Abstract
Sex differences in the etiology of human trait variation are a major topic of interest in the social and medical sciences given its far-reaching implications. For example, in genetic research, the presence of sex-specific effects would require sex-stratified analysis, and in clinical practice sex-specific treatments would be warranted. Here, we present a study of 2,335,920 twin pairs, in which we tested sex differences in genetic and environmental contributions to variation in 2,608 reported human traits, clustered in 50 trait categories. Monozygotic and dizygotic male and female twin correlations were used to test whether the amount of genetic and environmental influences was equal between the sexes. By comparing dizygotic opposite sex twin correlations with dizygotic same sex twin correlations we could also test whether sex-specific genetic or environmental factors were involved. We observed for only 3% of all trait categories sex differences in the amount of etiological influences. Sex-specific genetic factors were observed for 25% of trait categories, often involving obviously sex-dependent trait categories such as puberty-related disorders. Our findings show that for most traits the number of sex-specific genetic variants will be small. For those traits where we do report sexual dimorphism, sex-specific approaches may aid in future gene-finding efforts.
Similar content being viewed by others
Introduction
In the past decade, several studies have highlighted the importance of differential genetic and environmental effects on a variety of traits across males and females1,2,3,4,5. For example, a study performed in 806 subjects from a genetically isolated Hutterite population showed sex differences in X-linked and autosomal additive genetic effects on several anthropometric traits, such as height, fasting insulin, and triglycerides1. Similarly, several robust sex-specific genetic effects have been identified outside the sex chromosomes in human3 and animal studies6,7,8. In addition, sex-specific environmental effects have been observed in obesity, where boys and girls differ in susceptibility to their social environment9. The presence of sex-specific etiological effects in human traits has far-reaching implications. A strong contribution of sex-specific effects in trait variation would for instance imply a difference in etiology between males and females for the same disorder, which would require sex-specific treatments. To assess the overall importance of sex-specific genetic effects across human traits, estimates of sex-specific heritability and male-female genetic correlations are most informative. While heritability quantifies the relative contribution of genetic effects compared to environmental effects in a trait, male-female co-heritability quantifies to what extent the same genetic variants play a role in males and females. Both measures are largely independent and complementary, and can be assessed using pairs of monozygotic (MZ) and dizygotic (DZ) twins, including same-sex and opposite DZ twins.
Twin studies have contributed enormously to our understanding of the relative contribution of genetic and environmental effects across human traits. A recently published meta-analysis of virtually all twin studies10 provided sex-specific estimates for the contribution of genes and environment across all traits investigated thus far. However, this study did not include an in-depth analysis of the extent to which male and female heritability estimates differed, nor did it report on the co-heritability in males and females. In the present study we therefore use single-sex and opposite-sex twin correlations to systematically assess the overall contribution of sex-specific genetic effects as well as the male-female genetic overlap across all investigated domains of human traits.
The use of twin correlations allows testing of three sex-specific hypotheses. The first hypothesis is that the difference between the monozygotic twin correlation (rMZ) and dizygotic twin correlation (rDZ) is similar across males (M) and females (F) ((rMZM-rDZM) = (rMZF-rDZF)). This can be interpreted as a test of whether the influence of (additive) genetic effects on the population variance is the same in males and females (hypothesis of equal amount of heritability). The second hypothesis is that the difference between 2*rDZ and rMZ is similar across sexes, thus ((2rDZM-rMZM) = (2rDZF-rMZF))11. This can be interpreted as a test of whether the relative influence of the shared environment is the same in males and females (hypothesis of equal amount of shared environmental influences) The third hypothesis that can be tested with twin data is that the observed correlation in DZ same-sex twins (rDZSS), here defined as the male and female DZ twin correlation midpoint 1/2 rDZM + 1/2 rDZF, is the same as the observed correlation in DZ twins of opposite sex (rDZSS = rDOS). This is a test of whether the same genes and regulatory regions (and/or shared environmental factors) affect the trait of interest in males and females (hypothesis of full co-heritability).
Results
To assess the contribution of sex-specific genetic effects we used the MATCH database of twin correlations of Polderman et al.10, of which we selected all traits for which sex-specific twin correlations and their standard errors (SEs) were available. The three hypotheses as introduced above were tested per ‘trait’ as reported in the original study as well as per ‘trait category’ following the official ICF12 and ICD-1013 sub-chapter classification (see Methods for details). We report on 50 trait categories based on 2,608 reported traits to test our hypotheses (see Table 1).
Sex differences per trait
For each trait we tested whether rMZM-rDZM = rMZF-rDZF. Of all 2,608 traits the relative contribution of genetic influences was significantly different between males and females for 1% (37) of the traits after Bonferroni corrected p-value of 1.5 × 10−5, while 12% (309) of traits were significant at the uncorrected 0.05 level. The test for equal shared environment variance resulted in a significantly different contribution of the environment between males and females in 1% (35) of traits after correction (p < 1.5 × 10−5) and 12% (311) at the 0.05 level of significance (Fig. 1a and b).
Estimates of genetic and environmental influences for males versus females across individual study traits. Scatterplots of estimates of (a) heritability (h2 = 2(rMZ − rDZ)) and (b) amount of shared environment (c2 = 2rDZ − rMZ) for males versus females. Red dots indicate traits for which the female estimate is significantly larger than the male estimate after. Blue dots indicate traits for which male heritability is significantly larger than female heritability. Grey dots indicate no statistically significant difference after Bonferroni correction for 2,608 trait statistics. The bottom graphs show the distribution of male-female differences in genetic effects (c) and shared environmental effects (d).
Note that although it is impossible to have a negative h2 and c2 in the population, due to the sometimes large sampling error of the observed twin correlations, sample estimates of h2 and c2 can be negative. Although it is possible to truncate these negative values to zero, this would result in biased estimates of the sex differences in h2 and c2, making hypothesis testing less reliable. In other words, a significant sex difference in h2 or c2 driven by a negative value only reflects that the null hypothesis of no sex difference can be rejected. It does not imply that h2 or c2 are negative on the population level. In addition, some statistically significant sex-differences are close to zero, due to small standard errors. This illustrates that statistically significant sex-differences are not necessarily large. The size of these significant sex-differences should therefore be taken into account as well.
The distribution of the genetic and environmental differences between males and females was symmetrically distributed around zero with a sharp peak and long tails (Fig. 1c and d). The sex-differences in h2 and c2 are not normally distributed due to the large variation in standard errors. Drawing test statistics under the null hypothesis of no sex-differences from a normal distribution based on the same variation in standard errors, shows a similarly peaked distribution (see Supplementary Fig. S1).
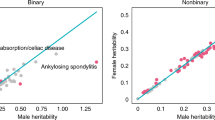
When testing for the contribution of sex-specific effects in these traits (rDZSS = rDOS) in 1,922 within-study comparisons, 3% (55) of traits were significantly different from each other at the Bonferroni corrected level of p < 2.6 × 10−5, while 23% (447 traits) were significant at an alpha of 0.05 (Fig. 2a). All statistically significant effects involved rDZSS ≥ rDOS, which is in the expected direction. This pattern was also reflected in a slightly skewed distribution of the difference between the opposite and same-sex correlation (Fig. 2b). See Figure S1 for the distribution under the symmetric null hypothesis.
Estimates of co-heritability between males and females across 1,922 individual study traits. (a) Scatterplot of all dizygotic (DZ) same-sex midpoint correlations (rDZSS = 1/2 rDZM + 1/2 rDZF) versus DZ opposite-sex correlations (rDOS). For each study trait it was tested whether rDZSS = rDOS. Red dots indicate that rDOS was significantly larger than rDZSS. Blue dots indicate that rDZSS was larger than rDOS. Grey dots indicate no statistically significant difference after Bonferroni correction for 1,922 individual study traits. (b) Distribution of all DZ same-sex midpoint and rDOS differences. Although most differences are close to zero, opposite-sex correlations are on average larger than same-sex DZ twin correlations.
Sex differences per trait category
Here we tested whether sex differences clustered within trait categories. Testing for heritability differences between males and females in 50 trait categories did not reveal any statistically different effects (Fig. 3a). Only the shared environmental estimate (2rDZ-rMZ) of the trait category Function of Brain was significantly higher for females than for males, although the difference was modest (−0.1) and both male and female estimates were close to zero (Fig. 3b).
Estimates of genetic and environmental influences for males versus females across 50 trait categories. Scatterplots of estimates of (a) heritability (h2 = 2(rMZ − rDZ)) and (b) amount of shared environment (c2 = 2rMZ − rDZ) for males versus females. Red dots indicate traits for which the female estimate is significantly larger than the male estimate. Blue dots indicate traits for which male heritability is significantly larger than female heritability. Grey dots indicate no statistically significant difference after Bonferroni correction for 50 trait category statistics. Except for Function of Brain no trait categories shows robust statistical evidence for sex-specific heritabilities or environmental influences.
The contribution of sex-specific effects (rDZSS = rDOS) could be tested in 36 trait categories revealing 9 categories (25%) with significant differences after Bonferroni correction, and 17 categories (47%) at the 0.05 significance level) (Fig. 4).
Nine trait categories (25%) show robust statistical evidence for reduced co-heritability between males and females. Scatterplot of all dizygotic (DZ) same-sex midpoint correlations (rDZSS = 1/2 rDZM + 1/2 rDZF) versus DZ opposite-sex correlations (rDOS) for a total of 36 trait categories. For each pair of it was tested whether rDZSS = rDOS. Red dots indicate that rDOS was significantly larger than rDZSS. Blue dots indicate that rDZSS was larger than rDOS. Grey dots indicate no statistically significant difference after Bonferroni correction for 36 trait categories.
Finally, Table 2 shows the eight trait categories with robust statistical evidence for differences in genetic and/or shared environmental factors between males and females.
Discussion
Although twin studies have reported sex-specific correlations for many traits, surprisingly few studies have systematically assessed the overall importance of sex-specific genetic and environmental influences. We present a comprehensive analysis of sex-specific genetic and environmental effects across all human traits that have been studied in twins to date, based on over two million twin pairs. Overall, we find little evidence for sex-related differences in the extent to which genetic or shared environmental influences contribute to the population variance. On the trait level only 1% of 2,608 traits showed robust statistical evidence for sex differences.
For some of the trait categories for which we did find sex-specific effects, differential sex effects can biologically be expected. For example, Disorders of Puberty, Not Elsewhere Classified includes traits like onset of puberty, which is measured differently in males and females (i.e. start of breast development in girls and change of voice in boys) and for which it is not unthinkable that different genes influence the specific bodily changes in boys and girls. Other traits, like Height, Weight Maintenance Functions, Eating Disorders, Food, Specific Personality Disorders, Recurrent Depressive Disorder, and Looking After One’s Health are measured similarly in males and females, but have been reported previously to be sexually dimorphic. For example, males and females have different fat percentages and fat distributions (Weight Maintenance Functions)14, different levels of physical activity and self-rated health (Looking After One’s Health)15. Although prevalence differences do not automatically imply etiological differences, it is noteworthy that food preferences, food intake (Food), and the prevalence of eating disorders are also different in males and females16, as well as in some personality disorders17. In addition, for all these trait categories the genetic contribution is much larger than that of shared environmental effects10. This suggests that sexual dimorphism for these categories is mainly driven by sex-specific genetic effects. In contrast, the categories Mental and Behavioural Disorders Due to Use of Tobacco and Looking After One’s Health show a relatively large contribution of shared environmental effects, suggesting that different environmental factors (as well as genetic factors) may contribute to the differences observed in males and females10.
Although our results show sex differences for some traits, it is yet unclear which genomic regions underlie these differences. Especially for sex dimorphic traits, sex differences may simply reflect differences on the sex chromosome.
Note that the heritability estimate (h2) and contribution of shared environmental factors (c2) are independent from the male-female genetic correlation and correlation due to shared environmental factors. While the former estimate how much genetic and shared environmental factors contribute in males and females, the latter estimates the extent to which the same factors contribute in males and females, irrespective of their magnitude.
Our results are in sharp contrast with the most prominent findings presented in a review by Gilks et al.18, which suggested that 50% of human traits have sex-specific heritabilities. An important difference between their review and our study is the number and type of traits. Gilks et al.18 analyzed 32 human traits that focused on anthropomorphic characteristics, where we analyzed a wide variety of over 2,600 traits. Furthermore, we based our analyses on over two millions of twin pairs, allowing robust assessment of sex-specific heritabilities in human traits. Of the 15 traits for which Gilks et al.18 reported sex differences in heritability we could confirm six sex-related etiological differences. These six traits were all part of the broader trait categories Height, Weight Maintenance Functions, and Eating Disorders.
The twin correlations used in this study have several levels of dependency. Many twin studies report on several, possibly related traits. Although this does not invalidate the individual p-values we report for example in Figs 1 and 2, it does imply that the Bonferroni correction will be somewhat conservative. The extent of this effect is difficult to assess, since it is not always known which correlations are (partly) based on the same sample.
Despite little evidence for sex differences in the relative contribution of genetic and shared environmental influences, one quarter of the investigated trait categories in our study do show evidence for differential genetic and/or environmental effects. Since many of these traits show small contributions of shared environment, it is likely that sex-specific genetic effects, as opposed to sex-specific environmental effects, play a role. Nonetheless, for a large majority of trait categories, including trait categories that are sexually dimorphic, we do not find evidence that the relative influences of genes and environment is different between males and females. This is consistent with recent studies based on SNP data that show large SNP-based genetic correlations between males and females across human traits19,20,21. For example, even the effect size of genetic variants related to sex-specific puberty-related traits like age at menarche and voice breaking show a relatively large correlation. These converging lines of evidence do not imply that sex-specific genetic variants do not exist for the majority of human traits. They do imply, however, that the overall contribution of sex-specific genetic variants to the genetic architecture is modest at best for most traits.
Although previous studies have performed similar analyses based on the classic twin model, the collection of phenotypes analyzed in this study is unprecedented by covering virtually all twin studies published to date, providing a comprehensive assessment of the sex differences across all human traits.
In conclusion, for the majority of traits, the relative influence of genes and environment is highly similar in males and females and genetic influences are typically not sex-specific. This suggests that for many traits the number of identified sex-specific genetic variants will be small compared to non-sex-specific variants. For those traits where we do report sexual dimorphism, sex-specific approaches may aid in future gene-finding efforts.
Materials and Methods
We used the database of Polderman et al.10, which is a comprehensive database of virtually all twin studies published between 1958 and 2012. The database includes reported twin correlations from 2,748 twin studies for 17,804 traits classified into >300 official trait categories according to the official ICF12 and ICD-1013. In the current study we focus on traits as reported in the original study and trait categories classified according to the official ICF and ICD-10 sub-chapter classification. None of the trait variables have been adjusted for covariates before computing the twin correlations. However, since correlations are insensitive to sex differences in trait mean and variance, same-sex and opposite sex correlations in DZ twins are comparable for sex dimorphic traits as well.
Since only twin correlations are consistently reported in twin studies, we computed the commonly used least squares estimates of the heritability (h2 = 2(rMZ − rDZ)) and relative contribution of shared environment (c2 = 2rDZ − rMZ) for individual traits as well as the meta-analyzed trait categories11. A key assumption of the classic twin model on which the least squares estimates of h2 and c2 are based is that shared environmental influences are similar in monozygotic and dizygotic twin pairs respectively. In their comprehensive review of the twin literature, Polderman et al.10 only included twin studies in which siblings were brought up in the same nuclear family. Moreover, a previous study on several traits did not find significant differences in shared environmental factors between monozygotic and dizygotic twins22, showing at least for some human traits that this key assumption of the classic twin model is tenable.
We selected all traits for which sex-specific twin correlations and their standard errors (SEs) were available: the monozygotic male (rMZM) and female (rMZF), dizygotic male (rDZM) and female (rDZF), and opposite sex (rDOS) twin correlations. SEs were computed on the correlation scale \(({\boldsymbol{se}}({\boldsymbol{r}})=\sqrt{\frac{1-{{\boldsymbol{r}}}^{2}}{{\boldsymbol{n}}-2}})\). From these twin correlations we derived the heritability (h2 = 2(rMZ − rDZ); se(h2) = 4(se(rMZ)2 + se(rDZ)2)) and contribution of shared environment (c2 = 2rDZ − rMZ; se(c2) = 4se(rDZ)2 + se(rMZ)2) for males and females separately. We estimated the same-sex correlation by meta-analyzing the male and female dizygotic twin correlations using a fixed effects model after Fisher-z transformation. We used these sex-specific statistics to test the following three hypothesis: 1) male and female heritabilities are equal (h2male = h2female), 2) male and female shared environmental effects are equal (c2male = c2female), and 3) the opposite-sex twin correlation equals the DZ same-sex correlation (rDZSS = rDOS). All hypotheses were tested with a Wald test (z = beta/se).
To account for similarity of traits within trait categories, we meta-analyzed all twin correlations within each trait category using a random effects model (see ref. 10 for details) and tested all three hypotheses for each trait category. We only included those traits for which at least 500 twin pairs were included in the meta-analysis and at least 10 studies contributed to the meta-analytic estimate. In addition, for each individual study at least 5 twin pairs needed to be present. This resulted in 50 trait categories (based on 2,608 individual trait statistics) with sufficiently reliable estimates to test our hypotheses (see Table 1). All three hypotheses were tested per trait as well as per trait category, based on the meta-analysis estimates of the relevant twin correlations. Bonferroni correction was applied per hypothesis for traits and trait categories respectively. See Supplementary Dataset S1 online for more elaborate descriptive statistics of the twin correlations and derived statistics.
Meta-analyzed data on the trait category level and R syntax used in the current study are included in this published article (and its Supplementary Information files). The raw data analysed and generated during the current study are available from the corresponding author on reasonable request.
Change history
21 December 2018
A correction to this article has been published and is linked from the HTML and PDF versions of this paper. The error has been fixed in the paper.
References
Pan, L., Ober, C. & Abney, M. Heritability estimation of sex-specific effects on human quantitative traits. Genet. Epidemiol. 31, 338–347 (2007).
Ober, C., Loisel, D. A. & Gilad, Y. Sex-specific genetic architecture of human disease. Nat. Rev. Genet. 9, 911–922 (2008).
Randall, J. C. et al. Sex-stratified Genome-wide Association Studies Including 270,000 Individuals Show Sexual Dimorphism in Genetic Loci for Anthropometric Traits. PLoS Genet 9, e1003500 (2013).
Dubois, L. et al. Genetic and Environmental Contributions to Weight, Height, and BMI from Birth to 19 Years of Age: An International Study of Over 12,000 Twin Pairs. PLoS ONE 7, e30153 (2012).
Rawlik, K., Canela-Xandri, O. & Tenesa, A. Evidence for sex-specific genetic architectures across a spectrum of human complex traits. Genome Biol. 17, 166 (2016).
Reinius, B. et al. An Evolutionarily Conserved Sexual Signature in the Primate Brain. PLoS Genet 4, e1000100 (2008).
Rinn, J. L. & Snyder, M. Sexual dimorphism in mammalian gene expression. Trends Genet. 21, 298–305 (2005).
Yang, X. et al. Tissue-specific expression and regulation of sexually dimorphic genes in mice. Genome Res. 16, 995–1004 (2006).
Wisniewski, A. B. & Chernausek, S. D. Gender in childhood obesity: Family environment, hormones, and genes. Gend. Med 6, 76–85 (2009).
Polderman, T. J. C. et al. Meta-analysis of the heritability of human traits based on fifty years of twin studies. Nat. Genet. 47, 702–709 (2015).
Falconer, D. S., Mackay, T. F. & Frankham, R. Introduction to quantitative genetics (4th edn). Trends Genet. 12, 280 (1996).
World Health Organization. International classification of functioning, disability and health: ICF. (World Health Organization, 2001).
World Health Organization. International statistical classification of diseases and health related problems (The) ICD-10. (World Health Organization, 2004).
Fried, S. K., Lee, M.-J. & Karastergiou, K. Shaping fat distribution: New insights into the molecular determinants of depot‐and sex‐dependent adipose biology. Obesity 23, 1345–1352 (2015).
Azevedo, M. R. et al. Gender differences in leisure-time physical activity. Int. J. Public Health 52, 8–15 (2007).
Hudson, J. I., Hiripi, E., Pope, H. G. & Kessler, R. C. The Prevalence and Correlates of Eating Disorders in the National Comorbidity Survey Replication. Biol. Psychiatry 61, 348–358 (2007).
Grant, B. F. et al. Prevalence, Correlates, and Disability of Personality Disorders in the United States: Results From the National Epidemiologic Survey on Alcohol and Related Conditions. J. Clin. Psychiatry 65, 948–958 (2004).
Gilks, W. P., Abbott, J. K. & Morrow, E. H. Sex differences in disease genetics: evidence, evolution, and detection. Trends Genet. 30, 453–463 (2014).
Muñoz, M. et al. Evaluating the contribution of genetics and familial shared environment to common disease using the UK Biobank. Nat. Genet. 48, 980–983 (2016).
Day, F. R. et al. Shared genetic aetiology of puberty timing between sexes and with health-related outcomes. Nat. Commun. 6, 8842 (2015).
Yang, J. et al. Genome-wide genetic homogeneity between sexes and populations for human height and body mass index. Hum. Mol. Genet. 24, 7445–7449 (2015).
Derks, E. M., Dolan, C. V. & Boomsma, D. I. A test of the equal environment assumption (EEA) in multivariate twin studies. Twin Res. Hum. Genet. Off. J. Int. Soc. Twin Stud 9, 403–411 (2006).
Acknowledgements
This work is supported by the Netherlands Organization for Scientific Research (NWO VICI 453-14-005).
Author information
Authors and Affiliations
Contributions
S.S., D.P. and T.J.C.P. wrote the main manuscript text. D.P. and S.S. performed the analyses and prepared the figures.
Corresponding author
Ethics declarations
Competing Interests
The authors declare that they have no competing interests.
Additional information
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Stringer, S., Polderman, T.J.C. & Posthuma, D. Majority of human traits do not show evidence for sex-specific genetic and environmental effects. Sci Rep 7, 8688 (2017). https://doi.org/10.1038/s41598-017-09249-3
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-017-09249-3
This article is cited by
-
Sex-biased genetic regulation of inflammatory proteins in the Dutch population
BMC Genomics (2024)
-
On the etiology of aesthetic chills: a behavioral genetic study
Scientific Reports (2022)
-
Brain serotonin deficiency and fluoxetine lead to sex-specific effects on binge-like food consumption in mice
Psychopharmacology (2022)
-
Sex differences in genetic architecture in the UK Biobank
Nature Genetics (2021)
-
Women’s socioeconomic position in ontogeny is associated with improved immune function and lower stress, but not with height
Scientific Reports (2020)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.