Abstract
Iron is an essential nutrient for bacterial survival and thus higher iron levels may precipitate bacterial infections. We investigated the association between the serum iron level and prognosis in patients with sepsis by using the single-centre Medical Information Mart for Intensive Care III (MIMIC-III) database. Sepsis patients with iron parameters measured on ICU admission were included and stratified according to quartiles of serum iron levels. A total of 1,891 patients diagnosed with sepsis according to the Sepsis-3 criteria were included in this study, 324 of whom were septic shock. After adjusting for confounding variables, higher iron quartile was associated with an increase in 90-day mortality in the Cox regression analysis. Moreover, a stepwise increase in the risk of 90-day mortality was observed as the quartiles of serum iron levels increased in the patients with sepsis. In conclusion, higher serum iron levels were independently associated with increased 90-day mortality in this large cohort of patients with sepsis.
Similar content being viewed by others
Introduction
Iron plays a pivotal role during infection because it functions as a critical cofactor in a variety of physiological metabolic reactions and is an essential nutrient for bacteria. Thus, during bacterial infections, both the host and bacteria compete for iron to thrive. In the human body, most iron is sequestered by haemoglobin1. Meanwhile, iron-binding proteins, antibodies and complement proteins in the body function jointly to retain iron2. Nevertheless, both free iron and protein-bound iron can be captured by bacteria through various mechanisms, such as siderophores2,3, which are secreted by pathogens and possess an affinity to iron that is higher than that of the host transport proteins4. Moreover, free iron is toxic because it readily accepts or donates electrons, leading to the generation of reactive oxygen species5.
Haemochromatosis, a hereditary iron overload condition, is known to cause susceptibility to infection and iron chelators may reverse this susceptibility6. Thus, a higher iron level may precipitate infections. Transferrin saturation (TSAT), a parameter reflects iron availability, was recently reported to be associated with prognosis of ICU patients7. Therefore, the likelihood that a higher serum iron level in patients with sepsis is associated with a poorer prognosis is an appealing hypothesis; though, few clinical studies were available as to this hypothesis so far. We hypothesized that higher iron level was associated with worse outcomes in patients with sepsis. Thus, we tested the abovementioned hypothesis by analysing a large database and exploring the association between serum iron levels and the 90-day mortality in patients with sepsis.
Results
Characteristics of the study population
A total of 1,891 patients diagnosed with sepsis according to the Sepsis-3 criteria were included in the present study and 324 (17.1%) patients were in septic shock. The baseline characteristics of all patients were presented in Table 1. Half of the patients were male, and approximate two-thirds of the patients were older than 60 years. Most of the patients were admitted from emergency room. One-fifth of the patients were treated by mechanical ventilation upon ICU admission, and the median Simplified Acute Physiology Score (SAPS) II and Sequential Organ Failure Assessment (SOFA) scores were 43 and 5, respectively. With respect to the primary infection sites (Table 2), respiratory, bloodstream and urinary infections accounted for the vast majority of the infections. When it comes to microbiology, Staphylococcus (762, 40.3%) and Escherichia coli (195, 10.3%) were the two leading pathogens.
Outcomes and Cox regression analysis
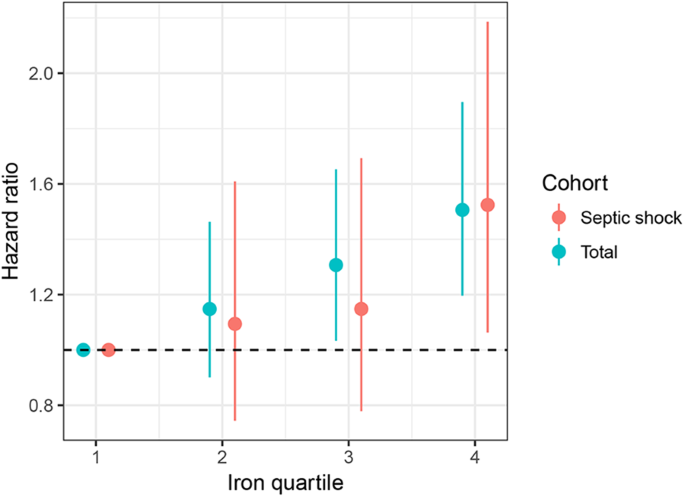
The median ICU and hospital stays were 3.8 days and 10.6 days, respectively. The overall 28- and 90-day mortality rates were 21.3% and 30.9%, respectively (Table 3). The mortality rates on days 28 and 90 were significantly increased as the iron quartiles increased (p = 0.001 and p < 0.001 for trend, respectively) (Table 3 and Fig. 1). Cox regression models were established to further determine the independent effect of iron levels on prognosis. After adjusting for possible confounding variables, the risk of death on day 90 increased in a stepwise manner as the iron quartiles increased (Table 4 and Fig. 2). Regarding the other iron parameters, transferrin (HR: 0.997, 95% CI: 0.996–0.999, p < 0.001) and haemoglobin (HR: 0.936, 95% CI: 0.899–0.975, p = 0.001) levels exerted a beneficial effect on the mortality of patients with sepsis, whereas ferritin levels had no significant impact on 90-day mortality (HR: 1.000, 95% CI: 0.999–1.000, p = 0.393) (Table 4). For septic shock cohort, the fourth quartiles of iron was associated with increased risk of death after adjustment for confounding factors (HR: 1.524, 95% CI: 1.063–2.186, p = 0.022, Fig. 2).
Discussion
In this large cohort of ICU patients, a higher serum iron quartile was associated with increased 90-day mortality in patients with sepsis. Furthermore, a dose-response analysis indicated that the risk of death on day 90 increased as the quartiles of serum iron levels upon ICU admission increased.
In animal models, bacterial proliferation has been shown to be driven by iron sufficiency and suppressed by iron starvation8,9,10. In a mouse model in which the hfe gene was deleted (hfe−/−), iron abnormally accumulates in tissues. The mortality of the hfe−/− mice was approximately doubled after the mice were subjected to caecal ligation and puncture, a typically clinical used animal model of intra-abdominal sepsis11. In addition, iron-overloaded mice tended to develop more severe Salmonella infections12. Undoubtedly, the high iron level was a risk factor in the infected mouse model.
Clinically, during conventional chemotherapy treatment, free iron levels > 2 μM were significantly correlated with a higher risk of sepsis caused by gram-negative bacilli for patients with acute leukaemia13. Furthermore, as described in previous studies6,14, patients with haemochromatosis, a hereditary disease characterized by an iron overload, were highly susceptible to infections by various pathogens, including Escherichia coli, Vibrio vulnificus, Vibrio cholera, Klebsiella species, Listeria monocytogenes, and Shigella species. Thus, elevated iron level was probably associated with a worse prognosis in patients with sepsis.
The iron level throughout the body is tightly controlled by systems that elaborately regulate iron absorption, systemic transport and cellular uptake and storage14. Because nearly all bacteria, fungi and protozoa require a continuous supply of host iron to successfully sustain an infection, humans have evolved powerful strategies, such as the immune system and various iron-binding proteins, to restrict the availability of iron to pathogens15. Micro-organisms, such as Klebsiella pneumonia and Escherichia coli, have developed intricate mechanisms to sequester iron from iron-proteins, such as transferrin, and counter the host’s iron restriction strategies. Siderophores (including aerobactin, enterobactin, salmochelin and yersiniabactin), which are excreted by bacteria, assist the pathogen in acquiring iron16. Neisseria meningitides can even obtain iron directly from transferrin via receptors, such as TbpA and TbpB, expressed on the bacterial outer membrane3. However, the majority of the transferrin that is not saturated with iron possesses a powerful bactericidal or bacteriostatic effect2. Transferrin has been recently shown to be a good indicator of organ failure, and higher transferrin level was associated with a decreased 30-day mortality rate in patients with decompensated cirrhosis17. Moreover, serum transferrin levels <150 mg/dl were associated with an increased risk of sepsis in burn patients18. Consistent with these findings, transferrin was associated with increased survival rates in patients with sepsis in the present study (Table 4).
Although higher iron levels independently facilitate the pathogenesis of infection, some other parameters such as ferritin, the abovementioned transferrin protein and transferrin saturation (TSAT), are associated with iron. In the human body, ferritin, which is regulated by hepcidin, is an iron-binding protein whose function is to store iron in tissue, whereas transferrin in the blood transports iron. Patients who received a kidney transplant and presented ferritin levels ≥500 ng/mL had higher incidence rates (per 1,000 transplant-days) of overall infection (p = 0.017), bacterial infection (p = 0.002), and bloodstream infection (p = 0.011) during the first post-transplant year19. Moreover, the one-year infection-free survival rate was significantly lower in these recipients (26% vs 41%; p = 0.004)19. As an acute phase reactant, however, ferritin did not show impact on the survival of sepsis (HR: 1.000, 95% CI: 0.999–1.000). The reason might be its complex regulation including growth hormones, hypoxia, anaemia, and endoplasmic reticulum stress response7. Moreover, ferritin was defined as the major iron storage protein within hepatocytes and macrophages20. The fluctuation of ferritin level was complicated by hepatic disorder, tumour and hematologic instability.
According to a recent prospective study of 155 ICU patients and 156 healthy individuals, TSAT was associated with increased mortality in ICU patients7. Actually, TSAT reflects serum iron availability and is highly correlated with iron levels (r = 0.860, p < 0.001) (see Supplementary Table S1). Generally, ICU patients, particularly septic patients, face a katabolic state that decreases iron consumption and increases iron release by destroying erythrocytes and other tissues, and this effect is further amplified by the administration of frequent blood transfusions7. Consistent with these findings, the regression and subgroup analyses in our study revealed that iron level was an independent risk factor for 90-day mortality. Moreover, a dose-dependent increase in the risk of death was observed as the iron level increased. The increase in iron-related mortality may be due to excess iron, which directly facilitates recurrent bacterial infections. Moreover, iron catalyses the chemical production of reactive oxygen species, such as the hydroxyl anion and superoxide, and thereby contributes to the development of multiorgan failure11. Thus, iron deprivation, i.e., via iron chelators, could be used to manage severe infections. For example, deferoxamine has been assessed as a chemotherapeutic adjuvant treatment in human malaria21, and DIBI, another iron chelator, showed a significant curative effect on animal models of sepsis22.
Although this study is a large cohort study designed to explore the relationship between the serum iron levels and outcomes in patients with sepsis, our study has several limitations. Firstly, the study was based on a publicly accessible, single-centre database, which may lead to concerns regarding the generalizability of the conclusions and the confounding bias caused by the missing data. However, this database has been explored by many authors worldwide, and articles have been published guaranteeing the data quality23,24, potentially facilitating the generalization of our findings. Secondly, we included only patients with sepsis for whom iron measurements were available, which may be responsible for some selection bias. Thirdly, we adopted only one measurement of the iron parameters on ICU admission rather than the trends, which may ignore the effect of iron levels on the trends. Fourthly, we could only extract the administration date rather than exact hours of antimicrobials in the database. Thus, we could not incorporate the time to start of antimicrobials to the Cox regression analysis. Finally, the database was relative old (patients between 2001 and 2012) and some management strategies have been improved for sepsis recently.
In summary, higher serum iron level was independently associated with an increased 90-day mortality in patients with sepsis.
Methods
Data source
This study was based on a publicly accessible critical care database named Medical Information Mart for Intensive Care III (MIMIC-III, version 1.4), which is a large, single-centre database containing information of 46,520 patients (aged 16 years or older) who were admitted to Beth Israel Deaconess Medical Center (a teaching hospital of Harvard Medical School in Boston, Massachusetts) between 2001 and 201225. The database contains data pertaining to general information (i.e., demographics, billing, ICD-9 codes, ICU types, SOFA scores, SAPS II scores, etc.), treatment process (i.e., medications, procedures, laboratory tests, fluid balance, image reports, etc.) and survival data. The data were extracted from the MIMIC-III database using structure query language (SQL) with pgAdmin4 PostgreSQL 9.6.
Study patients and definitions
All patients with sepsis for whom measurement of iron parameters were available after admission to the ICU were included in the analysis. Infectious patients were defined as individuals meeting one of the following criteria: (1) ICD-9 diagnostic code containing the terms “infection”, “pneumonia”, “meningitis”, “peritonitis”, “bacteraemia”, “sepsis” or “septic”, or (2) a positive microbiological culture26. According to the Sepsis-3 definition, patients with infections or suspected infections and an increase of SOFA score of 2 points or more were defined as sepsis27. Furthermore, patients with sepsis who required vasopressors to maintain a mean arterial pressure of 65 mm Hg or greater and with serum lactate level greater than 2 mmol/L in the absence of hypovolemia were categorized as septic shock27. Patients who received iron supplementation during the ICU stay were excluded.
For some patients for whom multiple iron measurements were available, the first measurement after ICU admission was used. Other data, including gender, age (categorized into 16–30, 31–59, and 60 years or above), admission type (elective, emergency and urgent), use of mechanical ventilation upon ICU admission, comorbidities, infection sites, pathogenic organisms, creatinine level, lactate level, white blood cell count, and SAPS II scores were extracted. Adequate antimicrobial treatments and tight glucose control were defined as previously described28,29. Missing values were imputed as the mean values, because all variables included less than 10% of missing observations. Patients were stratified according to the quartiles of serum iron level.
The primary outcome was all cause 90-day mortality. The secondary outcomes included 28-day mortality and the length of the hospital and ICU stays.
Statistical analysis
Shapiro-Wilk tests were performed and histograms were generated to determine the normality of the distribution of the continuous variables. Normally distributed continuous variables were reported as the means ± SD, whereas skewed variables were summarized as the median and interquartile range (IQR). Categorical variables were compared using a chi-square analysis or Fisher’s exact test to determine the differences among the quartiles. For comparisons among the four quartiles of iron level, analysis of variance (ANOVA) or the Kruskal-Wallis test was performed. Outcome data were compared using a trend analysis.
We compared the survival rates using log-rank tests and present the results as Kaplan–Meier curves. We developed multivariable Cox proportional hazard models for the overall population and patients with septic shock to determine the independent effect of the quartiles of serum iron level on 90-day mortality. Variables with p < 0.05 in the univariate analysis were further incorporated into multivariate Cox proportional hazard models. Iron parameters with a moderate to strong correlation strength (r > 0.3) were not simultaneously entered into the multivariable models to avoid collinearity (see Supplementary Table S1). The hazard ratios and their 95% CIs were calculated. All analyses were performed using R 3.3.3, and a p-value less than 0.05 was considered statistically significant.
Data availability
The data analysed in the study are fully available on the website.
References
Marx, J. J. Iron and infection: competition between host and microbes for a precious element. Best practice & research. Clinical haematology 15, 411–426 (2002).
Bullen, J., Griffiths, E., Rogers, H. & Ward, G. Sepsis: the critical role of iron. Microbes and infection 2, 409–415 (2000).
Gray-Owen, S. D. & Schryvers, A. B. Bacterial transferrin and lactoferrin receptors. Trends in microbiology 4, 185–191 (1996).
Paczosa, M. K. & Mecsas, J. Klebsiella pneumoniae: Going on the Offense with a Strong Defense. Microbiol Mol Biol Rev 80, 629–661, https://doi.org/10.1128/mmbr.00078-15 (2016).
Ganz, T. Systemic iron homeostasis. Physiol Rev 93, 1721–1741, https://doi.org/10.1152/physrev.00008.2013 (2013).
Wallace, D. F. The Regulation of Iron Absorption and Homeostasis. The Clinical biochemist. Reviews 37, 51–62 (2016).
Tacke, F. et al. Iron Parameters Determine the Prognosis of Critically Ill Patients. Critical care medicine 44, 1049–1058, https://doi.org/10.1097/ccm.0000000000001607 (2016).
Freidank, H. M., Billing, H. & Wiedmann-Al-Ahmad, M. Influence of iron restriction on Chlamydia pneumoniae and C. trachomatis. Journal of medical microbiology 50, 223–227, https://doi.org/10.1099/0022-1317-50-3-223 (2001).
Trivier, D., Davril, M., Houdret, N. & Courcol, R. J. Influence of iron depletion on growth kinetics, siderophore production, and protein expression of Staphylococcus aureus. FEMS microbiology letters 127, 195–199 (1995).
Goldoni, P., Visca, P., Pastoris, M. C., Valenti, P. & Orsi, N. Growth of Legionella spp. under conditions of iron restriction. Journal of medical microbiology 34, 113–118, https://doi.org/10.1099/00222615-34-2-113 (1991).
Wizorek, J. J., Turnbull, I. R. & Buchman, T. G. Iron overload before cecal ligation and puncture increases mortality. Shock (Augusta, Ga.) 20, 52–55, https://doi.org/10.1097/01.shk.0000065770.72937.fd (2003).
Nairz, M. et al. Genetic and Dietary Iron Overload Differentially Affect the Course of Salmonella Typhimurium Infection. Front Cell Infect Microbiol 7, 110, https://doi.org/10.3389/fcimb.2017.00110 (2017).
Belotti, A. et al. Non transferrin bound iron (NTBI) in acute leukemias throughout conventional intensive chemotherapy: kinetics of its appearance and potential predictive role in infectious complications. Leuk Res 39, 88–91, https://doi.org/10.1016/j.leukres.2014.11.003 (2015).
Ali, M. K. et al. Role of iron in the pathogenesis of respiratory disease. The international journal of biochemistry & cell biology 88, 181–195, https://doi.org/10.1016/j.biocel.2017.05.003 (2017).
Lasocki, S., Gaillard, T. & Rineau, E. Iron is essential for living! Critical care (London, England) 18, 678, https://doi.org/10.1186/s13054-014-0678-7 (2014).
Russo, T. A., Olson, R., MacDonald, U., Beanan, J. & Davidson, B. A. Aerobactin, but not yersiniabactin, salmochelin, or enterobactin, enables the growth/survival of hypervirulent (hypermucoviscous) Klebsiella pneumoniae ex vivo and in vivo. Infect Immun 83, 3325–3333, https://doi.org/10.1128/iai.00430-15 (2015).
Bruns, T. et al. Low serum transferrin correlates with acute-on-chronic organ failure and indicates short-term mortality in decompensated cirrhosis. Liver Int 37, 232–241, https://doi.org/10.1111/liv.13211 (2017).
Morath, M. A., Miller, S. F. & Finley, R. K. Jr. Nutritional indicators of postburn bacteremic sepsis. JPEN J Parenter Enteral Nutr 5, 488–491, https://doi.org/10.1177/0148607181005006488 (1981).
Fernandez-Ruiz, M. et al. Serum iron parameters in the early post-transplant period and infection risk in kidney transplant recipients. Transpl Infect Dis 15, 600–611, https://doi.org/10.1111/tid.12137 (2013).
Arosio, P., Elia, L. & Poli, M. Ferritin, cellular iron storage and regulation. IUBMB life 69, 414–422, https://doi.org/10.1002/iub.1621 (2017).
Smith, H. J. & Meremikwu, M. Iron chelating agents for treating malaria. The Cochrane database of systematic reviews, Cd001474, https://doi.org/10.1002/14651858.cd001474 (2003).
Islam, S. et al. Anti-inflammatory and anti-bacterial effects of iron chelation in experimental sepsis. The Journal of surgical research 200, 266–273, https://doi.org/10.1016/j.jss.2015.07.001 (2016).
Carrara, M., Baselli, G. & Ferrario, M. Mortality prediction in septic shock patients: Towards new personalized models in critical care. Conference proceedings: … Annual International Conference of the IEEE Engineering in Medicine and Biology Society. IEEE Engineering in Medicine and Biology Society. Annual Conference 2015, 2792–2795, https://doi.org/10.1109/embc.2015.7318971 (2015).
Warner, J. L. & Alterovitz, G. Phenome based analysis as a means for discovering context dependent clinical reference ranges. AMIA … Annual Symposium proceedings. AMIA Symposium 2012, 1441–1449 (2012).
Johnson, A. E. et al. MIMIC-III, a freely accessible critical care database. Sci Data 3, 160035, https://doi.org/10.1038/sdata.2016.35 (2016).
Zhang, Z., Chen, L. & Ni, H. Antipyretic therapy in critically ill patients with sepsis: an interaction with body temperature. PloS one 10, e0121919, https://doi.org/10.1371/journal.pone.0121919 (2015).
Singer, M. et al. The Third International Consensus Definitions for Sepsis and Septic Shock (Sepsis-3). Jama 315, 801–810, https://doi.org/10.1001/jama.2016.0287 (2016).
Kollef, M. H., Sherman, G., Ward, S. & Fraser, V. J. Inadequate antimicrobial treatment of infections: a risk factor for hospital mortality among critically ill patients. Chest 115, 462–474 (1999).
Finfer, S. et al. Intensive versus conventional glucose control in critically ill patients. N Engl J Med 360, 1283–1297, https://doi.org/10.1056/NEJMoa0810625 (2009).
Author information
Authors and Affiliations
Contributions
P.L., K.P., and J.Z. designed the study, extracted and analysed the data and drafted the article. Y.F., Q.S., and Y.C. assisted with data collection and manuscript preparation. S.W. and Y.Y assisted with the statistical analysis and commented on the manuscript. Y.J., X.H., J.Z. and Y.Y. commented on the manuscript.
Corresponding author
Ethics declarations
Competing Interests
The authors declare no competing interests.
Additional information
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Lan, P., Pan, Kh., Wang, Sj. et al. High Serum Iron level is Associated with Increased Mortality in Patients with Sepsis. Sci Rep 8, 11072 (2018). https://doi.org/10.1038/s41598-018-29353-2
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-018-29353-2
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.