Abstract
High levels of ammonia (NH3) have been suggested to elevate ambient particle pH levels to near neutral acidity (pH = 7), a condition that promotes rapid SO2 oxidation by NO2 to form aerosol sulfate concentration consistent with “London fog” levels. This postulation is tested using aerosol data from representative sites around the world to conduct a thorough thermodynamic analysis of aerosol pH and its sensitivity to NH3 levels. We find that particle pH, regardless of ammonia levels, is always acidic even for the unusually high NH3 levels found in Beijing (pH = 4.5) and Xi’an (pH = 5), locations where sulfate production from NO x is proposed. Therefore, major sulfate oxidation through a NO2-mediated pathway is not likely in China, or any other region of the world (e.g., US, Mediterranean) where the aerosol is consistently more acidic. The limited alkalinity from the carbonate buffer in dust and seasalt can provide the only likely set of conditions where NO2-mediated oxidation of SO2 outcompetes with other well-established pathways. The mildly acidic levels associated with excessive amounts of ammonia can promote high rates of SO2 oxidation through transition metal chemistry, this may be an alternative important aerosol chemical contributor to the extreme pollution events.
Similar content being viewed by others
Introduction
pH is a fundamental particle property that affects aerosol formation, composition, toxicity and nutrient delivery1,2,3,4,5,6. Sulfate is a ubiquitous inorganic aerosol species that strongly regulates aerosol acidity and is produced by aqueous and gas-phase oxidation of SO2 along well-established pathways. Aqueous pathways dominate depending on the pH level (O3 under alkaline and H2O2 under acidic conditions7). Aqueous oxidation of HSO3 − has recently been proposed as the major mechanism of haze formation in China, but requires fine particle pH levels that are close to neutral (pH 6–7) or higher8,9. It is well-known that upon emission, fresh dust or seasalt particles can have a pH level that exceeds 610,11, hence provide aerosol where NO2-mediated oxidation of sulfate is possible; however, the acidic sulfate that forms upon these particles rapidly depletes their alkaline carbonate buffer and limits any substantial NO2-mediated production of sulfate. Acidification is fast for submicron particles, since acidic gases (e.g., HNO3 and H2SO4) are rapidly scavenged by alkaline aerosols12,13, dust and seasalt are only minor ionic fractions compared to sulfate and nitrate1, and equilibrium states with gases are typically achieved within 30 minutes under ambient conditions14,15,16. The good agreements between model and observation for the semivolatile species partitioning of NH3-NH4 + and HNO3-NO3 − species, using aerosol bulk properties as model input, suggest the thermodynamic equilibrium states in many circumstances and that the ambient fine mode aerosol is consistently (and often strongly) acidic2,17,18,19. Unlike fine haze particles, fogs and cloud drops can have pH closer to neutral owing to dilution of H+ by the orders of magnitude more liquid water.
Wang, et al.8 and Cheng, et al.9 argue that very high levels of NH3 from intense agriculture (e.g., up to 50–60 ppbv in Beijing and Xi’an, China) can sufficiently elevate pH in fine mode aerosol (PM1 and PM2.5) to promote rapid sulfate formation from NO2 oxidation of SO2. We explore this by carrying out a thorough thermodynamic analysis with the ISORROPIA-II model18 for conditions of aerosol- and gas-phase constituents that characterize a broad range of aerosol acidities and drivers thereof. We limit our analysis to fine mode (PM2.5) aerosol, as the majority of the sulfate mass resides in that fraction1,20 (hence its pH being the most relevant for sulfate formation), and which is also the size range where thermodynamic analysis for acidity inference works best2,17,18,21.
Results
To understand the major drivers of aerosol acidity, we explore pH levels for aerosol of increasing chemical complexity, and its sensitivity to NH3 levels found throughout the world; we focus on two well-characterized “extremes” of anthropogenic influence: the relatively clean southeastern US and the heavily polluted regions of Beijing and Xi’an, China. In our analysis, we first focus on the simplest possible composition that is atmospherically relevant: aerosol dominated by NH4 +, HSO4 −/SO4 2−, i.e., where the effects of NO3 −, Cl− or nonvolatile cations (Na+, K+, Ca2+, Mg2+) is negligible. The summertime southeastern US meets this criteria, and was thoroughly studied by Weber, et al.21; the same study predicted that large amounts of NH3, ~ 160 µg m−3 (220 ppbv), is required for equilibrium with a deliquesced ammonium sulfate aerosol. Under such conditions, aerosol pH is equal to 3.2. The pH drops to about 0.1 for aerosol composed of deliquesced ammonium bisulfate, requiring a low gas-phase NH3 level of 0.06 µg m−3 (0.08 ppbv) to be in equilibrium. The transition from NH4HSO4 to (NH4)2SO4 aerosol increases equilibrium NH3 by 2700 times and aerosol acidity by roughly 3 pH units, regardless of SO4 2− level in the range of 0.1–10 µg m−3. Expanding the thermodynamic analysis to include the effects of other minor inorganic constituents and organic water found in the southeastern US aerosol do not change this finding; hence a 10-fold increase in NH3 increases aerosol pH by about one unit over a wide range of ambient NH3 and SO4 2− concentration (0.1–10 µg m−3)21.
For a more chemically-complex aerosol, where pH is controlled by the NH4 +, HSO4 −/SO4 2− and NO3 − system (wintertime Beijing and Xi’an meet this criteria;8,9), co-condensation of gas-phase NH3 occurs with HNO3 to form NH4NO3 aerosol if the ambient temperature is low enough and sufficient liquid water content is present18. This co-condensation also reduces the concentration of hydronium ions in the aerosol aqueous phase (i.e., increases pH) because the salts formed are less acidic than sulfate, and the additional condensed aerosol water further dilutes the aqueous phase2,17. The response of pH to NH3 in this more complex aerosol may differ from the simpler NH4 +, HSO4 −/SO4 2 system discussed above. To study this, we carry out pH calculations for T and RH conditions representative of the eastern US and Beijing during wintertime (~0 °C and 58% RH;2,8) under conditions of “low” (HNO3 + NO3 − = 2.2 µg m−3, characteristic of eastern US), and “high” (HNO3 + NO3 − = 26 µg m−3; characteristic of Beijing haze) total inorganic nitrate levels. The results of the simulations are shown in Fig. 1(a,b), respectively. Regardless of total NO3 − concentration, at any SO4 2− concentration from 0.1 to 100 µg m−3, a 10-fold increase in NH3 raises pH by one unit over a wide range of NH3 concentrations (0.1 to 1000 µg m−3). In Fig. 1(a), a weak sensitivity of pH to SO4 2− is predicted for SO4 2− above 10 µg m−3, similar to the situation found in the southeastern US in summer21. For this SO4 2− range, SO4 2− mass is high enough to dominate over any effect of NO3 − on water uptake and pH, and maintains aerosol pH at 2.5 or below; for lower SO4 2− concentrations, NO3 − becomes increasingly important (for constant NH3) and pH increases accordingly to levels that may range between 3 and 4.5 for atmospherically-relevant levels of NH3. At higher levels of total nitrate (Fig. 1b), the transition from SO4 2–controlled acidity (pH < 2.5) and NO3 –dominant acidity (pH > 3) occurs at levels above 100 μg m−3 SO4 2−. Therefore, for conditions of modest sulfate and high ammonia and total nitrate levels, acidity in Beijing tends to be reduced compared to the southeastern US and is largely controlled by a “nitrate-dominated” pH level.
Sensitivity of PM1 pH to gas-phase ammonia (NH3) and PM1 sulfate (SO4 2−) concentrations. The results are predictions from a thermodynamic analysis assuming equilibrium between the gas and particle phases for typical winter conditions (RH = 58%, T = 273.1 K) in (a) the eastern United States with low total NO3 − (HNO3 + NO3 −) concentrations, 2.2 µg m−3, and (b) Beijing haze pollution periods with high total NO3 −, 26 µg m−3. Boxes define observed concentration ranges for the Eastern US and Beijing and open symbols represent mean NH3 and SO4 2− conditions. Average total NO3 − for Eastern US, Beijing (BJ) clean, BJ transition, BJ polluted were 2.2, 6.6, 18, 26 μg m−3, respectively. Since total NO3 − during Beijing clean and transition periods were 6.6 µg m−3 and 18 µg m−3, respectively, graph (a) better represents the Beijing clean period and graph (b) better for the Beijing transition period.
Based on the above, the important question on what controls aerosol pH can be seen to be the relative amounts of HNO3 + ΝΟ3 −, SO4, and NH3 + NH4 + of the system considered. The boxes indicated in Fig. 1(a,b) define characteristic areas corresponding to eastern US and Beijing aerosol (similar RH and T in winter); the pH levels inside these boxes then characterize the inherent particle acidity level of each location. The NH3 in the eastern US normally ranges between 0.1 and 2 µg m−3 with some extremes as high as 3–4 µg m−3 according to field measurements22, and the Ammonia Monitoring Network (AMoN, http://nadp.sws.uiuc.edu/amon)23. NH3 levels in Beijing were observed to be much higher, up to 38 µg m−3 (51 ppbv), during a heavy haze event in 2015 (Table S2 in Wang, et al.8). SO4 2− concentration in the same event reached a maximum of 38 µg m−3. The lowest pH is predicted for the eastern US due to the lower NH3 and SO4 2− compared to Beijing in Fig. 1(a,b). However, for a wide range in NH3 and SO4 2−, particle pH for Beijing during clean, transition, and polluted periods are all around 4, and do not exceed 5. Although an extreme maximum of 300 µg m−3 SO4 2− was reported in another wintertime in Beijing in 20139, the weak dependency of pH on SO4 2− (>10 µg m−3) results in a somewhat lower pH but still within the sub-100 µg m−3 SO4 2− ranges discussed above.
The main conclusions derived from Fig. 1 do not change when the thermodynamic analysis is expanded to include a broader temperature range or the small amount of fine mode nonvolatile cations found in each region. This is shown in Fig. 2, which presents the equilibrium particle pH versus ammonia for summertime (T ~20 °C) and wintertime (T ~0 °C) conditions at different locations. Partitioning of NH3 and HNO3 towards particle-phase NH4 + and NO3 − is enhanced in lower temperatures, which as expected tends to increase particle pH. All lines become parallel for >20 µg m−3 NH3, exhibiting a sensitivity of roughly one unit pH unit increase per 10-fold increase in NH3. The slope of the eastern US summertime line (green) is constant throughout the entire NH3 range due to negligible effects of NO3 − or other nonvolatile cations on pH. The lowest range of NH3 and pH (0.9) is also found in the eastern US in summer. Due to the impact of high HNO3 and NO3 − observed in the southwest US, the lines shift to higher pH levels, despite a T, RH, and NH3 range similar to the eastern US. In that case the study mean PM2.5 pH (2.7) is nearly one unit higher than PM1 pH (1.9) owing to nonvolatile cations from seasalt being internally mixed with PM2.5, confirmed by particle mixing states measurements and thermodynamic simulations17. The difference between the southwestern US PM1 (red line) and PM2.5 (orange line) decreases with NH3, as the influence of seasalt on particle pH decreases as more and more ammonium nitrate forms. Biomass burning plumes observed in Greece reached the highest PM1 pH (2.8) from the effects of K+ and NH3 co-condensation with HNO3 24,25 and the corresponding sensitivity line (yellow) converges with the southwestern US. Some extreme concentrations of NH3 (e.g. 10 µg m−3) in the US would increase pH to 3.5 in summer conditions. In winter conditions, although the eastern US line (purple) is very close to the Beijing lines (blue) and Xi’an polluted (black) line, the actual pH is much lower in the eastern US due to a tenfold or more lower NH3 concentration (on the level of 0.10 µg m−3); by comparison, Beijing observed on average NH3 4.8 µg m−3 and 12.8 µg m−3 during clean and polluted periods respectively, and Xi’an observed even higher NH3 levels at 9.0 µg m−3 and 17.3 µg m−3 for clean and polluted periods. Owing to the high levels of NH3, the PM1 pH of Beijing is predicted to be 4.2 regardless of the air quality condition (clean or polluted), and the PM2.5 pH of Xi’an are predicted to be 4.6 and 5.4. The highest pH in Xi’an is caused by a large fraction of nonvolatile cations (Na+, Ca2+, K+, Mg2+; 31% to total aerosol ions by moles); given however that Xi’an data corresponds to PM2.5, and that the mixing state between the PM1 and PM2.5 can cause pH to vary up to 3 units1, it is likely that the aerosol pH in Xi’an exhibits a strong size-dependence that is not reflected in a simple bulk measurement and thermodynamic analysis used here. The maximum NH3 in Beijing and Xi’an increase pH up to 4.5 and 5.0, respectively, while the maximum NH3 in the southwestern US increases pH up to 3.3 in the summertime.
Equilibrium particle pH versus a wide range of ammonia (NH3) based on average aerosol and meteorological conditions (RH, T) at each site. The open symbols are the study mean pH and NH3, and shaded backgrounds show the upper limit of the pH range for each study (shading color matches color of study line given in the legend). Note that Xi’an polluted and WINTER PM1 lines overlap showing inherent consistency between the two (also true for Beijing). For the WINTER study (the only aircraft data shown), the point represents a predicted NH3 level 0.1 μg m−3 (pH = 2), whereas the reported campaign average pH (0.8 ± 1.0) is lower due to lower pH aloft (2).
Implications for sulfate formation mechanism
The sensitivity of pH to NH3 is found to be similar between China and eastern US, despite the 10-fold or higher mass loadings of aerosols and gases of the former during intense haze pollution events. We show that for a given set of meteorological conditions (temperature and RH), roughly a 10-fold decrease in NH3 concentrations is required to drop pH levels by one unit, revealing an inherent consistency between vastly different aerosol systems. The pH levels between the eastern US, Beijing and Xi’an can indeed be related to the inherently different concentrations of NH3 found in each environment. The average pH of Beijing PM1 is predicted to be 4.2 (the same in clean and polluted periods), and the highest pH is about 4.5 for the maximum NH3 levels observed. Nonvolatile cations do not appear to considerably affect PM2.5 pH at Xi’an (12% mole fraction to total ions for the polluted period) compared to Beijing PM1, except when these cations become a large fraction of PM2.5 (31% mole fraction found during the clean period). Overall, Xi’an PM2.5 may reach a slightly higher maximum pH (5.0) than Beijing, due to even higher NH3 levels than Beijing. However, for all the pH ranges we find, none are in the range to provide consistent and sufficient alkalinity for the NO2 oxidation pathway to overwhelm sulfate formation (Fig. 3) based on the model of Cheng, et al.9. Given this, and that most of the sulfate forms where particles are most acidic (PM1 or PM2.5), it is unlikely that NO2-mediated oxidation of SO2 is a major SO4 2− formation route. Under conditions where alkalinity is sufficient to promote NO2 oxidation, it does not form due to the large amounts of NH3, but rather only from the presence of nonvolatile cations, such as those found in mineral dust and seasalt and associated carbonates that maintain pH at levels above 6. Because these species are generally limited to particles sizes larger than 1 µm diameter1, this route is highly unlikely to contribute to PM1 sulfate production, including in Beijing20.
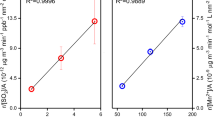
Aqueous-phase sulfate production by sulfur dioxide oxidation under characteristic conditions adapted from Cheng, et al.9 and plotted with pH ranges calculated in this study. Lines represent sulfate production rates calculated for different aqueous-phase reaction pathways with oxidants: hydrogen peroxide (H2O2), ozone (O3), transition metal ions (TMIs), and nitrogen dioxide (NO2). The gray-shaded area indicates characteristic pH ranges for aerosols during severe haze episodes in Beijing, calculated in this study. These conditions are contrasted to the lower pH of eastern US aerosol. The plot shows the NO2 pathway (red line) is not the main route for sulfate production.
The mildly acidic levels associated with excessive amounts of ammonia, however, could promote high rates of oxidation through transition metal chemistry, which overwhelms all other oxidation pathways for pH levels up to 4.5 (Fig. 3). The observed high levels of soluble transition metals that coincide with sulfate at the particle level in the PM2.5 range in US urban air masses1 and polluted air masses sampled off the coast of China24 supports that this may be an important pathway for explaining the high sulfate production rates, provided that the aerosol pH persists at the levels predicted here for sufficient time for the slow acid dissolution process of recalcitrant species such as iron6. Our analysis shows that aerosol with neutral pH is highly unlikely to be driven by excessive amounts of NH3; measurements of gas-phase and PM1/PM2.5 aerosol composition at rapid temporal resolution however are still required to show the frequency at which pH exceeds 4.5 during peak haze events, and whether it is possible to approach or exceed the pH 7.6 level in Beijing reported by Wang, et al.8. Our analysis also suggests this may not be likely, but measurements of size-resolved aerosol composition (including soluble transition metals) and gas-phase constituents at sufficient temporal resolution will provide the definitive observational constraints. We have shown that increasing NH3 does not lead to a substantially more neutral aerosol, minimizing the importance of a proposed SO2-NOx sulfate formation route. An alternative explanation for the recent China winter haze events is changes in weather patterns that have strengthened stagnation conditions26.
Methods
pH affects the equilibrated partitioning of semi-volatile compounds, such as NO3 − and NH4 +, between gas- and particle-phase. Based on this sensitivity, the current most reliable method for fine particle pH is via prediction through a thermodynamic model, such as ISORROPIA-II, with gas- and particle-phase concentrations, and meteorological conditions (RH&T) as model input. ISORROPIA-II computes the equilibrium composition of an NH4 +-SO4 2–-NO3 –-Cl–-Na+-Ca2+-K+-Mg2+-water inorganic aerosol (available online at: http://isorropia.eas.gatech.edu)19,27.
pH is defined as the hydrogen ion activity in an aqueous solution28, where \({{\gamma }}_{{{H}}^{+}}\) is the hydronium ion activity coefficient (assumed as 1; discussed further below), \({{H}}_{\mathrm{aq}}^{+}\) (mole L−1) is the hydronium ion mole fraction in particle liquid water, \({{H}}_{{air}}^{+}\) (µg m−3) is the hydronium ion concentration per volume of air, and \({{W}}_{{i}}\) and \({{W}}_{{o}}\) (µg m−3) are the bulk particle water concentrations associated with inorganic and organic species, respectively. \({{W}}_{{o}}\) needs to be calculated independently by Equation (5) in Guo, et al.18, while both \({{H}}_{{air}}^{+}\) and \({{W}}_{{i}}\) are the outputs of ISORROPIA-II. Particle liquid water (\({{W}}_{{i}}\)+\({{W}}_{{o}}\)), which is essential for pH calculation, is well predicted compared to the measurement18. Due to a small bias between 0 and −0.2 pH often found without considering \({{W}}_{{o}}\) in the pH calculation (the logarithmic nature of pH)2,17,18, in this study we only calculate pH based on \({{W}}_{{i}}\), a reasonable assumption given the lower organic mass fraction reported in Beijing (on average 20–60%)8,9 compared to the southeastern US (on average 60%)29 resulting in an even smaller effect of organic particle water on pH.
ISORROPIA-II assumes \({{\gamma }}_{{{H}}^{+}}\) as unity, however, the activity coefficients of the other water-soluble ions are calculated as ionic pairs (including H+, e.g. H+-NO3 −). The pH calculated from this method is proven to be similar to models that specifically calculate \({{\gamma }}_{{{H}}^{+}}\), such as E-AIM30, and observed and predicted gas-particle partitioning of semivolatile species are in good agreement2,17. We note that it is difficult to retrieve activity coefficients in concentrated aqueous solutions. The ISORROPIA-II has been tested by several ambient particle datasets with strong ionic strength, for example, the mean ionic strength 38 mole L−1 in the eastern US2. The ionic strength in Beijing haze polluted period (36 mole L−1) is on the same magnitude despite the much higher particle mass loadings (i.e. more particle water).
Details on how the model was run (e.g., forward mode, metastable aerosols), an extensive uncertainty analyses, and predictions of pH at various sites in the southeastern US are discussed in Guo, et al.18. The pH predictions are accurate to a high degree based on the consistency between the predicted and measured partitioning of NH3-NH4 + or HNO3-NO3 − examined in a number of studies in various locations from summer to winter conditions2,17,18,21,24,25,30. The thermodynamic model results are further supported by a single pair of semivolatile partitioning calculation, which appears as “S curves” and are thoroughly discussed in the section 3.6 of Guo, et al.2 and in the section 4.2 of Guo, et al.17, respectively. In applying ISORROPIA-II, we assumed no compositional dependence on particle size, treating the measured chemical constituents as bulk PM1 or PM2.5 properties, and that the aerosol (NH4 +, SO4 2−, NO3 −) was internally mixed and composed of a single aqueous phase that contained the inorganic species, without phase separations that could affect pH (along with partitioning of semi-volatile inorganic species). In Beijing and Xi’an, the large amounts of nitrates present in the aerosol (which exhibit very low efflorescence relative humidity) and other dissolved electrolytes and organics that further depress crystallization7 strongly favor the presence of a single aqueous phase. pH calculated under these assumptions (bulk properties, no phase separations, dissolved components in equilibrium with the gas phase) is supported by the ability of ISORROPIA-II to reproduce independently measured gas-and particle-phase semivolatiles concentrations (e.g. NH3, HNO3, HCl). It should be noted that Wang, et al.8 heavily relied on the usage of aerosol molar ratios as a proxy of acidity, which have been shown to not represent pH well2,18,21,30. pH levels reported in that study were carried out with ISORROPIA-II but in stable mode and were evaluated only by predicted equilibrium NH3 levels by the model. Evaluation of model pH based on predicted NH3 (or HNO3) alone is insufficient because gas-phase predictions are insensitive to pH errors (Fig. S1 in the supplemental material; also shown as Fig. S3 in Guo, et al.2 for HNO3). Aerosol-phase concentrations, such as NH4 +, NO3 −, Cl−, are however sensitive to the assumption of phase state assumed by ISORROPIA-II and should be used for evaluation purposes (Fig. S1), which were not carried out by Wang, et al.8. When carrying out such an evaluation (Fig. S1), the metastable option reproduces aerosol NH4 +, NO3 −, Cl− considerably better than assuming a stable aerosol, hence pH calculations from the metastable option of the model are more consistent with observed thermodynamic partitioning, hence used here. Comparing measured and predicted particle-phase fractions (e.g. ε(NH4 +) = NH4 +/(NH4 + + NH3)) provides a means for evaluation of the predicted pH. Cheng, et al.9 also carried out estimates of aerosol pH using ISORROPIA-II with the assumption of metastable aerosol, but a combination of forward and reverse-mode calculations were used; the strong dependence of pH with size1 and the extreme sensitivity of ammonia equilibrium vapor pressure to small errors in aerosol NH4 + when pH approaches neutral conditions30 also makes pH assessments that utilize reverse-mode calculations subject to considerable uncertainty.
The approach for generating the contour plots of Fig. 1 is as follows. Average RH, T, and total NO3 − (HNO3 + NO3 −) for the eastern US or Beijing in wintertime, along with a selected sulfate concentration, are input to ISORROPIA-II. Total NH4 + (NH3 + NH4 +) is left as the free variable. The equilibrium concentrations of various components (e.g., gas-phase NH3, and particle-phase NH4 +, SO4 2−, and NO3 −) and particle pH (along with other variables) are predicted by ISORROPIA-II. Data for the contour plots are generated by varying sulfate from 0.1 to 100 µg m−3 while equilibrated NH3 covers from a wide range between 0.1 and 1000 µg m−3 (0.13–1333 ppbv at STP). The calculation of the sensitivity lines in Fig. 2 utilizes a simpler approach than the above due to fixed sulfate concentration at the reported campaign averages, which can be found in the supplemental material Table S1.
References
Fang, T. et al. Highly Acidic Ambient Particles, Soluble Metals, and Oxidative Potential: A Link between Sulfate and Aerosol Toxicity. Environmental science & technology 51, 2611–2620, https://doi.org/10.1021/acs.est.6b06151 (2017).
Guo, H. et al. Fine particle pH and the partitioning of nitric acid during winter in the northeastern United States. Journal of Geophysical Research: Atmospheres 121, 10355–10376, https://doi.org/10.1002/2016jd025311 (2016).
Longo, A. F. et al. Influence of Atmospheric Processes on the Solubility and Composition of Iron in Saharan Dust. Environmental science & technology 50, 6912–6920, https://doi.org/10.1021/acs.est.6b02605 (2016).
Gwynn, R. C., Burnett, R. T. & Thurston, G. D. A time-series analysis of acidic particulate matter and daily mortality and morbidity in the Buffalo, New York, region. Environmental Health Perspectives 108, 125–133 (2000).
Jang, M., Czoschke, N. M., Lee, S. & Kamens, R. M. Heterogeneous atmospheric aerosol production by acid-catalyzed particle-phase reactions. Science 298, 814–817, https://doi.org/10.1126/science.1075798 (2002).
Meskhidze, N., Chameides, W. L., Nenes, A. & Chen, G. Iron mobilization in mineral dust: Can anthropogenic SO2 emissions affect ocean productivity? Geophysical Research Letters 30, 2085, https://doi.org/10.1029/2003gl018035 (2003).
Seinfeld, J. H. & Pandis, S. N. Atmospheric Chemistry and Physics: from Air Pollution to Climate Change 2nd Edition. (John Wiley & Sons, Inc., 2006).
Wang, G. et al. Persistent sulfate formation from London Fog to Chinese haze. Proceedings of the National Academy of Sciences of the United States of America 113, 13630–13635, https://doi.org/10.1073/pnas.1616540113 (2016).
Cheng, Y. et al. Reactive nitrogen chemistry in aerosol water as a source of sulfate during haze events in China. Science Advances 2, e1601530, https://doi.org/10.1126/sciadv.1601530 (2016).
Katoshevski, D., Nenes, A. & Seinfeld, J. H. A study of processes that govern the maintenance of aerosols in the marine boundary layer. Journal of Aerosol Science 30, 503–532, https://doi.org/10.1016/S0021-8502(98)00740-X (1999).
Stockdale, A. et al. Understanding the nature of atmospheric acid processing of mineral dusts in supplying bioavailable phosphorus to the oceans. Proceedings of the National Academy of Sciences of the United States of America 113, 14639–14644, https://doi.org/10.1073/pnas.1608136113 (2016).
Hanisch, F. & Crowley, J. N. The heterogeneous reactivity of gaseous nitric acid on authentic mineral dust samples, and on individual mineral and clay mineral components. Physical Chemistry Chemical Physics 3, 2474–2482, https://doi.org/10.1039/b101700o (2001).
Meskhidze, N., Chameides, W. L. & Nenes, A. Dust and pollution: A recipe for enhanced ocean fertilization? Journal of Geophysical Research 110, D03301, https://doi.org/10.1029/2004jd005082 (2005).
Fountoukis, C. et al. Thermodynamic characterization of Mexico City aerosol during MILAGRO 2006. Atmospheric Chemistry and Physics 9, 2141–2156, https://doi.org/10.5194/acp-9-2141-2009 (2009).
Cruz, C. N., Dassios, K. G. & Pandis, S. N. The effect of dioctyl phthalate films on the ammonium nitrate aerosol evaporation rate. Atmospheric Environment 34, 3897–3905, https://doi.org/10.1016/S1352-2310(00)00173-4 (2000).
Dassios, K. G. & Pandis, S. N. The mass accommodation coefficient of ammonium nitrate aerosol. Atmospheric Environment 33, 2993–3003, https://doi.org/10.1016/S1352-2310(99)00079-5 (1999).
Guo, H. et al. Fine particle pH and gas–particle phase partitioning of inorganic species in Pasadena, California, during the 2010 CalNex campaign. Atmospheric Chemistry and Physics 17, 5703–5719, https://doi.org/10.5194/acp-17-5703-2017 (2017).
Guo, H. et al. Fine-particle water and pH in the southeastern United States. Atmospheric Chemistry and Physics 15, 5211–5228, https://doi.org/10.5194/acp-15-5211-2015 (2015).
Fountoukis, C. & Nenes, A. ISORROPIA II: a computationally efficient thermodynamic equilibrium model for K+-Ca2+-Mg2+-NH4 +-Na+-SO4 2−NO3 −Cl−H2O aerosols. Atmospheric Chemistry and Physics 7, 4639–4659, https://doi.org/10.5194/acp-7-4639-2007 (2007).
Tian, S., Pan, Y., Liu, Z., Wen, T. & Wang, Y. Size-resolved aerosol chemical analysis of extreme haze pollution events during early 2013 in urban Beijing, China. Journal of Hazardous Materials 279, 452–460, https://doi.org/10.1016/j.jhazmat.2014.07.023 (2014).
Weber, R. J., Guo, H., Russell, A. G. & Nenes, A. High aerosol acidity despite declining atmospheric sulfate concentrations over the past 15 years. Nature Geoscience 9, 282–285, https://doi.org/10.1038/ngeo2665 (2016).
You, Y. et al. Atmospheric amines and ammonia measured with a chemical ionization mass spectrometer (CIMS). Atmospheric Chemistry and Physics 14, 12181–12194, https://doi.org/10.5194/acp-14-12181-2014 (2014).
Puchalski, M. A. et al. A statistical comparison of active and passive ammonia measurements collected at Clean Air Status and Trends Network (CASTNET) sites. Environmental Science Processes & Impacts 17, 358–369, https://doi.org/10.1039/c4em00531g (2015).
Bougiatioti, A. et al. The unappreciated effects of biomass burning on fine mode aerosol acidity, water and nitrogen partitioning. In review (2017).
Bougiatioti, A. et al. Particle water and pH in the Eastern Mediterranean: Sources variability and implications for nutrients availability. Atmospheric Chemistry and Physics 16, 4579–4591, https://doi.org/10.5194/acp-16-4579-2016 (2016).
Zou, Y., Wang, Y., Zhang, Y. & Koo, J. H. Arctic sea ice, Eurasia snow, and extreme winter haze in China. Science Advances 3, e1602751, https://doi.org/10.1126/sciadv.1602751 (2017).
Nenes, A., Pandis, S. N. & Pilinis, C. ISORROPIA: A new thermodynamic equilibrium model for multiphase multicomponent inorganic aerosols. Aquat Geochem 4, 123–152, https://doi.org/10.1023/A:1009604003981 (1998).
Stumm, W. & Morgan, J. J. Aquatic Chemistry: Chemical Equilibria and Rates in Natural Waters 3rd Edition. (John Wiley & Sons, Inc., 1996).
Xu, L. et al. Effects of anthropogenic emissions on aerosol formation from isoprene and monoterpenes in the southeastern United States. Proceedings of the National Academy of Sciences of the United States of America 112, 37–42, https://doi.org/10.1073/pnas.1417609112 (2015).
Hennigan, C. J., Izumi, J., Sullivan, A. P., Weber, R. J. & Nenes, A. A critical evaluation of proxy methods used to estimate the acidity of atmospheric particles. Atmospheric Chemistry and Physics 15, 2775–2790, https://doi.org/10.5194/acp-15-2775-2015 (2015).
Acknowledgements
This work was supported by the National Science Foundation (NSF) under grant AGS-1360730. AN acknowledges support from a Johnson Faculty Fellowship.
Author information
Authors and Affiliations
Contributions
H.G., A.N., R.W. conceived the study and wrote the manuscript. H.G. and A.N. carried out calculations and H.G. prepared the figures.
Corresponding authors
Ethics declarations
Competing Interests
The authors declare that they have no competing interests.
Additional information
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Guo, H., Weber, R.J. & Nenes, A. High levels of ammonia do not raise fine particle pH sufficiently to yield nitrogen oxide-dominated sulfate production. Sci Rep 7, 12109 (2017). https://doi.org/10.1038/s41598-017-11704-0
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-017-11704-0
This article is cited by
-
Effect of NOX, O3 and NH3 on sulfur isotope composition during heterogeneous oxidation of SO2: a laboratory investigation
Air Quality, Atmosphere & Health (2024)
-
Sources, Variations, and Effects on Air Quality of Atmospheric Ammonia
Current Pollution Reports (2024)
-
Study on the assimilation of the sulphate reaction rates based on WRF-Chem/DART
Science China Earth Sciences (2023)
-
Dust emission reduction enhanced gas-to-particle conversion of ammonia in the North China Plain
Nature Communications (2022)
-
Formation and evolution of secondary particulate matter during heavy haze pollution episodes in winter in a severe cold climate region of Northeast China
Environmental Science and Pollution Research (2022)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.