Urinary biomarkers for non-invasive assessment of dietary adherence and nutritional deficiencies. Analyst 144, 6595–6608 (2019).
DiBattista, A. et al. Temporal signal pattern recognition in mass spectrometry: a method for rapid identification and accurate quantification of biomarkers for inborn errors of metabolism with quality assurance. Anal. Chem. 89, 8112–8121 (2017).
Azab, S., Ly, R. & Britz-McKibbin, P. Robust method for high-throughput screening of fatty acids by multisegment injection-nonaqueous capillary electrophoresis-mass spectrometry with stringent quality control. Anal. Chem. 91, 2329–2336 (2019).
Harada, S. et al. Reliability of plasma polar metabolite concentrations in a large-scale cohort study using capillary electrophoresis-mass spectrometry. PLoS ONE 13, 1–16 (2018).
Mahieu, N. G. & Patti, G. J. Systems-level annotation of a metabolomics data set reduces 25 000 features to fewer than 1000 unique metabolites. Anal. Chem. 89, 10397–10406 (2017).
Yamamoto, M., Pinto-Sanchez, M. I., Bercik, P. & Britz-McKibbin, P. Metabolomics reveals elevated urinary excretion of collagen degradation and epithelial cell turnover products in irritable bowel syndrome patients. Metabolomics 15, 1–18 (2019).
Sumner, L. W. et al. Proposed minimum reporting standards for chemical analysis: Chemical Analysis Working Group (CAWG) Metabolomics Standards Initiative (MSI). Metabolomics 3, 211–221 (2007).
De Souza, R. J. et al. The influence of maternal and infant nutrition on cardiometabolic traits: Novel findings and future research directions from four Canadian birth cohort studies. Proc. Nutr. Soc. 78, 351–361 (2019).
Wahi, G. et al. Aboriginal birth cohort (ABC): a prospective cohort study of early life determinants of adiposity and associated risk factors among Aboriginal people in Canada. BMC Public Health 13, 608 (2013).
Subbarao, P. et al. The Canadian Healthy Infant Longitudinal Development (CHILD) study: examining developmental origins of allergy and asthma. Thorax 70, 998–1000 (2015).
Morrison, K. M. et al. The Family Atherosclerosis Monitoring In earLY life (FAMILY) study. Rationale, design, and baseline data of a study examining the early determinants of atherosclerosis. Am. Heart J. 158, 533–539 (2009).
Anand, S. S. et al. Rationale and design of South Asian Birth Cohort (START): A Canada-India collaborative study. BMC Public Health 13, 1 (2013).
De Souza, R. J. et al. Harmonization of food-frequency questionnaires and dietary pattern analysis in 4 ethnically diverse birth cohorts. J. Nutr. 146, 2343–2350 (2016).
Wellington, N. et al. Metabolic trajectories following contrasting Prudent and Western diets from food provisions: identifying robust biomarkers of short-term changes in habitual diet. Nutrients 11, 2407 (2019).
Al-Ibrahim, A. A. & Jackson, R. T. Healthy eating index versus alternate healthy index in relation to diabetes status and health markers in U.S. adults: NHANES 2007-2010. Nutr. J. 18, 26 (2019).
Broadhurst, D. et al. Guidelines and considerations for the use of system suitability and quality control samples in mass spectrometry assays applied in untargeted clinical metabolomic studies. Metabolomics 14, 48 (2018).
Kamleh, M. A., Ebbels, T. M. D., Spagou, K., Masson, P. & Want, E. J. Optimizing the use of quality control samples for signal drift correction in large-scale urine metabolic profiling studies. Anal. Chem. 84, 2670–2677 (2012).
Lewis, M. R. et al. Development and application of ultra-performance liquid chromatography-TOF MS for precision large scale urinary metabolic phenotyping. Anal. Chem. 88, 9004–9013 (2016).
Wehrens, R. et al. Improved batch correction in untargeted MS-based metabolomics. Metabolomics 12, 88 (2016).
De Souza R. J. et al. Maternal diet and the serum metabolome in pregnancy: robust dietary biomarkers generalizable to a multiethnic birth cohort. Curr. Dev. Nutr. https://doi.org/10.1093/cdn/nzaa144 (2020).
Chong, J. et al. MetaboAnalyst 4.0: towards more transparent and integrative metabolomics analysis. Nucleic Acids Res. 46, W486–W494 (2018).
Carayol, M. et al. Reliability of serum metabolites over a two-year period: A targeted metabolomic approach in fasting and non-fasting samples from EPIC. PLoS ONE 10, 1–10 (2015).
Braekke, K. et al. Asymmetric dimethylarginine in the maternal and fetal circulation in preeclampsia. Pediatr. Res. 66, 411–415 (2009).
Chong, Y. et al. Ethnic differences translate to inadequacy of high-risk screening for gestational diabetes mellitus in an Asian population: a cohort study. BMC Pregnancy Childbirth 14, 345 (2014).
Yu, Z. et al. Differences between human plasma and serum metabolite profiles. PLoS ONE 6, e21230 (2011).
Lindsay, K. L. et al. Longitudinal metabolomic profiling of amino acids and lipids across healthy pregnancy. PLoS ONE 10, 1–19 (2015).
Stevens, V. L., Hoover, E., Wang, Y. & Zanetti, K. A. Pre-analytical factors that affect metabolite stability in human urine, plasma, and serum: a review. Metabolites 9, 156 (2019).
Saoi, M. et al. Metabolic perturbations from step reduction in older persons at risk for sarcopenia: plasma biomarkers of abrupt changes in physical activity. Metabolites. 9, 134 (2019).
Azab, S. M. et al. Serum non-esterified fatty acids have utility as dietary biomarkers of fat intake from fish, fish oil and dairy in women. J. Lipid Res. 63, 933–944 (2020).
Sasaki, K. et al. Metabolomics platform with capillary electrophoresis coupled with high-resolution mass spectrometry for plasma analysis. Anal. Chem. 91, 1295–1301 (2019).
Höcker, O. et al. Enrichment-free analysis of anionic micropollutants in the sub-ppb range in drinking water by capillary electrophoresis-high resolution mass spectrometry. Anal. Bioanal. Chem. 412, 4857–4865 (2020).
Azab, S. M. et al. Serum metabolic signatures of chronic limb-threatening ischemia in patients with peripheral artery disease. J. Clin. Med. 9, 1877 (2020).
Acknowledgements
The authors thank the participants in all of the cohorts who generously donated their time, information, and serum samples. The study was funded by the Canadian Institutes of Health Research (CIHR) (Team Grant: DOHaD – Implications for Men, Women, Boys and Girls; 201511). PB-M. also acknowledges support from the Natural Sciences and Engineering Research Council of Canada (NSERC), and Genome Canada. Dr. Anand is supported by a Tier 1 Canada Research Chair in Ethnicity and Cardiovascular Disease and Heart and Stroke Foundation Chair in Population Health. The authors acknowledge the contributions of the cohort investigators who recruited participants from CHILD (A. Becker, P. Mandhane, S. Turvey, T. Moraes, M. Azad, D. Befus), FAMILY (K. Morrison), START (M. Gupta) and ABC (J. Wilson, G. Wahi).
Author information
Authors and Affiliations
Contributions
S.S.A. and P.B.-M. designed the research. M.S. conducted research and processed data with support from Z.K., B.G. and S.A. M.S. and P.B.-M. analyzed the data and performed statistical analyses that was reviewed by R.J.d.S. and S.S.A. M.S. and P.B.-M. wrote the paper with critical feedback from R.J.d.S., S.S.A. and S.A. P.B.-M. had primary responsibility for final content. S.A.A., K.K.T., S.A. and P.S. were principal investigators responsible for oversight of each cohort’s data. All listed authors read, edited, and approved the final manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Peer review information Nature Protocols thanks the anonymous reviewers for their contribution to the peer review of this work.
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Related links
Key references using this protocol
de Souza R. J. et al. Curr. Develop. Nutr. 4, nzaa144 (2020): https://doi.org/10.1093/cdn/nzaa144
Yamamoto M. et al. Metabolomics 15, 1–18 (2019): https://doi.org/10.1007/s11306-019-1543-0
Wellington N., et al. Nutrients 11, 2407 (2019): https://www.mdpi.com/2072-6643/11/10/2407
Saoi M., et al. Metabolites 9, 134 (2019): https://www.mdpi.com/2218-1989/9/7/134
DiBattista A. et al. J. Proteome Res. 18, 841–854 (2019): https://pubs.acs.org/doi/10.1021/acs.jproteome.8b00351
Key data used in this protocol
de Souza R. J. et al. Curr. Develop. Nutr. 4, nzaa144 (2020): https://doi.org/10.1093/cdn/nzaa144
Supplementary information
Supplementary Information
Supplementary Table 1.
Rights and permissions
About this article
Cite this article
Shanmuganathan, M., Kroezen, Z., Gill, B. et al. The maternal serum metabolome by multisegment injection-capillary electrophoresis-mass spectrometry: a high-throughput platform and standardized data workflow for large-scale epidemiological studies. Nat Protoc 16, 1966–1994 (2021). https://doi.org/10.1038/s41596-020-00475-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41596-020-00475-0
This article is cited by
-
Comparative characterization of the infant gut microbiome and their maternal lineage by a multi-omics approach
Nature Communications (2024)
-
Early sex-dependent differences in metabolic profiles of overweight and adiposity in young children: a cross-sectional analysis
BMC Medicine (2023)
-
Simultaneous determination of uric acid, xanthine, and caffeine in human urine samples using nickel ferrite/reduced graphene oxide modified electrode
Journal of Materials Science: Materials in Electronics (2023)
-
Metabonomics profile analysis in inflammation-induced preterm birth and the potential role of metabolites in regulating premature cervical ripening
Reproductive Biology and Endocrinology (2022)
-
Parenteral BCG vaccine induces lung-resident memory macrophages and trained immunity via the gut–lung axis
Nature Immunology (2022)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.