Abstract
The aim of this study was to investigate the effects of lysine restriction on inflammatory responses in piglets. 38 male piglets with similar body weight of 9.62 kg were randomly divided into control group (basal diet) and lysine-restricted group (diet containing 70% lysine of the control diet). The results showed that lysine restriction increased the serum concentration of IgG an IgM. Piglets fed the lysine-restricted diet exhibited overexpression of interleukin-8 (IL-8) in the kidney (P < 0.05) and IL-6 and IL-4 in the spleen (P < 0.05). The mRNA abundances of IL-4 in the kidney (P < 0.05) and IL-10 in the liver (P < 0.05) were significantly lower in the lysine-restricted group compared with the control group. Meanwhile, lysine restriction increased the mRNA level of Tlr8 in the kidney (P < 0.05) but decreased the mRNA level of Tlr8 in the liver (P < 0.05). Finally, lysine restriction markedly enhanced extracellular signal regulated kinases 1/2 (ERK1/2) phosphorylation in the kidney and liver and nuclear transcription factor kappa B (NF-κB) was activated in the liver and spleen in response to dietary lysine restriction. In conclusion, lysine restriction affected inflammatory responses in the kidney, liver, and spleen via mediating serum antibody volume, inflammatory cytokines, Tlrs system, and ERK1/2 and NF-κB signals in piglets.
Similar content being viewed by others
Introduction
Amino acids are critically important for the growth, health, and disease in piglets1. Lysine is one of the building blocks for synthesis of proteins, peptides and non-peptide molecules2, which are involved in various biochemical and physiological process. In our previous reports, we found that dietary different dosages of lysine influence intestinal morphology and expressions of amino acid transporters, which further mediate intestinal absorption and metabolism of amino acids3,4. More recently, lysine deficiency in vivo and in vitro was investigated in our lab and the results showed that lysine deficiency affects cell cycle arrest, apoptosis, and amino acid metabolism, which may be associated with the mammalian target of rapamycin (mTOR) signal5.
Dietary lysine deficiency also impairs both antibody responses and cell-mediated immune responses1,6,7. However, the effect of lysine restriction on inflammatory response is still obscure. Thus, the present study aimed to investigate the inflammatory status of the kidney, liver, and spleen in piglets after exposure to a lysine-restricted diet.
Results
Lysine restriction increased serum concentration of IgG and IgM
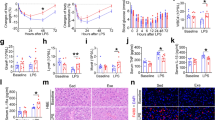
The serum concentration of IgG and IgM were significantly higher (P < 0.01) in piglets from the lysine-restricted group when compared with the control group (Table 1).
Lysine restriction upregulated pro-inflammatory cytokines
Expressions of pro-inflammatory cytokines (IL-1β, IL-6, IL-8, IL-12, tumor necrosis factor-α (TNF-α), and interferon-gamma (IFN-γ)) were determined in the kidney, liver, and spleen (Fig. 1). The results showed that lysine restriction markedly increased mRNA abundances of IL-8 in the kidney and IL-6 in the spleen (P < 0.05). Meanwhile, IL-12 and IFN-γ expressions in the kidney tended to decrease in lysine-restricted group, while the difference was insignificant (P > 0.05).
Lysine restriction influenced anti-inflammatory cytokines
Dietary lysine restriction decreased the mRNA level of IL-4 in the kidney (P < 0.05) and mRNA level of IL-10 in the liver (P < 0.05). While the IL-4 mRNA level in the spleen were markedly higher in the lysine-restricted group compared to the control group (P < 0.05) (Fig. 2).
Effects of lysine restriction on toll-like receptors (Tlrs) system
Tlrs are widely demonstrated to involve in the activation of inflammatory response. Thus, expressions of Tlr3, 4, 7, 8, 9, and Myd88 were determined in the kidney, liver, and spleen (Fig. 3). Lysine restriction increased the mRNA level of Tlr8 in the kidney (P < 0.05) but decreased the mRNA level of Tlr8 in the liver (P < 0.05). Furthermore, lysine restriction exhibited little effect on expression of other Tlrs and myeloid differentiation 88 (Myd88) in the kidney, liver, and spleen.
Lysine restriction induced the abundance of extracellular signal regulated kinases 1/2 (ERK1/2) and nuclear transcription factor kappa B (NF-κB) proteins
ERK1/2 signal was markedly activated in the kidney and liver and NF-κB signal was upregulated in the liver and spleen of lysine restricted piglets evidenced by the enhanced phosphorylation ratio of ERK1/2 and NF-κB (P < 0.01) (Fig. 4).
The ration of phosphorylated JNK abundance to total JNK in the kidney (A), liver (D), and spleen (G) of piglets fed a basal diet (100% lysine) or a lysine-restricted diet containing 70% lysine of the basal diet. The ration of phosphorylated ERK1/2 abundance to total ERK1/2 in the kidney (B), liver (E), and spleen (H) of piglets fed a basal diet (100% lysine) or a lysine-restricted diet containing 70% lysine of the basal diet. The ration of phosphorylated NF-κB abundance to NF-κB in the kidney (C), liver (F), and spleen (I) of piglets fed a basal diet (100% lysine) or a lysine-restricted diet containing 70% lysine of the basal diet. Each image shows three samples from 100% Lysine group and three samples from 70% Lysine group. *Different from control, P < 0.01.
Discussion
Lysine is the first limiting amino acid for piglets and is one of the building blocks for the synthesis of proteins8. For this reason, inadequate lysine intake can limit the synthesis of inflammatory-related proteins (including cytokines)9. Numerous studies have demonstrated that the intake of amino acid affect the inflammatory responses of animals7,10. What’s more, it was reported that the deficiency of dietary lysine also impaired animal immune responses9,11.
The serum antibody volume has been widely used to evaluated the humoral immunity12. IgG and IgM, two major serum immunoglobulins, are key components humoral immunity in all mammals13 and protect the extravascular compartment against pathogenic virus and microorganisms9. In this study, dietary lysine restriction decreased the serum concentration of IgG and IgM. Pro-inflammatory cytokines (including IL-6 and IL-8) serve as an important role in mediating inflammatory and immune responses14,15,16,17. IL-4 is involved in all major aspects of inflammatory responses18. IL-10, an anti-inflammatory cytokine, down-regulates macrophage activity in swine19,20. In this study, the mRNA abundance of IL-8 in the kidney and IL-6 in the spleen were significantly higher in the lysine-restricted group compared with the control group. We also found that lysine restriction markedly decreased the abundance of IL-4 in the kidney and IL-10 in the liver, but significantly increased the abundance of IL-4 in the spleen. Tlrs are a family of pathogen recognition receptors which promote innate immunity14. Tlrs activate the expression of pro-inflammatory, such as IL-6 and TNF-α21. Myd88 plays an important role in the Tlr signaling pathway22. Dietary arginine supplementation has effects on the activation of Tlrs23. In the present study, lysine restriction influenced the expression of Tlr8 in the kidney and liver of piglets. These results showed that lysine restriction affect inflammatory response via mediating serum antibody volume, inflammatory cytokines, and Tlrs. Notably, the current results showed a tissue-dependent of gene expressions, which might be caused by different functions of these tissues. For example, liver mainly contributes to metabolism and kidney involves in excretion and re-absorption. Similarly, we also noticed that expressions of Tlr system varied from different sections of intestine (duodenum, jejunum, and ileum)24.
NF-κB pathway plays an important role in inflammation by mediating synthesis of pro-inflammatory (i.e. IL-6 and IL-8)25. Mitogen-activated protein kinase (MAPK) pathway involves in nuclear translocation of NF-κB and contributes to the production of inflammatory cytokines26,27. ERKs and c-Jun N-terminal protein kinase (JNK) are members of MAPK family, which is associated with inflammation28. Amino acids have been demonstrated to activate NF-κB and MAPK signaling pathways to regulate expression of pro-inflammatory cytokines and inflammation23. Similarly, in this study, lysine restriction activated ERK1/2 and NF-κB signals, which might further involve in immune and inflammatory responses. Our previous study has revealed that lysine deficiency induced apoptosis5, which is highly associated with inflammatory response29. Thus, it is not surprising to uncover that lysine restriction induces inflammatory response.
Taken together, this study indicated that lysine restriction can induce inflammatory via mediating serum concentration of IgG and IgM, the expression of inflammatory cytokines, Tlrs, and ERK1/2 and NF-κB signals in the kidney, liver, and spleen of piglets.
Materials and Methods
Animals and Experimental Design
This study was conducted in accordance with the guidelines of the Institute of Subtropical Agriculture, Chinese Academy of Sciences. All experimental protocols were approved by animal ethical committee of the Institute of Subtropical Agriculture, Chinese Academy of Sciences. 38 male piglets (about 35-day old, 9.62 ± 0.30 kg) were randomly divided into 2 groups: a control group and a lysine-restricted group. Piglets in the control group were received the basal diet according to the NRC (2012) (Table 2), whereas piglets in the lysine-restricted group were fed a lysine-restricted diet containing 70% lysine of the control group. Piglets were individually housed in cages and had ad libitum access to drinking water and feed for 21 days. Then 7 animals were sampled randomly from each group. Blood samples from the overnight fasting piglets were collected in plastic uncoated tubes. Sera were obtained by centrifugation at 3000 rpm for 20 min and stored at −20 °C until analysis for IgG and IgM. After blood sampling, the piglets were sacrificed for kidney, liver, and spleen collection.
Real-Time Quantitative RT-PCR
Total RNA was isolated from liquid nitrogen–frozen kidney, liver, and spleen using TRIZOL reagent (Invitrogen, USA) and then treated with DNase I (Invitrogen, USA) according to the instructions of the manufacturer. Synthesis of the first strand (cDNA) was performed with PrimeScript Enzyme Mix 1, RT Primer Mix, and 5 × PrimerScript Buffer 2. The reverse transcription was conducted at 37 °C for 15 m, 85 °C for 5 s. Primers (Table 3) used in this study were presented in the previous study23,24,25. β-actin was used as a housekeeping gene to normalize target gene transcript levels. Real-time PCR was performed according to our previous study3. Briefly, 1 μl cDNA template was added to a total volume of 10 μl containing 5 μl SYBR Green mix, 0.2 μl Rox, 3 μl ddH2O, and 0.4 μl each of forward and reverse primers. We used the following protocol: (i) pre-denaturation programma (30 s at 95 °C); (ii) an amplification and quantification program consisting of repeated 40 cycles (5 s at 95 °C and 30 s at 60 °C); (iii) a melting curve program (extention at 72 °C). Relative expression was expressed as a ratio of the target gene to the control gene using the formula 2−(ΔΔCt), where ΔΔCt = (CtTarget − Ctβ-actin) treatment − (CtTarget − Ctβ-actin) control26. Relative expression was normalized and expressed relative to the expression in the control group.
Western blot analysis
The expression of protein in the kidney, liver and spleen was determined by Western blot analysis as described previously. Briefly, about 50 µg of total protein obtained from samples were extracted by a reducing SDS-PAGE electrophoresis. The proteins were transferred onto polyvinylidene difluoride membranes and blocked with 5% nonfat milk in tris-Tween-buffered saline buffer (20 mM tris, pH 7.5, 150 mM NaCl, and 0.1% Tween 20) for 1.5 hour. Then the primary antibodies were incubated overnight at 4°C and the horseradish peroxidase-conjugated secondary antibodies were subsequently incubated for 1.5 hour at room temperature before development of the blot using the Alpha Imager 2200 software (Alpha Innotech Corporation, CA, USA). We quantified the resultant signals and normalize the data to the abundance of β-actin according to our previous reports.
Statistical analysis
All data were analyzed between two groups using the student’s T test (SPSS 16.0 software). Data are expressed as the mean ± SEN. Differences of p < 0.05 are considered significant.
References
Wu, G. Functional amino acids in nutrition and health. Amino acids 45, 407–411, https://doi.org/10.1007/s00726-013-1500-6 (2013).
Liao, S. F., Wang, T. & Regmi, N. Lysine nutrition in swine and the related monogastric animals: muscle protein biosynthesis and beyond. SpringerPlus 4, 147, https://doi.org/10.1186/s40064-015-0927-5 (2015).
He, L. Q. et al. Effects of dietary L-lysine supplementation on lysine transport by the piglet small intestine in vitro. J. Anim. Sci. 94, 106–110, https://doi.org/10.2527/jas2015-0207 (2016).
He, L. et al. Effects of dietary L-lysine intake on the intestinal mucosa and expression of CAT genes in weaned piglets. Amino acids 45, 383–391, https://doi.org/10.1007/s00726-013-1514-0 (2013).
Yin, J. et al. Effects of Lys deficiency and Lys-Lys dipeptide on cellular apoptosis and amino acids metabolism. Molecular nutrition & food research, https://doi.org/10.1002/mnfr.201600754 (2016).
Chen, C., Sander, J. E. & Dale, N. M. The effect of dietary lysine deficiency on the immune response to Newcastle disease vaccination in chickens. Avian diseases 47, 1346–1351, https://doi.org/10.1637/7008 (2003).
Konashi, S., Takahashi, K. & Akiba, Y. Effects of dietary essential amino acid deficiencies on immunological variables in broiler chickens. The British journal of nutrition 83, 449–456 (2000).
Wu, G. Amino acids: metabolism, functions, and nutrition. Amino acids 37, 1–17, https://doi.org/10.1007/s00726-009-0269-0 (2009).
Li, P., Yin, Y. L., Li, D., Kim, S. W. & Wu, G. Amino acids and immune function. The British journal of nutrition 98, 237–252, https://doi.org/10.1017/s000711450769936x (2007).
Zhong, Z. et al. L-Glycine: a novel antiinflammatory, immunomodulatory, and cytoprotective agent. Current opinion in clinical nutrition and metabolic care 6, 229–240, https://doi.org/10.1097/01.mco.0000058609.19236.a4 (2003).
Datta, D., Bhinge, A. & Chandran, V. Lysine: Is it worth more? Cytotechnology 36, 3–32, https://doi.org/10.1023/a:1014097121364 (2001).
Kong, X. F. et al. Dietary supplementation with Chinese herbal ultra-fine powder enhances cellular and humoral immunity in early-weaned piglets. Livest. Sci. 108, 94–98, https://doi.org/10.1016/j.livsci.2007.01.002 (2007).
Deng, Z. Y. et al. Effect of polysaccharides of cassiae seeds on the intestinal microflora of piglets. Asia Pacific journal of clinical nutrition 16(Suppl 1), 143–147 (2007).
Allam, M., Julien, N., Zacharie, B., Penney, C. & Gagnon, L. Enhancement of Th1 type cytokine production and primary T cell activation by PBI-1393. Clinical immunology (Orlando, Fla.) 125, 318–327, https://doi.org/10.1016/j.clim.2007.07.017 (2007).
Candel-Marti, M. E., Flichy-Fernandez, A. J., Alegre-Domingo, T., Ata-Ali, J. & Penarrocha-Diago, M. A. Interleukins IL-6, IL-8, IL-10, IL-12 and periimplant disease. An update. Medicina oral, patologia oral y cirugia bucal 16, e518–521 (2011).
Clop, A. et al. Identification of genetic variation in the swine toll-like receptors and development of a porcine TLR genotyping array. Genetics, selection, evolution: GSE 48, 28, https://doi.org/10.1186/s12711-016-0206-0 (2016).
Tanaka, T., Narazaki, M. & Kishimoto, T. IL-6 in inflammation, immunity, and disease. Cold Spring Harbor perspectives in biology 6, a016295, https://doi.org/10.1101/cshperspect.a016295 (2014).
Oeser, K., Maxeiner, J., Symowski, C., Stassen, M. & Voehringer, D. T cells are the critical source of IL-4/IL-13 in a mouse model of allergic asthma. Allergy 70, 1440–1449, https://doi.org/10.1111/all.12705 (2015).
Singh, P. & Ramamoorthy, S. Immune gene expression in swine macrophages expressing the Torque Teno Sus Virus1 (TTSuV1) ORF-1 and 2 proteins. Virus research 220, 33–38, https://doi.org/10.1016/j.virusres.2016.04.004 (2016).
Sun, J., Madan, R., Karp, C. L. & Braciale, T. J. Effector T cells control lung inflammation during acute influenza virus infection by producing IL-10. Nature medicine 15, 277–284, https://doi.org/10.1038/nm.1929 (2009).
Martinez-Robles, E. et al. Genotypic distribution of common variants of endosomal toll like receptors in healthy Spanish women. A comparative study with other populations. Gene 578, 32–37, https://doi.org/10.1016/j.gene.2015.12.004 (2016).
Wu, D. et al. Identification of TLR downstream pathways in stroke patients. Clinical biochemistry 46, 1058–1064, https://doi.org/10.1016/j.clinbiochem.2013.05.059 (2013).
Ren, W. et al. Dietary arginine supplementation of mice alters the microbial population and activates intestinal innate immunity. The Journal of nutrition 144, 988–995, https://doi.org/10.3945/jn.114.192120 (2014).
Yin, J. et al. Hydrogen peroxide-induced oxidative stress activates NF-kappa B and Nrf2/Keap1 signals and triggers autophagy in piglets. RSC Adv. 5, 15479–15486, https://doi.org/10.1039/c4ra13557a (2015).
Tak, P. P. & Firestein, G. S. NF-kappaB: a key role in inflammatory diseases. The Journal of clinical investigation 107, 7–11, https://doi.org/10.1172/jci11830 (2001).
Lanna, A., Gomes, D. C. & Muller-Durovic, B. A sestrin-dependent Erk-Jnk-p38 MAPK activation complex inhibits immunity during aging. 18, 354–363, https://doi.org/10.1038/ni.3665 (2017).
Suzuki, M. et al. The role of p38 mitogen-activated protein kinase in IL-6 and IL-8 production from the TNF-alpha- or IL-1beta-stimulated rheumatoid synovial fibroblasts. FEBS letters 465, 23–27 (2000).
Seki, E., Brenner, D. A. & Karin, M. A liver full of JNK: signaling in regulation of cell function and disease pathogenesis, and clinical approaches. Gastroenterology 143, 307–320, https://doi.org/10.1053/j.gastro.2012.06.004 (2012).
Davidovich, P., Kearney, C. J. & Martin, S. J. Inflammatory outcomes of apoptosis, necrosis and necroptosis. Biological chemistry 395, 1163–1171, https://doi.org/10.1515/hsz-2014-0164 (2014).
Acknowledgements
We are thankful for the support of Public Service Technology Center, Institute of Subtropical Agriculture, Chinese Academy of Sciences. This study was supported by the National Basic Research Program of China (973) (2013CB127301), National Natural Science Foundation of China (No. 31472106), and Key Projects in the National Science & Technology Pillar Program (2013BAD21B04). We would like to thank the Public Service Technology Center, Institute of Subtropical Agriculture, Chinese Academy of Sciences and members of the laboratory of Yin Y.L. for helpful discussions.
Author information
Authors and Affiliations
Contributions
H.H. and J.Y. contributed equally to this study. H.H. and J.Y. conducted the study; J.Z., B.W., X.H., J.Y., and W.F. helped to perform the experiment and write the paper; J.Y., T.L., and Y.Y. designed the experiment and revised the manuscript.
Corresponding authors
Ethics declarations
Competing Interests
The authors declare that they have no competing interests.
Additional information
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Han, H., Yin, J., Wang, B. et al. Effects of dietary lysine restriction on inflammatory responses in piglets. Sci Rep 8, 2451 (2018). https://doi.org/10.1038/s41598-018-20689-3
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-018-20689-3
This article is cited by
-
Low-protein diets supplemented with methionine and lysine alter the gut microbiota composition and improve the immune status of growing lambs
Applied Microbiology and Biotechnology (2021)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.