Abstract
The diversity of coral reef and soft sediment ecosystems in the Red Sea has to date received limited scientific attention. This study investigates changes in the community composition of both reef and macrobenthic communities along a cross shelf gradient. Coral reef assemblages differed significantly in species composition and structure with location and depth. Inner shelf reefs harbored less abundant and less diverse coral assemblages with higher percentage macroalgae cover. Nutrient availability and distance from the shoreline were significantly related to changes in coral composition and structure. This study also observed a clear inshore offshore pattern for soft sediment communities. In contrast to the coral reef patterns the highest diversity and abundance of soft sediment communities were recorded at the inshore sites, which were characterized by a higher number of opportunistic polychaete species and bivalves indicative of mild disturbance. Sediment grain size and nutrient enrichment were important variables explaining the variability. This study aims to contribute to our understanding of ecosystem processes and biodiversity in the Red Sea region in an area that also has the potential to provide insight into pressing topics, such as the capacity of reef systems and benthic macrofaunal organisms to adapt to global climate change.
Similar content being viewed by others
Introduction
The Red Sea is a confined water body by the Arabian Peninsula and African mainland, stretching from 30°N to 12°40′N over 1900 km and reaching a maximum width of 335 km1. Its latitude extension and arid continental setting make the Red Sea experience high rates of evaporation, a wide range of seasonal shallow water temperature regimes from 18 to 32 °C and above global ocean average saline conditions in the range of 37 to 42 psu2, 3. There is limited coastal runoff of freshwater into the Red Sea therefore the water loss of 2 m year−1 by evaporation is replenished almost entirely by oceanic waters from the Mediterranean via the Suez Canal and from the Indian Ocean by the narrow Bab-al-Mandab Strait4, 5. These combined conditions make the Red Sea one of the world’s warmest and most saline habitats in which extensive coral reefs occur, and therefore also a region of increasing interest for scientists working on climate change.
Much of the information on the Red Sea lies within technical reports, and there is relatively little information available to researchers using modern channels6. The Red Sea region is also relatively inaccessible due to permitting regulations and therefore remains a poorly studied system6. Further, the vast majority of published research for coral assemblages originates from an approximately 6 km stretch of the coastline in the far northern Red Sea near the Gulf of Aqaba while the literature on the soft sediment benthos of the Red Sea has been reported as very limited. Despite these research limitations, the Red Sea has long been recognized ecologically as one of the world’s biodiversity hotspots and an area of high endemism7,8,9. More than 364 scleractinian coral species (hard corals) have been recorded; of which 5.8% were suggested to be endemic9. Additionally, new reports of endemic scleractinian corals support that high levels of speciation exist in the Red Sea10. A few early studies have provided initial records on the abundance of soft sediment macrobenthos in the region and also provided support for high levels of soft sediment benthic species diversity11, 12. There is still a current lack of understanding of the general ecology of the Red Sea despite the region’s high biodiversity6. Increasingly anthropogenic impacts are affecting the ecology of the Red Sea region including rapid human population growth and urbanization, coastal construction, tourism, reef over-usage13 and destructive fisheries14. Thus, marine resource management priorities and the urgent need for monitoring programs have been identified as of high importance for the Red Sea region15.
As part of a recent initiative between the Red Sea Research Centre and Saudi Aramco, regional assessments have been undertaken to characterise the Red Sea environment for marine spatial planning efforts and to provide baseline information for future monitoring programs. In this study, we assessed regional biodiversity patterns from both coral reef and soft sediment data collected across a shelf gradient in the Farasan Islands. Spatial patterns in the distribution and abundance of species have been reported to change significantly over cross shelf gradients16, however the underlying drivers of species change across these gradients has not previously been fully assessed. There has been an increasing focus worldwide on regional assessments of biodiversity that can link the role of environmental variables in structuring community composition17. The understanding of regional biodiversity patterns and species distribution relative to their habitat is fundamental18 providing data that could inform science-based management approaches6. Most diversity studies across coastal shelves have, however, focused on single taxonomic groups, such as corals, with very few studies assessing multi-taxon diversity patterns in a marine context (exceptions include19, 20). Multi-taxon studies addressing the dynamic structuring of ecological communities have been beneficially utilized in testing and implementing high-quality conservation strategies19. Within this study, we present results of coral reef surveys following a longitudinal gradient at the Farasan Islands, ranging from inshore to offshore locations. We also present cross shelf biodiversity data collected from soft sediment macrobenthic assemblages investigating environmental variables that influence species distribution. The specific aims of this paper are to: (a) provide baseline information on both coral reef and soft sediment biodiversity patterns for the Red Sea region where there is limited information published; (b) to assess soft sediment and coral reef diversity patterns across a shelf gradient; and (c) to identify the role of environmental variables in structuring coral reef and soft sediment benthic communities. The present study aims to contribute to a better understanding of ecosystem processes and current biodiversity in the southern Red Sea region, an area that has the potential to provide insight into pressing topics such as the capacity of reef systems and benthic macrofaunal organisms to adapt to global climate change. This is, to our knowledge, the first synoptic study of benthic soft sediment faunal species richness and benthic coral reef diversity in the Red Sea that also relates biodiversity patterns to environmental and hydrographic variables.
Material and Methods
Study area
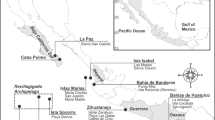
Soft sediment and reef surveys were conducted in the southern Red Sea to assess cross shelf gradient biodiversity patterns. The study area included sites around the Jazan City region and the Farasan Island Marine Sanctuary (Fig. 1). The wider Jazan province has a population of approximately 1.5 million and covers an area of 40,000 km2. Jazan City is an urbanized area with agricultural activities also present in the region. Three of the main islands at the Farasan Island Marine Sanctuary are permanently inhabited (Farasan, 369 km2; Sajid, 109 km2; Qummah, 14.3 km2) with a total population of approximately 4,500 inhabitants. Despite being considered a Marine Sanctuary, most of the inhabitants engage in fishing and agricultural activities. The Farasan Island plateau slopes gently over a distance of 120 km never reaching water depths greater than 200 m21.
Map showing the location of the reef and soft sediment sites sampled across the Farasan shelf in the southern Red Sea. This figure was created using the ArcGIS software (ArcMap 10.3.1) by ESRI, Inc. (ESRI.com), GEBCO, DeLorme, US Geological Survey (USGS) and the National Oceanic and Atmospheric Administration (NOAA) photo library.
Reef survey
Quantitative surveys of benthic reef community
Coral reefs were assessed in February and September 2014. A total of 11 reef locations, six in the inner shelf (IS; IS1, IS2, IS3, IS4, IS5, IS6) and five in the outer shelf (OS; OS1, OS2, OS3, OS4, OS5), were surveyed using a 1 m wide photo quadrate belt-transect (Fig. 1). The transect line ideally followed the depth horizons at 3–5 m and 8–10 m. At some locations only the shallow transect was completed because a limestone reef foundation did not exist at deeper locations. Three replicate 20 m by 5 m transects comprised the surveyed 60 m2 along a horizontal stretch of slope at each reef site and depth. Along each transect, a photo (1 m2) was taken every two meters with a Canon G16 16 megapixel digital camera in Nauticam housing. The benthic community was assessed based on 90 benthic groups including scleractinian coral genera, abundant soft coral genera and other benthic community groups. Quantification and identification of benthic groups along each transect replicate utilized the software Coral Point Count with Excel extensions (CPCe22), with 40 randomly distributed points per 1 m2 frame. Coral identification was undertaken at family level for Fungidae or genus level for other Scleractinia following Veron23 despite the current revisions on coral’s taxonomy being undertaken worldwide. Counted points were averaged for each site and depth to calculate percentage cover of each group including corals (calcifying corals including Millipora), soft corals, sponges, coralline algae, turf algae, macroalgae and the abiotic components sand, rock and rubble. Forty points were randomly distributed on each substrate image and the features underlying the points user-identified. Overall, per photo-transect, benthic community composition was analyzed in terms of number of Operational Taxonomic Units (OTUs), cover of corals (both soft and scleractinian), sponges, hydroids and other invertebrates (e.g. bivalves, echinoderms), algae (subdivided by the following functional groups: macroalgae, turf, coralline algae) (see Table 1 for main benthic categories). Water samples for nutrients and Chlorophyll a were also collected from surface waters at each reef site (see water sampling methods below).
Size frequency distribution of corals
Negatively skewed size-frequency distributions in coral populations have been associated with unfavorable environmental conditions. Using the same photoquadrat data, we compared the size-frequency distributions between near shore and offshore reefs in the four most abundant genera (Acropora, Echinopora, Porites and Galaxea) to explore the occurrence of any differences in the skewness of the size distribution of the corals as a proxy for different environmental conditions. The colony diameter (maximum and minimum) was measured using the software ImageJ to estimate the colony area (projected surface area). Colony area was estimated using the half-sphere formula (Colony area = 2 × [(maximum diameter/2 × minimum diameter/2) × π]) following Lozano-Cortés & Berumen24. The projected surface area data was logarithmically transformed and from these data, size-frequency distributions were constructed25 and its skewness (g1) calculated26.
Soft sediment macrobenthic survey
Sediment samples from 13 stations (Fig. 1) were collected across an inshore to offshore transect from Jazan to the Farasan Islands using a 0.1 m2 Van Veen grab (two replicates at each site) and sieved through a 1 mm mesh screen. A total of 14 soft sediment stations were sampled. Sites located in the inner shelf include JAZ2, JAZ3, JAZ4, JAZ6, JAZ7, JAZ8, FI1, FI2, FI3, FI4, FI5 while sites located in the outer shelf included FI6, FI7, FI8. Samples were preserved in 96% ethanol. Sediment sub-samples from each replicate were taken for grain-size analysis, organic matter content (loss on ignition) and metals. Conductivity Temperature Depth (CTD) vertical casts were carried out at each station. The CTD casts recorded conductivity, temperature, depth and oxygen of the water column. Water samples were also taken for nutrients and chlorophyll a from the surface and the bottom layers (see below).
In the laboratory, each sample was hand-sorted and then organisms identified using a stereomicroscope. Macrofauna were generally identified to the species level where possible or family level (see Supplementary Material). Sediment grain size was determined by wet sieving and calculation of dry weight percentage fractions following the Wentworth scale27. Grain size fractions were gravel (>2 mm), very coarse sand (<2 mm and >1 mm), coarse sand (<1 mm and >500 μm), medium sand (<500 μm and >250 μm), fine sand (<250 μm and >125 μm), very fine sand (<125 μm and >63 μm) and silt-clay (<63 μm). Loss on ignition (LOI) was measured by dry sediment loss after combustion at 450 °C (American Public Health Association 21st Edn 2540 D + E Mod28, 29).
Water sample analysis
Water samples for chlorophyll a and nutrient quantification (NO3, NO2, PO4, SiO2) were taken at each station and/or reef. Water was collected from the surface in the case of coral reefs and from both the surface and bottom layers in sediment stations. Approximately 50 mL water was filtered through 0.22 μm membrane filters to collect nutrient samples which were frozen at −20 °C until analysis. To assess gradients in the primary productivity, surface water samples for chlorophyll a analysis were also taken in September 2014 and February 2015. For the analysis, 0.5–3 L of water were filtered through GF/F filters, wrapped in aluminum foil, and frozen at −20 °C on the research vessel and then to −80 °C (at the laboratory) until analysis. Chlorophyll a was extracted using 90% acetone30, 31. Following extraction, the raw fluorescence was measured with a Trilogy fluorometer (Turner Designs). Nutrient samples were analyzed using a Continuous Flow Analyzer (SEAL AutoAnalyser 3 with XY2/3 Sampler)31.
Data analysis
Hypotheses about changes in the structure and composition of benthic assemblages in the southern Red Sea were tested using a combination of multivariate and univariate techniques. Multivariate analyses were carried out with the statistical package PRIMER v6 and the PERMANOVA+ add-on package32, 33.
For coral reefs, shelf-position, depth, chlorophyll a and nutrients, sand, coralline algae and rock cover were used as potential explanatory variables. Nutrient and chlorophyll a data collected from the CTD casts were used in the analysis. Because nitrates and nitrites were highly correlated we used the nitrite measurements in subsequent statistical analyses where the percentage variation explained was highest. We analyzed the following univariate descriptors: number of taxa (OTUs), abundance (as % cover), Shannon-Wiener diversity, Margalef species richness and Pielou’s equitability. To visualize patterns of change in the composition and structure of the targeted assemblages across the shelf gradient, unconstrained (Principle Coordinates PCO) and constrained (distance-based redundancy analysis dbRDA34) ordination methods were applied. For reef datasets, the multivariate methods were based on Bray-Curtis similarities calculated from square-root transformed data. Benthic categories were related to the spatial patterns in composition and abundance of reef associated assemblages via Distance-based Linear Models (DistLM)33 where the most parsimonious model based on the ‘Best’ procedure and the R2 selection criterion was plotted using dbRDA. We also calculated the index of multivariate dispersion (MVDISP) based on Bray-Curtis similarity, Jaccard, Sorensen and Chi-Square distance.
The size frequency data was used to assess coral colony size differences between nearshore versus offshore reefs within the same coral genus using a non-parametric Mann–Whitney t-test. Kolgomorov-Smirnov test for two samples was carried out to compare the size-frequency distributions between the two types of reefs for each coral genus. These analyses were not carried out for the coral genus Galaxea due to the low number of colonies (only 2) found in the nearshore reefs. All the analyses were performed using the computer software Statistica 8.0.
Soft sediment macrobenthic community structure was characterized regarding abundance (N), total number of taxa (S), diversity (Shannon-Wiener index H’), and equitability (Pielou’s J’) indices. Ordination techniques, including Principle Component Ordination (PCO) were performed to better visualize the patterns of change in the macrobenthic assemblages. Distance-based linear modeling (DistLM) with step-wise and adjusted R2 selection criteria were performed to estimate the explained variation in macrobenthic assemblage structure by each environmental variable. The direction and magnitude of the relationship between environmental variables and assemblages (given by the vectors for each environmental variable) were visualized in distance-based redundancy analysis (dbRDA) biplots. Environmental data (depth, distance to the main shoreline, nutrients, and grain-size fractions) were normalized before the analyses, as they have different units and scales. For assemblages inhabiting sediments multivariate methods were also based on the Bray-Curtis similarities calculated from the square-root transformed values.
Results
Coral Reef Assemblages
A total of 41 scleractinian OTUs were identified, along with 10 soft coral OTUs. Porites massive form was the consistently dominant reef-building coral across depth and shelf. Depending on the habitat, Acropora (three OTUs, Acropora, Acropora tabular and Acropora branching), Echinopora, Stylophora, Galaxea and Pavona, also accounted on average for more than 1% of the cover per reef. Scleractinian corals mainly dominated reefs in the outer shelf, while sand or macroalgae mainly dominated the inner shelf reefs. Inner shelf reefs harbored less abundant, less diverse benthic megafaunal assemblages than outer shelf reefs (Table 2). In general, the outer shelf reefs supported higher numbers of rare taxa (i.e. singletons+doubletons). An increase in the mean biological cover and number of taxa was also observed from 2–5 m to 8–10 m depth transects. The same pattern was detected for both hard and soft corals and to a lesser extent, coralline algae. The macroalgae/hard coral cover ratio was higher in inner shelf reefs compared to outer shelf reefs (Table 2). High levels of dominance characterized the inner shelf reefs but no clear pattern regarding depth was detected. Overall, 31 taxa were shared among all the groups of reefs (i.e., inner shelf 2–5 m and 8–10 m; outer shelf 2–5 m and 8–10 m). The outer shelf reefs supported 14 exclusive taxa, against one (Padina) in the inner shelf reefs (Fig. 2).
Cluster diagram of sites based on benthic composition of Bray-Curtis dissimilarity and associated benthic species by site indicating cross shelf gradient from Outer Shelf (OS) to Inner Shelf (IS). Note colors groupings on histogram where Blue = Corals, Red and Orange = Sponges, Green = Algae. S = Sand, R = Rubble, MA = Macroalgae, SC = Soft Coral, HC = Hard Coral, CA = Coralline Algae, BA = Brown Algae, TF = Turf Algae.
Cluster ordination analyses performed on Bray-Curtis measures showed greater similarity in the outer reefs compared with inner shelf sites (Fig. 2). Overall, a consistent pattern emerged across the measures applied, i.e., lower heterogeneity in deeper than shallower transects and in outer than inner shelf reefs. The outer shelf reefs (particularly at the deeper transects) were mainly associated with higher cover of hard corals, soft corals and coralline algae, while most of the inner shelf reefs were associated with either upright sponges, cf. Dictyota or Sargassum (Fig. 2). For all the measures applied in the multivariate analyses, the MVDISP showed a consistent and increasing pattern, i.e., 2–5 m inner shelf >2–5 m outer shelf >8–10 m outer shelf >8–10 m inner shelf (Table 3).
Changes in size frequency and skewness
The corals surveyed ranged in diameter from 3 to 175 cm (Table 4). Statistically significant differences were detected between the size of the colonies from nearshore and offshore reefs for Acropora, Echinopora and Porites (Table 4). Smaller colonies were recorded in the nearshore reefs (Table 4). Juvenile coral density (mean ± SD) differed significantly between types of reefs for Acropora and Echinopora (p < 0.001) with lower densities recorded in the nearshore reefs (Table 4). The size-frequency distribution per coral genus was also statistically different for Acropora (p < 0.001) and Echinopora (p < 0.005) based on the Kolgomorov-Smirnov test. Only Porites showed negative values of skewness for the nearshore reefs. This more negatively-skewed size distribution is probably a result of the lack of abundant juveniles (<5 cm diameter, 58 in nearshore vs 234 in offshore reefs) and adult corals (>10 cm diameter, 34 in nearshore vs 415 in offshore) in the nearshore reefs in comparison with the offshore reefs.
Relationship between reef community patterns and environmental variables
Inshore reefs were associated with higher concentrations of nutrients (nitrites or phosphates) (Fig. 3). The distance to the main shoreline clearly separates both reef groups, suggesting a strong inshore to offshore pattern (Fig. 3). A number of environmental factors including nutrients, chlorophyll, depth and distance from the shore were significantly related to the composition of the benthic patterns (Table 5).
Soft sediment assemblages
Environmental patterns
The sampled area presents relatively high variability in terms of the environmental parameters analyzed. Depth varied from 5 m (JAZ6) to 138 m (FI5) and samples were distributed along an inshore to offshore gradient ranging from 2 to 127 km from the main shoreline (Table 6). Levels of phosphates (PO4) in the water were generally low (<0.1 μM) with only a few stations presenting values above 0.2 (FI5, FI7 and FI8). Variability was also high regarding the sediment particle size, with some stations, even within the same area, being either dominated by fine or sandy fractions (Table 6). This high variability of the sediment particle size between sites and the general pattern of a higher percentage of gravel and coarse sand in the coastal sites may explain why there was not a clear relationship between water column nutrient levels and organic content in the sediments for the nearshore JAZ sites.
Macrobenthic assemblages
In the subset of the samples selected, 154 taxa were identified. This number is highly underestimated, as Polychaeta, Decapoda and Echinodermata, some of the most abundant groups, were not identified to the genus or species level (family level for polychaetes and decapods; class level for echinoderms). Overall, Polychaeta (contributing from 49.5% to 65.8%, in Jazan and FI IS, respectively) and Amphipoda (contributing from 12.3% to 19.1%, in Jazan and FI IS, respectively) were the dominant groups in terms of abundance. Only at Jazan, bivalves (28% of the total abundance) were more abundant than amphipods (Fig. 4a). In terms of the number of taxa, Polychaeta was once more the dominant group across the study area, contributing to more than 45% (FI OS) of each area’s total number of taxa and reaching 69% in the FI IS (Fig. 4b). Amphipoda was in general the second highest contributor (7% to 14% of total number of taxa), followed by Decapoda (9% to 11% of total number of taxa) and Bivalvia (2% to 7% of total number of taxa) (Fig. 4b). When analyzed per area, Jazan was characterized by the highest number of taxa (25.5 ± 6.64), followed by FI IS and FI OS (Fig. 4c). Abundance was also higher in Jazan (Fig. 4d). In both cases, variability was high. The extremely high abundance values found in some of the replicates collected in the Jazan area (e.g., JAZ6 R1, 440 individuals), resulted in depressed equitability (J’) (Fig. 4e). Total polychaetes and opportunistic polychaetes were more abundant at the Jazan area, followed by the FI IS (Fig. 4g,h). Opportunistic polychaetes recorded included Capitellidae, Cirratulus sp., Chaetozone sp., Notomastus sp. amongst others. See Supplementary Table S1 for a full list of opportunistic polychaete species recorded by site.
Relative abundance of the main soft sediment taxonomic groups in the different study areas (a) and relative proportion of the main taxonomic groups in terms of the number of taxa in the different study areas (b). Boxplots for the univariate variables number of taxa (S, taxa × 0.1 m2) (c), abundance (N, no. of individuals × 0.1 m2) (d), Pielou’s equitability (J’) (e) and Shannon-Wiener diversity (H’) (f), the total number of Polychaeta (h) and the total number of opportunistic Polychaeta (g) based on soft sediment macrofauna in the different areas. JAZ, Jazan; FI IS, Farasan Islands-Inner shelf; FI OS, Farasan Islands-Outer shelf.
The multivariate analysis on the square-root transformed data identified four main groups (Fig. 5). Sites clearly grouped from inshore to offshore in the ordination. The near shore Jazan sites (JS: JAZ2, JAZ6) grouped together while sites further offshore from Jazan were grouped (JF: JAZ3, JAZ4, JAZ7, JAZ8). Similarly the Farasan Island soft sediment sites also clustered across the shelf with Farasan Inshore (FI; FI1, FI2, FI4, FI5) sites grouping together and Farasan Offshore sites grouping (FO; FI6, FI7, FI8). SIMPER analysis highlighted the higher abundance of bivalves and polychaetes (in general, but also the opportunistic Capitellidae and Cirratulidae) in Jazan and to a less extent in the FI IS area.
Relationship between biological patterns and environmental variables
Distance-based linear modeling (DistLM) analysis showed that all environmental variables were significant for the adjusted R2 selection criterion. The sequential tests showed that a combination of variables that reflected nutrient enrichment (Chlorophyll a) and grain size (silt-clay, medium sand, and gravel) were significant to the adjusted R2 reduced model, which explained 20% of the total variation. The first two dbRDA axes explained 45% of the fitted variation. The dbRDA analysis separated near shore sites (JAZ2, JAZ6) from the Jazan area based on higher chlorophyll a content and reduced Redox potential (Fig. 6). Within the Farasan Islands, sediments at the inner shelf sites (FI1, FI2) presented higher levels of fine particles and also had higher organic contents within the sediments. Depth and phosphates were significantly related with the deeper sites at the offshore FI7 and FI8 sites (Fig. 6).
Distance-based redundancy analysis plot of square-root-transformed macrobenthic data with Bray-Curtis similarity index. Vectors are shown only for the most relevant environmental variables in the model. Length and direction of vectors indicate the strength and direction of the relationship. PO4, phosphates; NO2, nitrites; Chla, chlorophyll a; LOI, loss on ignition; Redox, reduction potential; Fines; >63 μm grain size fraction.
Discussion
Cross shelf systems present the strongest persistent gradients in environmental conditions such as salinity, temperature, and nutrients in the marine environment and represent a transitional zone from coastal, estuarine and river influences to truly oceanic conditions19. The importance of cross shelf environmental conditions is reflected in the variation in diversity and composition of marine assemblages from in to offshore environments20, 35. Previous studies have reported distinct cross shelf distributional patterns for a range of taxonomic groups including larval fish, siphonophores, corals, sponges, soft corals, macroalgae, oysters and adult fish populations36,37,38,39,40,41,42,43.
This study investigates changes in the community composition of both coral reef and macrobenthic communities along a cross shelf gradient in the Farasan Islands and is, to our knowledge, the first synoptic study of soft sediment and reef diversity in the Red Sea region. We found clear cross shelf differences in community composition and structure for both reef and soft sediment habitats. Community composition of coral reef assemblages differed significantly in species composition, size distribution, and structure with location and depth while a clear inshore-offshore pattern of changing community composition and diversity was also observed for soft sediment communities. We consider these cross shelf biodiversity patterns in relation to key environmental factors including anthropogenic disturbance for both coral reef and soft sediment communities.
Coral reef cross shelf patterns
Cross shelf biodiversity patterns for coral reef assemblages were recorded. Inner shelf reefs harbored less abundant and less diverse soft and hard coral assemblages with a higher macroalgae cover than outer shelf reefs. These cross shelf biodiversity patterns are in agreement with previously reported studies from the Red Sea44 and global studies16, 45,46,47. For example a number of studies have documented near shore sites that are dominated by macroalgae with relatively low hard coral cover compared to much higher coral cover and diversity at offshore sites36, 48, 49. The general trend is a loss of coral species diversity and a transition to dominance by macroalgal species. Such shifts from coral reef systems to alternate states (e.g., dominance of macroalgae, turf algae) as a result of multiple stressors have been documented worldwide50. Major stressors associated with the degradation of reefs have included pollution from nutrients51, sediments52, 53, fishing and altered trophic structures54, 55 as well as elevated sea surface temperatures56,57,58. These stressors can drive coral reef degradation directly, through increased coral mortality, or indirectly, by increasing susceptibility to coral diseases59. At longer time scales (decades), stress forces coral reef ecosystem degradation by reducing resiliency after disturbances, such as storms60 and bleaching events61. The combination of these processes has been the loss of coral reef health and the promotion of phase shifts to alternate ecological states62. In this study, there was also evidence of negatively skewed size-frequency distributions in nearshore coral populations associated with poor environmental conditions, a similar finding to other studies documenting reduced colony size in disturbed environments63,64,65.
We investigated the key environmental parameters influencing the observed coral shelf biodiversity patterns within the Red Sea, a region where nearly 60% of the reefs have been identified as at risk from anthropogenic activities66. Environmental factors correlated with coral diversity loss were related to elevated nutrient levels and distance from the shoreline. Distance from the shoreline included aspects of depth as well as changing levels of disturbance such as turbidity and enrichment. Variation in depth has been previously identified as a significant parameter in the diversity and distribution of various taxa including scleractinian corals19 and meiofaunal communities67. Higher nutrient availability and chlorophyll a concentrations at near shore sites were also associated with lower diversity of coral species and a higher dominance of algal cover. This trend indicates a potential impoverishment of these reef assemblages driven by human-activities in the nearshore region. This pattern supports literature findings that nearshore reefs, closest to terrestrial impacts, would show the greatest signs of degradation relative to reefs located in offshore areas. Previous studies have indicated that the main source of nutrients in the Red Sea come from the Indian Ocean, through the Gulf of Aden, which have a higher influence on the offshore locations due to the shelf bathymetry and oceanography circulation patterns on the shelf 5, 68,69,70,71,72. Higher nutrient concentrations were therefore expected in the offshore locations, however, measured nutrient and chlorophyll a concentrations found to be important factors influencing reef assemblages were higher in the inner shelf region. There has been the general perception that the flux of anthropogenic nutrient inputs relative to oceanic sources of nutrients such as upwelling is small and therefore anthropogenic inputs can have relatively little effect on the productivity of coastal waters. Alternatively recent studies investigating the relative role of anthropogenic nutrient sources from rival natural sources on small scales suggest that anthropogenic nutrients can provide a significant source of nitrogen for near shore productivity in coastal waters73, 74. Sources of anthropogenic disturbance and nutrient enrichment in the inner shelf region may occur due to the proximity to Jazan City, an urbanized area in the south of the Red Sea. The province of Jazan also has agricultural farming activity. Since, Jazan is located in a wadi area and is one of the locations with higher annual rainfall (131 mm/year) in Saudi Arabia75, surface run-off and storm water may therefore contribute to coastal nutrient enrichment in this region. This finding is similar to another Red Sea study68 that also predicted higher nutrient concentrations at offshore sites due to oceanographic sources of nutrients, but reported higher chlorophyll a, phosphorus, and particulate carbon concentrations at the near shore sites, attributed to a domestic wastewater treatment facility and industrial facilities. Further studies conducted in the Red Sea have identified land-based nutrient loading (particularly nitrogen levels) as a variable responsible for coral diversity loss or coral mortality21, 76,77,78. Numerous laboratory and field experiments79,80,81 have concluded that corals are negatively affected by increased levels of nutrients. Case studies of in situ observations demonstrate shifts from coral dominance to algal dominance and suggest linkages with chronic nutrient loading, including case studies in the Pacific Ocean82, 83, the Red Sea84, 85, the Caribbean79, 86, the Indian Ocean87 and the Atlantic Ocean88. As well as elevated nutrient enrichment in the coastal zone, there is also the potential for elevated turbidity in the near shore southern Red Sea. This is due to the wider coastal shelf in the southern region where sediments are less readily lost to deeper water. The shallow bathymetry created by these sediment deposits in the southern Red Sea combined with their fine nature results in substrate instability and associated turbidity in exposed conditions, which can limit the opportunity for the development of coral reefs. Currently there are few large-scale studies of water quality and biotic responses to increasing nutrients and turbidity from terrestrial runoff, and the links between reef health and water quality at larger scales have remained the subject of debate89. Our study demonstrates a loss of coral health and clear shifts from reef to macroalgae dominated communities at nearshore sites associated with potential anthropogenic nutrient enrichment. As noted above Jazan is in a flooding area with higher than normal precipitation rates for the country, which may contribute to higher levels of coastal nutrients from run-off. Fishing disturbance particularly from trawling activities can contribute to damage of corals. The relative role of other mechanisms that may also affect coral health including turbidity and depth that are captured by the variable ‘distance from the shore’ will also require further investigation.
Soft sediment macrobenthic cross shelf patterns
This study also observed a clear inshore to offshore pattern of changing community composition and diversity for soft sediment macrobenthic communities. In contrast to coral reef patterns, the highest diversity and abundance of soft sediment communities were recorded at the inshore sites. Many studies have investigated spatial distribution patterns of macrobenthic communities in transitional estuarine systems and have found sediment composition, salinity, depth and organic content as relevant environmental factors explaining macrofaunal patterns18, 90,91,92. There are fewer comparable studies that have investigated cross shelf regional biodiversity patterns in coastal areas. Regional studies of the macrobenthic patterns from the Norwegian continental shelf identified environmental variables best correlated with macrofaunal communities, which included median grain size, silt-clay content of sediment and depth93,94,95. Relationships between the distribution of soft-bottom macrofauna and sediment characteristics, in general, have been studied for decades, and historically particle size has often been identified as an important variable explaining macrobenthic distribution96,97,98. It is also recognized that at any given location, a number of different interacting factors will be involved in explaining faunal patterns98. In this study, a combination of nutrient enrichment and grain size were identified as important factors affecting the ecology of the soft sediment macrobenthic communities. Sites closer to the shore (Jazan) were characterized by a higher number of bivalves and opportunistic polychaete species, as well as lower equitability, which may be indicative of mild disturbance. A peak of opportunistic species is often associated with mild disturbance such as organic enrichment as summarized in ref. 99 seminal review of the effects of organic enrichment and pollution on marine benthic communities. Currently, the high levels of diversity and abundance observed in the inshore Jazan area appear to support the hypothesis that the system is affected by mild enrichment. The region is not in a highly disturbed state where overall species richness is reduced and only high densities of small fast growing opportunistic species occur. In general, the macrofaunal species diversity recorded in this study was high, especially considering the taxonomic resolution of the study. Increasing the taxonomic resolution and expanding the spatial coverage of research in the Red Sea is likely to substantially increase the current number of macrofaunal taxa recorded supporting the overall high biodiversity and endemism previously reported for this region.
Management implications
Globally, both terrestrial and aquatic ecosystems are experiencing declining biodiversity100, 101. This decline highlights the need to understand processes regulating diversity and the consequences of species loss for ecosystem function102, 103. Although recent studies have confirmed the existence of diversity gradients and investigated species richness patterns for numerous marine groups23, 104, the processes that shape these patterns have not been adequately resolved105. Bellwood & Hughes (2001) highlight that if we wish to protect global biodiversity, we must understand the processes that maintain diversity at these scales. The protection of habitat over large regional-scale areas has therefore been identified as a major goal for management strategies105. Achieving this outcome requires an improved understanding of the dynamics of reef and soft sediment ecosystems, of the processes that support or undermine resilience, and of the socio-economic drivers and governance systems that regulate water quality and rates of extraction of resources50.
There has been significant debate in the coral reef literature over the relative importance of environmental variables and stressors that regulate community structure and resilience. Human population growth and associated increases in nutrient loading106, destructive fishing60, compounded with ocean warming58 and stochastic environmental effects have been broadly debated to explain the increasing degradation of coral reefs worldwide. Discriminating among various stressors is critical to determine conservation strategies and to eventually ameliorate the accelerating degradation of coral reefs. In an attempt to differentiate between acute versus chronic stressors, several researchers have conducted broad correlative and statistical assessments of communities over large regional scales. These studies have suggested a clear interaction between eutrophication in conjunction with declining herbivorous organisms due to overfishing as direct causes for maintaining present undesirable phase shifts on coral reefs107. In this study, we identified factors that resulted in the loss of reef diversity and increases in opportunistic macrobenthic species influenced by anthropogenic disturbances including nutrient enrichment. Regional management strategies for coastal areas in the Red Sea therefore benefit from the active management of water quality and will also benefit from the protection of large reef and benthic areas.
In many parts of the world including the Kingdom of Saudi Arabia, water quality guideline values and targets have or are being developed as a tool to improve integrated coastal management (see ref. 108). A guideline value is defined as a numerical value of a water quality measure (e.g., light, nutrients, sediment, and agrochemicals) that will support and maintain the biological integrity of a water body and its benthic and pelagic ecosystems. Chlorophyll concentration is highly correlated with particulate nitrogen, particulate phosphorous, and suspended solids53, 71 and commonly used as a measure of water column productivity. High macroalgal cover is also widely accepted as an indicator of reef degradation, has been linked to high nutrient inputs and loss of herbivores, and causes mortality of corals and other reef and benthic organisms109. Nutrient threshold points (where increasing water column nutrients reach critical resilience levels such that they reduce recovery from phase shifts) have been widely postulated as approx. 1.0 um DIN110,111,112 for potential macroalgal overgrowth of coral-reef communities. Another useful tipping-point indicator is water column chlorophyll a, where levels in excess of 0.4–0.45 ug l−1 also indicate detrimental over-enrichment of nutrients112, 113. Within this study we recorded water column chlorophyll a levels up to 1.54 ug l−1 beyond the critical values reported in the literature for potential over enrichment effects requiring further assessment with other regions within the Red Sea.
Despite the extent of coral reefs in the Red Sea, the levels of biodiversity and endemism of the region are sorely understudied6. The lack of available information represents, in many cases, a significant hurdle for its conservation and management6. This study has identified changes in the biodiversity and community composition of both soft sediment macrofaunal and coral reef biodiversity at regional scales. These cross shelf patterns were related to nutrient enrichment and potential near shore anthropogenic disturbance providing important information for the management of marine coastal environments in the Red Sea.
References
Rasul, N. M., Stewart, I. C. & Nawab, Z. A. The Red Sea (eds Rasul, N. M. A. & Stewart, I. C. F.) 1–28 (Springer-Verlag, Berlin, Heidelberg, 2015).
DeVantier, L. & Pilcher, N. J. The status of coral reefs in Saudi Arabia. PERGSGA Technical Series Report, Jeddah. 1–45 (2000).
Edwards, A. Red Sea Vol. 1 (Pergamon Press, 1987).
Ahmad, F. & Sultan, S. Surface heat fluxes and their comparison with the oceanic heat-flow in the red-sea. Oceanologica Acta 12, 33–36 (1989).
Sofianos, S. S. & Johns, W. An Oceanic General Circulation Model (OGCM) investigation of the Red Sea circulation, 1. Exchange between the Red Sea and the Indian Ocean. Journal of Geophysical Research 107, 3196–3111 (2002).
Berumen, M. L. et al. The status of coral reef ecology research in the Red Sea. Coral Reefs 32, 737–748 (2013).
Sheppard, C. R. C., Wilson, S. C., Salm, R. V. & Dixon, D. Coral Reefs of the Indian Ocean: Their Ecology and Conservation (eds McClanahan, T. R., Sheppard, C. R. C. & Obura, D. O.) (Oxford University Press, New York, 2000).
Allen, G. R. Conservation hotspots of biodiversity and endemism for Indo-Pacific coral reef fishes. Aquatic Conservation: Marine and Freshwater Ecosystems 18, 541–556 (2008).
DiBattista, J. D. et al. A review of contemporary patterns of endemism for shallow water reef fauna in the Red Sea. Journal of Biogeography 43, 423–439 (2016).
Terraneo, T. et al. Pachyseris inattesa sp. n. (Cnidaria, Anthozoa, Scleractinia): a new reef coral species from the Red Sea and its phylogenetic relationships. ZooKeys 433, 1–30 (2014).
Coles, S. L. & McCain, J. C. Environmental Factors Affecting Benthic Infaunal Communities of the Western Arabian Gulf. Marine Environmental Research 29, 289–315 (1990).
McCain, J. C. Marine ecology of Saudi Arabia: the nearshore, soft-bottom benthic communities of the northern area, Arabian Gulf, Saudi Arabia. Fauna of Saudi Arabia 6, 79–101 (1984).
Riegl, B. & Velimirov, B. How many damaged corals in Red Sea reef systems? A quantitative survey. Hydrobiologia 216–217, 249–256 (1991).
Riegl, B. & Luke, K. E. Ecological parameters of dynamited reefs in the northern Red Sea and their relevance to reef rehabilitation. Marine Pollution Bulletin 37, 488–498 (1998).
Sheppard, C. Coral reefs in the Gulf are mostly dead now, but can we do anything about it? Marine Pollution Bulletin 105, 593–598 (2015).
Becking, L. E. et al. Beta diversity of tropical marine benthic assemblages in the Spermonde Archipelago, Indonesia. Marine Ecology – An Evolutionary Perspective 27, 76–88 (2006).
Przeslawski, R., McArthur, M. A. & Anderson, T. J. Infaunal biodiversity patterns from Carnarvon Shelf (Ningaloo Reef), Western Australia. Marine and Freshwater Research 64, 573 (2013).
Ysebaert, T. et al. Large-scale spatial patterns in estuaries: estuarine macrobenthic communities in the Schelde estuary, NW Europe. Estuarine, Coastal and Shelf Science 57, 335–355 (2003).
Cleary, D. F. R. et al. Variation in the diversity and composition of benthic taxa as a function of distance offshore, depth and exposure in the Spermonde Archipelago, Indonesia. Estuarine, Coastal and Shelf Science 65, 557–570 (2005).
Adjeroud, M. Factors influencing spatial patterns on coral reefs around Moorea, French Polynesia. Marine Ecology Progress Series 159, 105–119 (1997).
Bruckner, A. W., Riegl, B., Purkis, S., Williams, A. & Renaud, P. Khaled bin Sultan living oceans foundation atlas of Saudi Arabian Red Sea marine habitats. (Panoramic Press, Pheonix, 2011).
Kohler, K. E. & Gill, S. M. Coral Point Count with Excel extensions (CPCe): A Visual Basic program for the determination of coral and substrate coverage using random point count methodology. Computers & Geosciences 32, 1259–1269 (2006).
Veron, J. Corals of the World. Australian Institute of Marine Science 1–3, 1–207 (2000).
Lozano-Cortés, D. F. & Berumen, M. L. Colony size-frequency distribution of pocilloporid juvenile corals along a natural environmental gradient in the Red Sea. Marine Pollution Bulletin 105, 546–552 (2016).
Bak, R. P. & Meesters, E. H. Coral population structure: the hidden information of colony size-frequency distributions. Marine Ecology Progress Series 162, 301–306 (1998).
Meesters, E. H. et al. Colony size-frequency distributions of scleractinian coral populations: spatial and interspecific variation. Marine Ecology Progress Series 209, 43–54 (2001).
Doeglas, D. J. Grain-size indices, classification and environment. Sedimentology 10, 83–100 (1968).
APHA. Standard Methods for the Examination of Water and Wastewater (American Public Health Association, 2005).
Kristensen, E. & Anderson, F. Ø. Determination of organic carbon in marine sediments: a comparison of two CHN-analyser methods. Journal of Experimental Marine Biology and Ecology 109, 15–23 (1987).
Sartory, D. P. “Spectrophotometric analysis of chlorophyll a in freshwater phytoplankton” (Hydrological Research Institute, Pretoria 1982).
EPA. Methods for chemical analysis of water and wastes. Method 365.2 (U.S. Environmental Protection Agency, Washington, DC., 1983).
Anderson, M., Gorley, R. N. & Clarke, R. K. Permanova+ for Primer: Guide to Software and Statistical Methods (2008).
Clarke, K. R. & Gorley, R. N. Primer v6: User Manual/Tutorial. PRIMER-E Ltd, Plymouth, UK (2006).
Legendre, P. & Anderson, M. J. Distance-based redundancy analysis: Testing multispecies responses in multifactorial ecological experiments. Ecological Monographs 69, 1–24 (1999).
Thibault-Botha, D., Lutjeharms, J. R. E. & Gibbons, M. J. Siphonophore assemblages along the east coast of South Africa; mesoscale distribution and temporal variations. Journal of Planktonic Research 26, 1115–1128 (2004).
Done, T. J. Patterns in the distribution of coral communities across the central Great Barrier Reef. Coral Reefs 1, 95–107 (1982).
Williams, D. M. Patterns in the distribution of fish communities across the central Great Barrier Reef. Coral Reefs 1, 35–43 (1982).
Dinesen, Z. D. Patterns in the distribution of soft corals across the central Great Barrier Reef. Coral Reefs 1, 229–236 (1983).
Williams, D. M. & Hatcher, A. I. Structure of fish communities on outer slopes of inshore, mid-shelf and outer shelf reefs of the Great Barrier Reef. Marine Ecology Progress Series 10, 239–250 (1983).
Dayton, P. K., Carleton, J. H., Mackley, A. G. & Sammarco, P. W. Patterns of settlement, survival and growth of oysters across the Great Barrier Reef. Marine Ecology Progress Series 54, 75–90 (1989).
Wilkinson, C. R. & Cheshire, A. C. Patterns in the distribution of sponge populations across the central Great Barrier Reef. Coral Reefs 8, 127–134 (1989).
Hernández-Miranda, E., Palma, A. T. & Ojeda, F. P. Larval fish assemblages in nearshore coastal waters off Central Chile: temporal and spatial patterns. Estuarine, Coastal and Shelf Science 56, 1075–1092 (2003).
Sampey, A., Meekan, M. G., Carleton, J. H., McKinnon, A. D. & McCormick, M. I. Temporal patterns in distributions of tropical fish larvae on the North-west shelf of Australia. Marine and Freshwater Research 55, 473–487 (2004).
PERSGA, Regional Organization for the Conservation of the Environment of the Red Sea and Gulf of Aden. State of the Marine Environment, Report for the Red Sea and Gulf of Aden (2006).
Harriott, V. J., Smith, S. D. A. & Harrison, P. L. Patterns of coral community structure of subtropical reefs in the Solitary Islands Marine Reserve, Eastern Australia. Marine Ecology Progress Series 109, 67–76 (1994).
Smith, T. B. et al. Assessing coral reef health across onshore to offshore stress gradients in the US Virgin Islands. Marine Pollution Bulletin 56, 1983–1991 (2008).
Roberts, T. E., Moloney, J. M., Sweatman, H. P. A. & Bridge, T. C. L. Benthic community composition on submerged reefs in the central Great Barrier Reef. Coral Reefs 34, 569–580 (2015).
Pichon, M. & Morrissey, J. Benthic zonation and com- munity structure of South Island reef, Lizard Island (Great Barrier Reef). Bulletin of Marine Science 32, 581–593 (1981).
Sandin, S. A. et al. Baselines and degradation of coral reefs in the Northern Line Islands. PLoS One 3, e1548 (2008).
Bellwood, D. R., Hughes, T. P. & Folke, C. Confronting the coral reef crisis. Nature 429, 827–833 (2004).
Pastorok, R. A. & Bilyard, G. R. Effects of sewage pollution on coral-reef communities. Marine Ecology Progress Series 21, 175–189 (1985).
Rogers, C. S. Responses of coral reefs and reef organisms to sedimentation. Marine Ecology Progress Series 62, 185–202 (1990).
Fabricius, K. E. Effects of terrestrial runoff on the ecology of corals and coral reefs: review and synthesis. Marine Pollution Bulletin 50, 125–146 (2005).
Jackson, J. B. C. et al. Historical overfishing and the recent collapse of coastal ecosystems. Science 293, 629–638 (2001).
Pandolfi, J. M. et al. Global trajectories of the long-term decline of coral reef ecosystems. Science 301, 955–958 (2003).
Glynn, P. W. Coral reef bleaching: ecological perspectives. Coral Reefs 12, 1–17 (1993).
Glynn, P. W. Coral reef bleaching: facts, hypotheses and implications. Global Change Biology 2, 495–509 (1996).
Hoegh-Guldberg, O. Climate change, coral bleaching and the future of the world’s coral reefs. Marine and Freshwater Research 50, 839–866 (1999).
Harvell, C. D. et al. Climate warming and disease risks for terrestrial and marine biota. Science 296, 2158–2162 (2002).
Hughes, T. P. Catastrophes, phase shifts, and large-scale degradation of a Caribbean coral reef. Science 265, 1547–1551 (1994).
Hughes, T. P. et al. Phase shifts, herbivory, and the resilience of coral reefs to climate change. Current Biology 17, 360–365 (2007).
Birkeland, C. Ratcheting down the coral reefs. BioScience 54, 1021–1027 (2004).
Crabbe, M. J. C. Scleractinian coral population size structures and growth rates indicate coral resilience on the fringing reefs of North Jamaica. Marine Environmental Research 67, 189–198 (2009).
Bauman, A. G. et al. Variation in the size structure of corals is related to environmental extremes in the Persian Gulf. Marine Environmental Research 84, 43–50 (2013).
Lozano-Cortés, D. F. & Zapata, F. A. Abundance and composition of juvenile scleractinian corals on a fringing reef (little reef) in San Andres island Colombia. Marine Biological Research 11, 304–312 (2015).
Burke, L. M., Reytar, K., Spalding, M. & Perry, A. Reefs at Risk Revisited. World Resources Institute (Washington, D.C., 2011).
Semprucci, F., Colantoni, P., Sbrocca, C., Baldelli, G. & Balsamo, M. Spatial patterns of distribution of meiofaunal and nematode assemblages in the Huvadhoo lagoon (Maldives, Indian Ocean). Journal of the Marine Biological Association of the United Kingdom 94, 1377–1385 (2014).
Sawall, Y. et al. Coral communities, in contrast to fish communities, maintain a high assembly similarity along the large latitudinal gradient along the Saudi Red Sea coast. Journal of Ecosystem and Ecography 4, 1–7 (2014).
Naqvi, S. W. A., Hansen, H. P. & Kureishy, T. W. Nutrient-uptake and regeneration ratios in the Red-Sea with reference to the nutrient budgets. Oceanologica Acta 9, 271–275 (1986).
Churchill, J. H., Bower, A. S., McCorkle, D. C. & Abualnaja, Y. The transport of nutrient-rich Indian Ocean water through the Red Sea and into coastal reef systems. Journal of Marine Research 72, 165–181 (2014).
Kurten, B. et al. Ecohydrographic constraints on biodiversity and distribution of phytoplankton and zooplankton in coral reefs of the Red Sea, Saudi Arabia. Marine Ecology 36, 1195–1214 (2015).
Kürten, B. et al. Carbon and nitrogen stable isotope ratios of pelagic zooplankton elucidate ecohydrographic features in the oligotrophic Red Sea. Progress in Oceanography 140, 69–90 (2016).
Howard, M. D. A. et al. Anthropogenic nutrient sources rival natural sources on small scales in the coastal waters of the Southern California Bight. Limnology Oceanography 59, 285–297 (2014).
Semprucci, F. et al. Meiobenthos and free-living nematodes as tools for biomonitoring environments affected by riverine impact. Environmental Monitoring and Assessment 187 (2015).
Hasanean, H. & Almazroui, M. Rainfall: Features and Variations over Saudi Arabia, A Review. Climate 3, 578–626 (2015).
Wielgus, J. The coral reef of Eilat (northern Red Sea) requires immediate protection. Marine Ecology Progress Series 263, 307 (2003).
Wielgus, J., Glassom, D. & Fishelson, L. Long-term persistence of low coral cover and abundance on a disturbed coral reef flat in the northern Red Sea. Journal of Experimental Marine Biology and Ecology 297, 31–41 (2003).
Bruckner, A. W. & Dempsey, A. C. The Red Sea (eds Rasul, N. M. & Stewart, I. C. F.) 471–486 (Springer-Verlag, Berlin, Heidelberg, 2015).
Tomascik, T. & Sander, F. Effects of eutrophication on reef-building corals II. Structure of scleractinian coral communities on fringing reefs, Barbados, West Indies. Marine Biology 94, 53–75 (1987).
Jokiel, P., Dobinsky, Z. & Stambler, N. Nutrient limitation in the symbiotic association between zooxanthellae and reef building corals. Pacific Science 48, 215–218 (1994).
Koop, K. et al. ENCORE: The Effect of Nutrient Enrichment on Coral Reefs. Synthesis of Results and Conclusions. Marine Pollution Bulletin 42, 91–120 (2001).
Grigg, R. W. Coral reefs in an urban embayment in Hawaii: A complex case history controlled by natural and anthropogenic stress. Coral Reefs 14, 253–266 (1995).
Green, A. L., Birkeland, R. H., Randall, R. H., Smith, B. D. & Wilkins, S. Years of coral reef degradation in Pago Pago Harbour: A quantitative record. Proceedings of the Eighth International Coral Reef Symposium, Panama 2, 1883–1888 (1997).
Mergener, H. Man-made Influences on and natural changes in the settlement of the Aqaba Reefs (Red Sea). Proceedings of the Fourth International Coral Reef Symposium 1, 193–207 (1981).
Walker, D. I. & Ormond, R. F. G. Coral death from sewage and phosphate pollution at Aqaba, Red Sea. Marine Pollution Bulletin 13, 21–25 (1982).
Lapointe, B. E., Littler, M. M. & Littler, D. S. Macroalgal over- growth of fringing coral reefs at Discovery Bay, Jamaica: Bottom-up versus top-down control. Proceedings of the Eighth International Coral Reef Symposium 1, 927–932 (1997).
Naim, O. Seasonal responses of a fringing community to eutrophication (Reunion Island, Western Indian Ocean). Marine Ecology Progress Series 99, 137–151 (1993).
Lapointe, B. E., Matzie, W. R. & Clark, M. W.. Phosphorus inputs and eutrophication on the Florida Reef tract. In Proceedings of the Colloquium on Global Aspects of Coral Reefs: Health, Hazards and History 106–112 (1994).
De’ath, R. & Fabricius, K. E. Water quality as a regional driver of coral biodiversity and macroalgae on the Great Barrier Reef. Ecological Applications 20, 840–850 (2010).
Thrush, S. F. et al. Habitat change in estuaries: predicting broad-scale responses of intertidal macrofauna to sediment mud content. Marine Ecology Progress Series 263, 101–112 (2003).
Ellis, J. I. et al. Predicting macrofaunal species distributions in estuarine gradients using logistic regression and classification systems. Marine Ecology Progress Series 316, 69–83 (2006).
Carvalho, S. et al. Factors structuring temporal and spatial dynamics of macrobenthic communities in a eutrophic coastal lagoon (Óbidos lagoon, Portugal). Marine Environmental Research 71, 97–110 (2011).
Ellingsen, K. Biodiversity of a continental shelf soft-sediment macrobenthos community. Marine Ecology Progress Series 218, 1–15 (2001).
Ellingsen, K. & Gray, J. Spatial patterns of benthic diversity: is there a latitudinal gradient along the Norwegian continental shelf? Journal of Animal Ecology 71, 373–389 (2002).
Ellingsen, K. Soft-sediment benthic biodiversity on the continental shelf in relation to environmental variability. Marine Ecology Progress Series 232, 15–27 (2002).
Gray, J. S. Animal sediment relationships. Oceanography Marine Biology Annual Review 12, 223–261 (1974).
Rhoads, D. C. Organism-sediment relations on the muddy sea floor. Oceanography Marine Biology Annual Review 12, 263–300 (1974).
Snelgrove, P. & Butman, C. Animal sediment relationships revisited – cause versus effect. Oceanography Marine Biology Annual Review 32, 111–177 (1994).
Pearson, T. H. & Rosenberg, R. Macrobenthic succession in relation to organic enrichment and pollution of the marine environment. Oceanography Marine Biology Annual Review 16, 229–311 (1978).
Gaston, K. J. Global patterns in biodiversity. Nature 6783, 220–227 (2000).
Tilman, D. Causes, consequences and ethics of biodiversity. Nature 405, 208–211 (2000).
Chapin, F. S. et al. Consequences of changing biodiversity. Nature 6783, 234–242 (2000).
McCann, K. S. The diversity-stability debate. Nature 405, 228–233 (2000).
Palumbi, S. R. Molecular biogeography of the Pacific. Coral Reefs 16, 47–52 (1997).
Bellwood, D. R. & Hughes, T. P. Regional-scale assembly rules and biodiversity of coral reefs. Science 292, 1532–1535 (2001).
Lapointe, B. E. Simultaneous top-down and bottom-up forces control macroalgal blooms on coral reefs. Limnology Oceanography 44, 1586–1592 (1999).
Burkepile, D. E. & Hay, M. E. Herbivore versus nutrient control of marine primary producers: context dependent effects. Ecology 87, 3128–3139 (2006).
RCES. Royal Commission Environmental Standards. Regulations and Standards. Environmental Protection and Control Department pp. 108 (2010).
McClanahan, T., Polunin, N. & Done, T. Ecological states and the resilience of coral reefs. Conservation Ecology 6, 18 (2002).
Bell, P. R. F. Eutrophication and coral reefs: some examples in the Great Barrier Reef lagoon. Water Resources 26, 555–568 (1992).
Lapointe, B. E., Littler, M. M. & Littler, D. S. Modification of benthic community structure by natural eutrophication: The Belize Barrier Reef. Proceedings of the Seventh International Coral Reef Symposium 1, 323–334 (1993).
Bell, P. R. F. & Elmetri, I. Ecological indicators of large- scale eutrophication in the Great Barrier Reef lagoon. Ambio 24, 208–215 (1995).
GBRMPA. Great Barrier Reef Outlook Report. Great Barrier Reef Marine Park Authority (2010).
Acknowledgements
This research has been developed using funding from the Saudi Aramco KAUST Marine Environmental Observations (SAKMEO) research program. We would like to thank Ali M. Qasem (Saudi Aramco) for information regarding the industrial facilities in the Jizan area. This paper has been reviewed and approved by Saudi Aramco Public Relation Department (Tracking number 16-3669). We wish to thank the crew of the R.V. Thuwal for their assistance in collecting the field samples. Finally we would also like to thank the two anonymous reviewers for their comments on this manuscript that improved the quality of the paper.
Author information
Authors and Affiliations
Contributions
Conceived and designed the experiments: H.A., S.C. Performed the experiments: H.A., S.K., S.C. Analyzed the data: H.A., S.K., D.L.C., Z.A., J.C., J.E., S.C. Contributed reagents/materials/analysis tools: ARAMCO. Wrote the paper: J.E., H.A., S.Y., S.C., B.J.
Corresponding author
Ethics declarations
Competing Interests
Dr. Ellis, Dr. Carvalho, Dr. Curdia, Ms. Kurten, Ms. Alsaffar, Dr. Jones and Dr. Anlauf’s work have been funded in part by Saudi Aramco. The funding from Saudi-Aramco contributes to the Saudi Aramco-KAUST center for Marine Environmental Observations (SAKMEO) program. Dr. Lozano-Cortes is an employee of Saudi Aramco.
Additional information
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Rights and permissions
This work is licensed under a Creative Commons Attribution 4.0 International License. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in the credit line; if the material is not included under the Creative Commons license, users will need to obtain permission from the license holder to reproduce the material. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/
About this article
Cite this article
Ellis, J., Anlauf, H., Kürten, S. et al. Cross shelf benthic biodiversity patterns in the Southern Red Sea. Sci Rep 7, 437 (2017). https://doi.org/10.1038/s41598-017-00507-y
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-017-00507-y
This article is cited by
-
Moving beyond traditional macrofaunal community structure studies in the Indian Ocean continental shelf: a research synthesis based on research weaving
Environmental Science and Pollution Research (2023)
-
Size structure of the coral Stylophora pistillata across reef flat zones in the central Red Sea
Scientific Reports (2022)
-
Geochemical Characterization of Al-Lith Coastal Sediments, Red Sea, Saudi Arabia
Arabian Journal for Science and Engineering (2020)
-
Consistent variability in beta-diversity patterns contrasts with changes in alpha-diversity along an onshore to offshore environmental gradient: the case of Red Sea soft-bottom macrobenthos
Marine Biodiversity (2019)
-
Cross-shelf investigation of coral reef cryptic benthic organisms reveals diversity patterns of the hidden majority
Scientific Reports (2018)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.