Abstract
Impaired hearing and cognition are disabling conditions among older adults. Research has presented inconsistent conclusions regarding hearing impairment posing a risk for cognitive impairment. We aimed to assess this from published evidence via searching PubMed and Embase, from the inception of the databases indexed to December 2, 2016. For those high-quality studies retrieved, relative risk (RR) and 95% confidence intervals (CIs) were combined to estimate the risk of cognitive impairment. Eleven cohort studies were included in the present study. Pooled results found that elderly people with disabled peripheral and central hearing function had a higher risk of cognitive impairment (for moderate/severe peripheral hearing impairment: RR = 1.29, 95% CI: 1.04–1.59 during a follow-up ≤6 years. RR = 1.57, 95% CI: 1.13–2.20 during a follow-up >6 years; for severe central hearing impairment, RR = 3.21, 95% CI: 1.19–8.69) compared to those with normal hearing function. We also recorded a dose-response trend for cognitive impairment as hearing thresholds rose. No evident bias from potential confounding factors was found with one exception: the length for clinical follow-up. Although results are preliminary because qualifying studies were few, statistical findings were consistent with older people identified as having greater levels of hearing loss, having a corresponding higher risk of cognitive impairment.
Similar content being viewed by others
Introduction
Age-related hearing loss (ARHL), a hearing impairment caused by aging and neurodegeneration, is characterized in older adults as difficulty in understanding speech and detecting sound1. Depending upon the auditory pathways involved, ARHL can be categorized as peripheral ARHL or central ARHL, and the clinical manifestations are often mixed2. ARHL has become a major sensory condition, within the world’s rapidly growing aging population1. American prevalence rates showed that hearing impairment affected 29.3% of the population at age 60 to 69 years3 and the percentage was increased to 63.1% in people aged 70 years or over4. Surveys from other countries also revealed the leading role of ARHL among disabling conditions associated with older demographics5,6. Apart from reduced hearing sensitivity and speech understanding, ARHL has a series of consequences including reduced ability to detect and localize safety and/or warning alarms1, and compromised communication efficiency linked to comorbid psychosocial issues such as social isolation and depression7. Although there are numerous rehabilitative alternatives for ARHL, patients and their families may not always seek such options; therefore, the condition is largely underestimated, especially during an early stage of ARHL2. Patients generally fail to obtain sufficient screening and intervention probably because they regard ARHL as simply a part of entering their senior years, unaware of its potentially far-reaching consequences1,8.
However, ARHL may be widely associated with neurodegenerative, functional, physical, and psychosocial impairment9. For instance, ARHL serves as one of the substantial markers of frailty (a nonspecific state of vulnerability, decreased physiological reserve, and reduced resistance to stressors10) in older age with adverse outcomes like cognitive impairment7. Cognitive impairment affects many domains such as: memory, attention, executive function, perception, and semantic knowledge, which also constitute some of the primary targets of dementia and Alzheimer’s disease (AD)11. Epidemiological evidence supports the high possibility of cognitive impairment progressing into dementia and AD12, both of which have rapidly-increasing prevalence with age and the absence of disease-modifying treatment2,8,13. Prevention of dementia and AD has become a public health priority due to its irreversibility and its burden upon individuals, families, and society14.
An increasing number of findings have suggested an association between ARHL and cognitive impairment, indicating that ARHL may be a potential early marker of AD2,7. Thus, collecting evidence from observational studies could be an important initiative to assess whether ARHL serves as a modifiable factor among the strategies of preventing dementia. An initial review appeared to provide some support for the relationship between ARHL and cognitive impairment; however, it also identified some studies which argued against this correlation15,16,17,18,19. More recent studies14,20,21,22 have emerged. They applied more recognized measures to evaluate the auditory and mental status of various populations. However, research with negative results23,24 has prevented us from definitively concluding that hearing function is connected to the risk of cognitive impairment during the later life of adults.
Previous systematic reviews and meta-analyses25,26 have offered some perspectives demonstrating that hearing impairment and cognitive problems are associated. To our knowledge, the first meta-analysis to explore hearing loss and cognitive function25, without putting restrictions on age or methodology when retrieving studies, concluded that individuals with hearing loss had worse cognitive performance. A recent study26 gathered some prospective cohort studies to support the conclusion that hearing impairment increases the risk of both cognitive disorders and AD, though it analyzed only four heterogeneous studies. Categories of hearing function and possible confounding factors were not explored in either study.
The present meta-analysis of cohort studies was undertaken to explore research inconsistencies regarding ARHL and cognitive impairment, statistically. By including more recent data, we appraised the hearing function-cognition relationship in older adults and its dose-response trend with more participants. We also incorporated both peripheral and central hearing function as independent variables to carry out an analysis for the risk of cognitive impairment by category. Additionally, the present study considered potential confounding factors such as race and sex in meta-regression because, for example, melanin and estrogen may work in the pathogenesis of ARHL27,28. Follow-up durations were considered because both ARHL and cognitive impairment are conditions that deteriorate with age2. Apart from the hearing function-cognition relationship, we also analyzed the initial findings from these retrieved studies about the effect of hearing aid use on the incidents of cognitive impairment.
Results
Study selection, characteristics and quality assessment
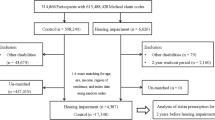
Figure 1 presents the flow chart used for determining eligible research. Through databases and manual searching, 973 studies were found after subtracting duplicates. Investigators next went through titles and abstracts to exclude the studies that had missing data, a small sample size (n < 100), a mean age at baseline below 60 years, or studies that were non-observational and not consistent with our aim. Full text of the remaining 111 were further analyzed. Of these, those with estimates that could not be combined, that were not cohort-designed, and those which used estimates other than odds ratio (OR), relative risk (RR), and hazard ratio (HR), were also omitted at this stage. Finally, eleven studies9,14,20,21,24,29,30,31,32,33,34 were included, seven of which9,14,20,24,29,30,31 were combined in the following meta-analyses. Eight studies20,21,24,29,31,32,33,34 were analyzed in meta-regression.
Table 1 summarizes studies that were selected for analysis. Eleven related studies were enrolled into the present study. All prospective cohort studies included participants across Europe32, North America14,20,21,29,30,31,33, Oceania9,24, and Asia34. All cohorts, except for one21, included both genders. Some studies evaluated hearing function with insurance records32 and self-reporting33,34, and the rest used pure-tone audiometry. Five cohorts were followed-up ≤6 years20,21,24,31,32. Cognitive status was qualified or quantified by the Mini-Mental State Examination (MMSE) and its revised version9,20,21,24,29,31,33,34, or the Diagnostic and Statistical Manual (DSM) and the National Institute of Neurological and Communicative Disorders and Stroke and the Alzheimer’s Disease and Related Disorders Association (NINCDS-ADRDA)14,30,33. All studies were multi-adjusted of covariates with three exceptions24,31,34. Quality assessment is presented in Supplementary Table S1 and all included studies scored more than five stars on the Newcastle-Ottawa Scale (NOS).
Overall Results
Figure 2 shows the cumulative risk for cognitive impairment from peripheral hearing functioning of the better ear at baseline. When the hearing threshold was greater than 40 decibels hearing level (dB HL) for the pure-tone average (PTA) at 0.5, 1, 2, and 4 kHz (subjects identified as having moderate/severe hearing impairment29), the risk of cognitive impairment in older subjects increased 29–57% compared to those with normal hearing (follow-up ≤6 years, RR = 1.29, 95% confidence interval (CI): 1.04–1.59; follow-up >6 years, RR = 1.57, 95% CI: 1.13–2.20, Fig. 2a). Older people were also at a risk of cognitive impairment when the hearing level was abnormal (subjects with PTA > 25 dB HL identified to have hearing impairment35; RR = 1.29, 95% CI: 1.12–1.50, Fig. 2b). In seniors, the estimated incidence of cognitive impairment had a 12% increase when PTA was modeled continuously (for every 10 dB increase in hearing loss, RR = 1.12, 95% CI: 1.04–1.22, Fig. 2c). No significant heterogeneity was seen among the studies except in Fig. 2c (in subgroup of follow-up ≤6 years, I2 = 0%, P = 0.45, in subgroup of follow-up >6 years, I2 = 0%, P = 0.71, Fig. 2a; I2 = 0%, P = 0.38, Fig. 2b; I2 = 45%, P = 0.16, Fig. 2c).
Forest plot showing the risk of incident cognitive impairment from peripheral auditory function. (a) Pooled relative risk from moderate/severe hearing impairment (PTA >40 dB HL). (b) Pooled relative risk from hearing impairment (PTA >25 dB HL). (c) Pooled relative risk per 10 dB of hearing loss. CI = confidence interval, dB HL = decibels hearing level, IV = inverse variance, PTA = pure-tone average, SE = standard error.
Figure 3 demonstrates the pooled RR of cognitive impairment when participants showed abnormality in one of the central auditory processing (CAP) tests at baseline. Here, central auditory function was obtained by Synthetic Sentence Identification with Ipsilateral Competing Message (SSI-ICM). SSI-ICM <80% correct is considered consistent with central auditory dysfunction (CAD) based on this test’s normative data30,31. The RR for incidence of cognitive impairment in older people was 2.42 in the moderate CAD group compared with the normal function group (SSI-ICM < 80% correct, RR = 2.42, 95% CI: 1.14–5.11, Fig. 3a). When CAP was severely abnormal on the SSI-ICM test (<50% correct), the risk elevated to 3.21 (RR = 3.21, 95% CI: 1.19–8.69, Fig. 3b). No obvious heterogeneity was detected among these studies (I2 = 0%, P = 0.91, Fig. 3a; I2 = 18%, P = 0.27, Fig. 3b).
Forest plot showing the risk of incident cognitive impairment from one central auditory processing test. Combined relative risk from (a) moderate impaired central auditory processing (SSI-ICM <80% correct) and (b) severe impaired central auditory processing (SSI-ICM <50% correct). CI = confidence interval, IV = inverse variance, SE = standard error, SSI-ICM = Synthetic Sentence Identification with Ipsilateral Competing Message.
Figure 4 demonstrates that we recorded no significant reduction in the risk for cognitive impairment among people who were using hearing aids (RR = 0.85, 95% CI: 0.66–1.10). There was no evidence of heterogeneity in this analysis (I2 = 0%, P = 0.89).
Subgroup and Sensitivity Analysis
Table 2 provides a dose-response association between peripheral hearing loss and cognitive impairment concluded from 3 studies14,20,29. Analyses were performed with groups stratified by the severity of hearing loss, evaluated with PTA results of thresholds in the better ear (Categories of ≤25 dB HL, 26–40 dB HL, 41–70 dB HL and >70 dB HL mean normal, mild, moderate, and severe hearing loss, respectively). Relative risk from the studies was combined with the risk ranging from 1.19 (95% CI: 0.96–1.48) to 4.94 (95% CI: 1.09–22.40) across three hearing loss categories.
Table 3 suggests the influence of potential confounding factors while assessing the relationship between peripheral hearing loss and cognitive impairment with meta-regression. We recorded no evident bias arising from the ear sides associated with PTA results (better ear, worse ear, one ear, two ears, or nonspecific), ethnicity (mixed cohorts, Black-excluded cohorts, or Asian cohorts), gender (mixed cohorts, male cohorts, or female cohorts), level of adjustment (confounding factors being fully adjusted, partially adjusted, or not adjusted), subjectivity of the hearing measurement (pure-tone audiometry versus self-report or others), and instruments for cognitive status (MMSE or related versions versus others) with one exception: maximum time to follow-up (when cohorts were divided into subgroups that had been followed-up ≤6 years or followed-up >6 years, follow-up durations were a likely source of confounding when analyzing peripheral hearing loss and cognitive impairment, P = 0.004). The data for meta-regression were gathered in Supplementary Table S2.
Table 4 shows the sensitivity analysis we have performed. Overall estimates remained relatively unchanged when a single study was omitted sequentially in the meta-analysis. A heterogeneity change in P heterogeneity and I2 was observed when evaluating the risk of cognitive impairment for every 10 dB increase in hearing loss.
Publication Bias
Publication bias analysis was not applied here due to a limited number of qualifying studies.
Discussion
Our major findings showed that, after statistical analyses, both peripheral and central hearing dysfunction appear to contribute to the risk of cognitive impairment in the aging population. The overall risk of cognitive impairment increased 29% (follow-up ≤6 years) or 57% (follow-up >6 years) in senior participants who had disabled peripheral hearing function compared with people of normal hearing function. The risk remained significant when adopting the World Health Organization (WHO) standard of hearing impairment or when PTA was modeled continuously. The analysis showed that the association between central hearing dysfunction and cognitive impairment was stronger (RR = 3.21, 95% CI: 1.19–8.69 for SSI-ICM <50% correct; RR = 2.42, 95% CI: 1.14–5.11 for SSI-ICM <80% correct). A dose-response trend was found between peripheral hearing function and cognition. There was no obvious heterogeneity among most studies, excluding the analysis combining RR for every 10 dB increase in hearing loss (Fig. 2c). After analyzing potential confounding factors including: the ear sides associated with PTA results, ethnicity, gender of cohorts, level of adjustment, as well as hearing and cognitive evaluations, the risk of cognitive impairment remained. Specifically, a maximum follow-up dependent association was found in the meta-regression and the subgroup analysis. However, using hearing aids did not significantly lower the risk of cognitive impairment in elderly people.
Previous meta-analyses25,26 had collected encouraging findings of the hearing function-cognition relationship, as it applied to cognition domains or the onset of AD. The first meta-analysis25 found that individuals with hearing loss had poorer cognitive manifestations whether hearing was treated or untreated. However, this conclusion was based on adults from a wide range of age groups, applying varied measures on hearing and cognitive status, and varied statistical methods. Another meta-analysis26 – which gathered limited but robust-appearing data from prospective studies – also chose a generalizing approach to analyze the relationship between hearing loss and AD. These two studies explored neither categories of hearing function nor possible confounding factors.
Our combined estimates were consistent with previous reviews2,7, confirming (by statistical analysis) the hearing function-cognition relationship in older people. In addition, we included more recent high-quality cohort studies and explicitly-classified hearing function as an independent variable. The present study has sought to elucidate research inconsistencies associated with previous studies investigating potential links between ARHL and cognition in the following ways: firstly, there have been unresolved discrepancies regarding whether hearing loss clearly correlates to cognitive decline. This has been preliminarily reconciled after our statistical analysis. We also observed a trend of increased risk for cognitive impairment when extending follow-up. Secondly, CAD symptoms have not been well-considered in the hearing function-cognition relationship, as ARHL has appeared to be regarded in terms of mostly being peripheral hearing dysfunction36. However, from the evidence accumulated here, CAD has been shown to potentially increase the risk of cognitive impairment. Thirdly, we found that there was a dose-response association that more damaged peripheral hearing function contributed to a greater risk of cognitive impairment. Lastly, we statistically analyzed the influence of several potential confounding factors that previous studies did not cover. From the summarized data, we hope that our findings can help others understand the hearing function-cognition connection in older adults, in a clearer way. The possibility that hearing intervention may be a way to delay cognitive impairment could be further explored based on this relationship. This is especially essential because hearing rehabilitative interventions are largely underutilized8,37.
The relationship between hearing function and cognition in older adults has been observed since at least the 1960s. At that time, the first research about this connection found that subjects with organic mental syndrome (deficits in memory and intellect) had a higher prevalence of deafness38. The association has been seen in additional observational studies since then. A preliminary summarizing study19 reviewed existing literature to explore this relationship, despite the varied methods and limited sample sizes those investigations had applied. However, considering at least three opposing views16,17,18 exist within the preliminary study, it is difficult to conclude the presence of a solid correlation. Although the risk from hearing impairment on cognition cannot be addressed by randomized controlled trials (RCT) due to ethical concerns regarding possible harm to participants’ wellbeing (studies could not be designed to deliberately assign “hearing impairment” to the participants), recent well-designed cohort studies14,20,24,29,30,31 have emerged to serve as an alternative to explore the problem as thoroughly as possible. Still, some studies drew negative conclusions about the hearing function-cognition relationship21,32,34. Furthermore, few studies included the tests of central auditory function as part of the hearing evaluations, although CAD is prevalent (central auditory function declined with age in the elderly39, with the prevalence of 23–76.4%40,41); additionally, CAP and cognitive function are able to interact centrally (degraded speech communication in older people is partially influenced by additional central networks, including cognitive processing efforts42).
There are some hypothesized mechanisms that support the hearing function-cognition relationship. The frailty hypothesis states that ARHL is one of the markers of frailty – characterized by vulnerability to stressors – which could exert adverse health outcomes like cognitive frailty through inflammatory, vascular, hormonal, nutritional, and metabolic pathways2,7,43. The peripheral-central impairment hypothesis suggests that poor encoding of sound from the impaired cochleae has severe consequences including: demanding more cognitive resources for auditory perceptual processing, influencing brain structure, and reducing social engagement44,45,46,47,48,49. Moreover, some manifestations of CAD itself could be a sign of cognitive dysfunction30 (this has yet to be fully explored because some findings indicate that results of CAP tests are largely influenced by peripheral hearing function50, while others support the objectivity of the tests51). Additionally, the common factors hypothesis shows that hearing and cognitive disorders are correlated because they share similar neurodegenerative processes resulting from aging, vascular diseases, and oxidative stress8,28,52.
Our findings do have some limitations. Firstly, there were a limited number of the included studies, especially studies discussing CAD, which may make it difficult to show a conclusive connection. There were not enough studies for a publication bias analysis. Of the qualifying CAD-related studies, only two from the same research team were analyzed and only one CAP test was selected without further differential diagnoses to ensure CAD. Secondly, there was little data from cohorts with comprehensive controls including: ethnicity, gender, history of noise exposure, hereditary information, ototoxic drug use, lifestyle, socioeconomic status, and so on. This may impact the present findings because ARHL has been influenced by those factors27,28 although our analysis of some confounding variables did not result in significant heterogeneity.
Thirdly, the measures of cognition varied. Qualifying or quantifying instruments applied by the included studies incorporated the following classifications, diagnostic manuals, or screening tools: MMSE, Modified Mini-Mental State Examination (3MS), International Classification of Diseases (ICD), Cognitive Abilities Screening Instrument (CASI), DSM, and NINCDS-ADRDA. This could lead to potential heterogeneity for example, as screening tools such as MMSE, 3MS, and CASI neither play a role in diagnosing cognitive impairment (they do not have the same dementia definitions as the DSM53,54), nor do they predict mild cognitive impairment ultimately developing into dementia. Also, they cannot exclude cognitive difficulties from causes other than age-related cognitive decline55,56,57,58. Additionally, those cognition screening tools show a possible insufficient validity to find cognitive impairment across populations, compared with neuropsychological test batteries. For example, for ethnicity, MMSE had lower specificity within non-White groups59 despite previous evidence ascribing the ethnic differences to other causes60,61. Similarly, different norms of 3MS between White and Black populations may be worthy of consideration62,63. Therefore, though some studies14,20,29 ensured race was fully adjusted for in the cohorts, to eliminate possible bias, care should be applied when interpreting the cognitive function of the present included participants. Other limitations associated with evaluating cognitive status have included: a lack of studies which have extensively analyzed AD and other types of dementia as the outcomes, plus possible overestimation of cognitive impairment via verbal administration of some instruments and its dependence on preserved hearing function. The latter concern has been raised by previous studies25,64,65, although others20,66 have claimed that verbal administration is not an issue if questions are delivered by experienced examiners in quiet environments. Some studies have attempted to address this by cautiously applying multiple sources of data, and by adjusting total scores to account for any potential confounding aspects associated with sensory problems33. These aspects may make it difficult to reach a comprehensive conclusion until further studies emerge to clarify the field.
Fourthly, some combined studies14,20,29 presented potential factors likely contributing to relative heterogeneity; therefore, this may introduce bias. Lastly, the expected protective effect of hearing aid use was not significant in the results. This is perhaps because the included studies selected individuals from more hearing damaged populations, and there was an absence of variables like hearing aid fitting details, sufficiency in the hearing aid use, and other unmeasured factors20. Therefore, the potential effect of hearing aid use on cognition remains unknown. A well-designed RCT may help to determine the significance of hearing rehabilitation for cognitive decline, and findings from on-going studies67,68,69 will be eagerly awaited.
In conclusion, our findings suggest that older people with peripheral and central hearing impairment have a higher risk of cognitive impairment from a statistical perspective. With the limitations mentioned above, hopefully this preliminary finding will be strengthened by future studies.
Methods
Literature Search
According to the guidelines for the Preferred Reporting Items for Systematic Reviews and Meta-analyses (PRISMA)70 and the Meta-analysis of Observational Studies in Epidemiology (MOOSE)71, we searched the databases of PubMed and Embase from the inception of the databases indexed to December 2, 2016. Free text terms with the meaning of hearing loss without restriction were used: “auditory defect” or “auditory impairment” or “auditory dysfunction” or “deafness” or “deaf” or “hearing damage” or “hearing defect” or “hearing difficulty” or “hearing loss” or “hypacusia” or “hypacusis” or “impaired hearing” or “hearing impairment” or “hearing decline” or “hearing disability” or “poor hearing” or “presbycusis” or “hearing deficit” or “hearing trouble” or “hearing limitation” or “hearing handicap”. Mesh and Emtree words were also searched. The same strategies were applied to identify cognition disorder and dementia (the synonyms used for cognitive impairment and dementia were “cognition disorder” or “cognitive defect” or “cognitive deficit” or “cognitive disability” or “cognitive disorder” or “cognitive dysfunction” or “cognitive impairment” or “dementia” or “cognitive decline” or “cognitive difficulty” or “compromising cognition” or “cognitive compromise” or “cognitive trouble” or “troubled cognition” or “cognitive limitation” or “limited cognition” or “cognition limit” or “amentia” or “Alzheimer disease”). The age filters of the results were limited to middle-aged and aged individuals. For some major reviews2,7,19, we went through the contents and bibliographies, finding the related research by citation searching.
Study selection
Two investigators (J.Y. and Y.S.) separately screened the studies. Duplicates were excluded; studies were then removed for reasons such as: having a sample size of less than 100 (in order to include high-quality studies in reference to a case-control study72,73,74), missing data, mean age at baseline not reaching 60 years, being irrelevant to our aim, and being non-observational studies. Next, the full texts of the remaining studies were reviewed to find: (1) prospective cohort studies; (2) extracted estimates were OR, RR, HR, or with enough data to calculate them, and their 95% CIs (odds ratio: ratio of the odds of cognitive impairment for the hearing impairment group to the odds of cognitive impairment for the control group; relative risk: ratio of hearing loss to cognitive impairment probability and normal hearing function to cognitive impairment probability; hazard ratio: the effect on the cognitive impairment rate of the difference between the hearing loss group and the control group estimated by the Cox hazards model74,75); (3) extracted results were possible to combine and adjusted with covariates. Divergences between the two investigators were discussed and resolved with the whole author group.
Data extraction
We extracted the following characteristics from each study: (1) participants of the study; (2) baseline information of the study; (3) the measures employed to evaluate hearing and cognitive function; (4) adjusted variables; (5) follow-up; (6) adjusted estimates of OR, RR, and HR with 95% CIs. If necessary, authors of some included studies were contacted for more detailed information.
Quality assessment
Quality assessment was made with standards from the Newcastle-Ottawa Scale (NOS)76. Selection (maximum 4 asterisks), comparability (maximum 2 asterisks), and outcome quality (maximum 3 asterisks) from the included studies were calculated. The scales were tabulated in values as the number of asterisks each study had gained. A maximum of 9 asterisks can be given for an individual study. The score for a high-quality study was defined as more than 5 asterisks77.
Two investigators (J.Y. and S.S.) carried out data extraction and quality assessment independently.
Statistical analysis
Studies which had used OR or HR to calculate the incidence of cognitive disorder were pooled as approximate RR because hearing loss resulting in cognitive impairment is a rare event. Meta-analysis was conducted with the inverse variance (IV) method. In the studies including covariates, we extracted the most comprehensively adjusted estimates. Heterogeneity was explored with the Cochrane Q-statistic among studies: Pheterogeneity < 0.10 and I2 > 50% indicating significant heterogeneity78,79,80. In our analyses, we used the random-effect models because there was variation among these studies in characteristics like sample size, follow-up, population characteristics, and definitions for cognitive impairment. We estimated the dose-response trend based on risks calculated by categories of peripheral hearing function. Covariates as potential confounding factors were retrieved and analyzed in groups with meta-regression. A confounding factor was recognized with a meta-regression P < 0.0581. Sensitivity analysis was conducted by the one-study-out method: omitting one study at a time, sequentially, to test the combined RR from the remaining studies so as to see the stableness of each meta-analysis82.
Meta-analysis was performed by Cochrane Review Manager (RevMan, Version 5.3. Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration, 2014) and Stata (MP 14.1, Stata Corp, College Station, TX, USA). Statistical significances were set at P < 0.05 for all the analyses unless otherwise specified.
References
Gates, G. A. & Mills, J. H. Presbycusis. Lancet. 366, 1111–1120 (2005).
Panza, F., Solfrizzi, V. & Logroscino, G. Age-related hearing impairment-a risk factor and frailty marker for dementia and AD. Nat Rev Neurol. 11, 166–175 (2015).
Swenor, B. K., Ramulu, P. Y., Willis, J. R., Friedman, D. & Lin, F. R. The prevalence of concurrent hearing and vision impairment in the United States. JAMA Intern Med. 173, 312–313 (2013).
Lin, F. R., Thorpe, R., Gordon-Salant, S. & Ferrucci, L. Hearing loss prevalence and risk factors among older adults in the United States. J Gerontol A Biol Sci Med Sci. 66, 582–590 (2011).
Chen, H. et al. The contributions of diseases to disability burden among the elderly population in China. J Aging Health. 26, 261–282 (2014).
Homans, N. C. et al. Prevalence of age-related hearing loss, including sex differences, in older adults in a large cohort study. Laryngoscope. 127, 725–730 (2017).
Panza, F. et al. Age-related hearing impairment and frailty in Alzheimer’s disease: Interconnected associations and mechanisms. Front Aging Neurosci. 7, 113 (2015).
Lin, F. R. & Albert, M. Hearing loss and dementia – who is listening? Aging Ment Health. 18, 671–673 (2014).
Karpa, M. J. et al. Associations between hearing impairment and mortality risk in older persons: The Blue Mountains Hearing Study. Ann Epidemiol. 20, 452–459 (2010).
Rodriguez-Manas, L. et al. Searching for an operational definition of frailty: A delphi method based consensus statement: The frailty operative definition-consensus conference project. J Gerontol A Biol Sci Med Sci. 68, 62–67 (2013).
Piirainen, S. et al. Psychosocial stress on neuroinflammation and cognitive dysfunctions in Alzheimer’s disease: The emerging role for microglia? Neurosci Biobehav Rev. 77, 148–164 (2017).
Lopez, O. L. et al. Incidence of dementia in mild cognitive impairment in the cardiovascular health study cognition study. Arch Neurol. 64, 416–420 (2007).
Prince, M. et al. The global prevalence of dementia: A systematic review and metaanalysis. Alzheimers Dement 9, 63–75.e62 (2013).
Lin, F. R. et al. Hearing loss and incident dementia. Arch Neurol 68, 214–220 (2011).
Herbst, K. G. & Humphrey, C. Hearing impairment and mental state in the elderly living at home. Brit Med J. 281, 903–905 (1980).
Thomas, P. D. et al. Hearing acuity in a healthy elderly population: Effects on emotional, cognitive, and social status. J Gerontol. 38, 321–325 (1983).
Jones, D. A., Victor, C. R. & Vetter, N. J. Hearing difficulty and its psychological implications for the elderly. J Epidemiol Community Health. 38, 75–78 (1984).
Vesterager, V., Salomon, G. & Jagd, M. Age-related hearing difficulties. Ii. Psychological and sociological consequences of hearing problems–a controlled study. Audiology. 27, 179–192 (1988).
Gennis, V., Garry, P. J., Haaland, K. Y., Yeo, R. A. & Goodwin, J. S. Hearing and cognition in the elderly. New findings and a review of the literature. Arch Intern Med. 151, 2259–2264 (1991).
Lin, F. R. et al. Hearing loss and cognitive decline in older adults. JAMA Intern Med. 173, 293–299 (2013).
Lin, M. Y. et al. Vision impairment and combined vision and hearing impairment predict cognitive and functional decline in older women. J Am Geriatr Soc. 52, 1996–2002 (2004).
Valentijn, S. A. et al. Change in sensory functioning predicts change in cognitive functioning: Results from a 6-year follow-up in the Maastricht Aging Study. J Am Geriatr Soc. 53, 374–380 (2005).
Anstey, K. J., Luszcz, M. A. & Sanchez, L. Two-year decline in vision but not hearing is associated with memory decline in very old adults in a population-based sample. Gerontology. 47, 289–293 (2001).
Hong, T., Mitchell, P., Burlutsky, G., Liew, G. & Wang, J. J. Visual impairment, hearing loss and cognitive function in an older population: Longitudinal findings from the Blue Mountains Eye Study. PLoS One. 11, e0147646 (2016).
Taljaard, D. S., Olaithe, M., Brennan-Jones, C. G., Eikelboom, R. H. & Bucks, R. S. The relationship between hearing impairment and cognitive function: A meta-analysis in adults. Clin Otolaryngol. 41, 718–729 (2016).
Zheng, Y. et al. Hearing impairment and risk of Alzheimer’s disease: A meta-analysis of prospective cohort studies. Neurol Sci. 38, 233–239 (2017).
Fetoni, A. R., Picciotti, P. M., Paludetti, G. & Troiani, D. Pathogenesis of presbycusis in animal models: A review. Exp Gerontol. 46, 413–425 (2011).
Yamasoba, T. et al. Current concepts in age-related hearing loss: Epidemiology and mechanistic pathways. Hear Res. 303, 30–38 (2013).
Deal, J. A. et al. Hearing impairment and incident dementia and cognitive decline in older adults: The Health ABC Study. J Gerontol A Biol Sci Med Sci 72, 703–709 (2017).
Gates, G. A., Anderson, M. L., McCurry, S. M., Feeney, M. P. & Larson, E. B. Central auditory dysfunction as a harbinger of Alzheimer dementia. Arch otolaryngol Head Neck Surg. 137, 390–395 (2011).
Gates, G. A. et al. Central auditory dysfunction, cognitive dysfunction, and dementia in older people. Arch otolaryngol Head Neck Surg. 122, 161–167 (1996).
Fritze, T. et al. Hearing impairment affects dementia incidence. An analysis based on longitudinal health claims data in Germany. PLoS One. 11, e0156876 (2016).
Gurgel, R. K. et al. Relationship of hearing loss and dementia: A prospective, population-based study. Otol Neurotol. 35, 775–781 (2014).
Lyu, J. & Kim, H.-Y. Gender-specific incidence and predictors of cognitive impairment among older Koreans: Findings from a 6-year prospective cohort study. Psychiatry Investig. 13, 473 (2016).
World Health Organization. Deafness and hearing loss http://www.who.int/mediacentre/factsheets/fs300/en/ (2016-11-28) (2015).
Gates, G. A. Central presbycusis: An emerging view. Otolaryngol Head Neck Surg 147, 1–2 (2012).
Lin, F. R. Hearing loss in older adults: Who’s listening? JAMA. 307, 1147–1148 (2012).
Kay, D. W. K., Beamish, P. & Roth, M. Old age mental disorders in Newcastle upon Tyne, ii: A study of possible social and medical causes. Br J Psychiatry. 110, 668–682 (1964).
Gates, G. A., Feeney, M. P. & Mills, D. Cross-sectional age-changes of hearing in the elderly. Ear Hear. 29, 865–874 (2008).
Cooper, J. C. Jr. & Gates, G. A. Hearing in the elderly–the Framingham cohort, 1983-1985: Part ii. Prevalence of central auditory processing disorders. Ear Hear. 12, 304–311 (1991).
Golding, M., Carter, N., Mitchell, P. & Hood, L. J. Prevalence of central auditory processing (CAP) abnormality in an older Australian population: The Blue Mountains Hearing Study. J Am Acad Audiol. 15, 633–642 (2004).
Peelle, J. E. & Wingfield, A. The neural consequences of age-related hearing loss. Trends Neurosci. 39, 486–497 (2016).
Panza, F. et al. Cognitive frailty: A systematic review of epidemiological and neurobiological evidence of an age-related clinical condition. Rejuvenation Res. 18, 389–412 (2015).
Peelle, J. E., Troiani, V., Grossman, M. & Wingfield, A. Hearing loss in older adults affects neural systems supporting speech comprehension. J Neurosci. 31, 12638–12643 (2011).
Wingfield, A., Tun, P. A. & McCoy, S. L. Hearing loss in older adulthood – what it is and how it interacts with cognitive performance. Curr Dir Psychol Sci. 14, 144–148 (2005).
Mick, P., Kawachi, I. & Lin, F. R. The association between hearing loss and social isolation in older adults. Otolaryngol Head Neck Surg. 150, 378–384 (2014).
Wingfield, A. & Grossman, M. Language and the aging brain: Patterns of neural compensation revealed by functional brain imaging. J Neurophysiol. 96, 2830–2839 (2006).
Eckert, M. A. et al. White matter hyperintensities predict low frequency hearing in older adults. J Assoc Res Otolaryngol. 14, 425–433 (2013).
Lin, F. R. et al. Association of hearing impairment with brain volume changes in older adults. NeuroImage. 90, 84–92 (2014).
Cox, L. C., McCoy, S. L., Tun, P. A. & Wingfield, A. Monotic auditory processing disorder tests in the older adult population. J Am Acad Audiol. 19, 293–308 (2008).
Gates, G. A., Anderson, M. L., Feeney, M. P., McCurry, S. M. & Larson, E. B. Central auditory dysfunction in older persons with memory impairment or Alzheimer dementia. Arch Otolaryngol Head Neck Surg. 134, 771–777 (2008).
Zhong, Y. et al. Age-related decline of the cytochrome c oxidase subunit expression in the auditory cortex of the mimetic aging rat model associated with the common deletion. Hear Res. 294, 40–48 (2012).
Alzheimer’s Association. Diagnostic Procedures http://www.alz.org/professionals_and_researchers_diagnostic_procedures.asp (2017-06-12).
American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders, 4th edition. (Washington, DC: American Psychiatric Press, 1994).
Folstein, M. F., Folstein, S. E. & McHugh, P. R. “Mini-mental state”. A practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res. 12, 189–198 (1975).
Arevalo-Rodriguez, I. et al. Mini-Mental State Examination (MMSE) for the detection of Alzheimer’s disease and other dementias in people with mild cognitive impairment (MCI). Cochrane Database Syst Rev. Cd010783 (2015).
McDowell, I., Kristjansson, B., Hill, G. B. & Hebert, R. Community screening for dementia: the Mini Mental State Exam (MMSE) and Modified Mini-Mental State Exam (3MS) compared. J Clin Epidemiol. 50, 377–383 (1997).
Teng, E. L. et al. The Cognitive Abilities Screening Instrument (CASI): A practical test for cross-cultural epidemiological studies of dementia. Int psychogeriatr. 6, 45-58; discussion 62 (1994).
Parker, C. & Philp, I. Screening for cognitive impairment among older people in black and minority ethnic groups. Age Ageing. 33, 447–452 (2004).
Espino, D. V., Lichtenstein, M. J., Palmer, R. F. & Hazuda, H. P. Ethnic differences in Mini-Mental State Examination (MMSE) scores: Where you live makes a difference. J Am Geriatr Soc. 49, 538–548 (2001).
Murden, R. A., McRae, T. D., Kaner, S. & Bucknam, M. E. Mini-Mental State exam scores vary with education in blacks and whites. J Am Geriatr Soc. 39, 149–155 (1991).
Brown, L. M., Schinka, J. A., Mortimer, J. A. & Graves, A. B. 3MS normative data for elderly African Americans. J Clin Exp Neuropsychol. 25, 234–241 (2003).
Jones, T. G. et al. 3MS normative data for the elderly. Arch Clin Neuropsychol. 17, 171–177 (2002).
Fulton, S. E., Lister, J. J., Bush, A. L. H., Edwards, J. D. & Andel, R. Mechanisms of the hearing–cognition relationship. Semin Hear. 36, 140–149 (2015).
Jorgensen, L. E., Palmer, C. V., Pratt, S., Erickson, K. I. & Moncrieff, D. The effect of decreased audibility on MMSE performance: A measure commonly used for diagnosing dementia. J Am Acad Audiol. 27, 311–323 (2016).
Gordon-Salant, S. Hearing loss and aging: New research findings and clinical implications. J Rehabil Res Dev. 42, 9–24 (2005).
Lin, F. et al. Development of the ACHIEVE Healthy Aging Study: A randomized controlled trial to determine if hearing loss treatment can reduce the risk of cognitive decline and dementia in older adults. Alzheimers Dement. 11, P756–P757 (2015).
Lin, F. R. et al. Results from the ACHIEVE-P study: A pilot study for a randomized controlled trial to determine if hearing loss treatment can reduce the risk of cognitive decline in older adults. Alzheimers Dement. 12, P829–P830 (2016).
Deal, J. A. et al. A randomized feasibility pilot trial of hearing treatment for reducing cognitive decline: Results from the aging and cognitive health evaluation in elders pilot study. Alzheimers Dement. 3, 410–415 (2017).
Moher, D., Liberati, A., Tetzlaff, J., Altman, D. G. & Group, P. Preferred reporting items for systematic reviews and meta-analyses: The PRISMA statement. PLoS medicine. 6, e1000097 (2009).
Stroup, D. F. et al. Meta-analysis of observational studies in epidemiology: A proposal for reporting. Meta-analysis of observational studies in epidemiology (MOOSE) group. JAMA. 283, 2008–2012 (2000).
Uhlmann, R. F., Larson, E. B., Rees, T. S., Koepsell, T. D. & Duckert, L. G. Relationship of hearing impairment to dementia and cognitive dysfunction in older adults. JAMA. 261, 1916–1919 (1989).
Woodward, M. Epidemiology: Study Design and Data Analysis, Third Edition. 321 (CRC press, 2013).
Lu, Y., Fang, J., Tian, L. & Jin, H. Advanced Medical Statistics. Vol. 5, 233–318 (World Scientific, 2015).
Spruance, S. L., Reid, J. E., Grace, M. & Samore, M. Hazard ratio in clinical trials. Antimicrob Agents Chemother. 48, 2787–2792 (2004).
Wells, G. A. et al. The Newcastle-Ottawa Scale (NOS) for Assessing the Quality of Nonrandomised Studies in Meta-Analyses (2011 ) http://www.ohri.ca/programs/clinical_epidemiology/oxford.asp (2016-7-14).
Song, J. et al. Association between cadmium exposure and renal cancer risk: A meta-analysis of observational studies. Sci Rep. 5, 17976 (2015).
Begg, C. B. & Mazumdar, M. Operating characteristics of a rank correlation test for publication bias. Biometrics. 50, 1088–1101 (1994).
DerSimonian, R. & Laird, N. Meta-analysis in clinical trials. Control Clin Trials. 7, 177–188 (1986).
Higgins, J. P., Thompson, S. G., Deeks, J. J. & Altman, D. G. Measuring inconsistency in meta-analyses. Brit Med J. 327, 557–560 (2003).
Cai, X. et al. Selenium exposure and cancer risk: An updated meta-analysis and meta-regression. Sci Rep. 6, 19213 (2016).
Higgins, J. P. T. & Green, S. Cochrane Handbook for Systematic Reviews of Interventions Version5.1.0 http://www.handbook.cochrane.org (2016-8-27ro) (2010)).
Acknowledgements
This work was supported by grants from the Key Project of National Natural Science Foundation of China (81230021) and the Major State Basic Research Development Program of China (973 Program) (2011CB504504).
Author information
Authors and Affiliations
Contributions
W.-J.K. conceived and designed the work; J.Y., W.-J.K., and J.H.P. prepared the manuscript; J.Y., W.-J.K., Y.S., and S.S. conducted data analysis; J.Y. and Y.S. prepared figures and tables; All authors reviewed and approved the manuscript.
Corresponding author
Ethics declarations
Competing Interests
The authors declare that they have no competing interests.
Additional information
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Yuan, J., Sun, Y., Sang, S. et al. The risk of cognitive impairment associated with hearing function in older adults: a pooled analysis of data from eleven studies. Sci Rep 8, 2137 (2018). https://doi.org/10.1038/s41598-018-20496-w
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-018-20496-w
This article is cited by
-
Links across disabilities: unveiling associations between functional domains
BMC Public Health (2024)
-
Electroencephalogram-based objective assessment of cognitive function level associated with age-related hearing loss
GeroScience (2023)
-
Combined effects of handgrip strength and sensory impairment on the prevalence of cognitive impairment among older adults in Korea
Scientific Reports (2022)
-
Genome-wide association meta-analysis identifies five novel loci for age-related hearing impairment
Scientific Reports (2019)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.