Abstract
Mucormycosis is a life-threatening fungal infection caused by various ubiquitous filamentous fungi of the Mucorales order, although Rhizopus spp. and Mucor spp. are the most prevalent causal agents. The limited therapeutic options available together with a rapid progression of the infection and a difficult early diagnosis produce high mortality. Here, we developed an adult zebrafish model of Mucor circinelloides infection which allowed us to confirm the link between sporangiospore size and virulence. Transcriptomic studies revealed a local, strong inflammatory response of the host elicited after sporangiospore germination and mycelial tissue invasion, while avirulent and UV-killed sporangiospores failed to induce inflammation and were rapidly cleared. Of the 857 genes modulated by the infection, those encoding cytokines, complement factors, peptidoglycan recognition proteins, and iron acquisition are particularly interesting. Furthermore, neutrophils and macrophages were similarly recruited independently of sporangiospore virulence and viability, which results in a robust depletion of both cell types in the hematopoietic compartment. Strikingly, our model also reveals for the first time the ability of mucormycosis to induce the apoptosis of recruited macrophages but not neutrophils. The induction of macrophage apoptosis, therefore, might represent a key virulence mechanism of these fungal pathogens, providing novel targets for therapeutic intervention in this lethal infection.
Similar content being viewed by others
Introduction
Mucormycosis is a life-threatening fungal infection caused by various ubiquitous filamentous fungi of the Mucorales order, although Rhizopus spp. and Mucor spp. are the most prevalent causal agents1. This fungal infection is a rare disease, but the advances in medical care and an increasingly aging population have resulted in an increased incidence1,2, which is also favored by the absence of effective pharmacological treatments compared to more frequent fungal infections, such as candidiasis and aspergillosis. The limited therapeutic options available together with a rapid progression of the disease and a problematic early diagnosis produce unacceptable mortality, higher than 90% in disseminated infections3,4,5. Mucormycosis is an infection affecting all ages, from premature neonates to older adults, with varying clinical forms2. Unlike other filamentous fungal pathogens that infect immunocompromised patients, Mucorales show a broader and more heterogeneous target population, which include immunocompetent persons. The most important underlying conditions that increase the risk of mucormycosis are diabetes mellitus, suppressed immunity (e.g., because of transplantation, malignancies, HIV, steroids, or neutropenia)4,6, elevated serum levels of iron under treatment with deferoxamine and the breakdown of the skin barrier by burns or local trauma injuries. Other risk factors for mucormycosis include intravenous drug abuse, malnutrition, premature birth, and long-term application of the broad-spectrum antifungal voriconazole6. This heterogeneity of risk factors and the reduced treatments available suggest that this currently rare infection hides the potential to become a devastating infection if a strain acquired a higher virulence. In fact, mucormycosis outbreaks have been increasingly frequent in recent years mainly due to traumas as in the tornado in Joplin(Missouri, USA), where 13 persons were affected7. Therefore, more studies to deepen in the biology of Mucorales and the progression of the Mucormycosis are required to identify virulence factors that could be targets of new drugs because most drugs in the market have no or low effectiveness against Mucorales.
Characterization of the infection by Mucorales requires a fungal model amenable for both genetic manipulation and large-scale genomic screening to identify pathogenicity factors, in addition to a suitable animal model that allows studying the disease progression and defense response of the animal to the fungus. Within Mucorales, Mucor circinelloides represent the best model for genetic manipulation because several genetic tools have been developed, including gene knockout by homologous recombination and gene knockdown by RNAi8. Moreover, it is the second most important agent in Mucormycosis and virulence has been linked to genetic differences between subspecies. A phylogenetic analysis based on multi-locus sequence typing established three different M. circinelloides subspecies corresponding to the M. circinelloides f. lusitanicus (Mcl), M. circinelloides f. griseocyanus (Mcg), and M. circinelloides f. circinelloides (Mcc). Strains of Mcc were found to be more prevalent among clinical M. circinelloides isolates, and more virulent than Mcl and Mcg strains in a diabetic murine model9. However, most common laboratories strains belong to Mcl, which shows a sporangiospore size dimorphism that is linked to virulence in larvae of the wax moth, Galleria mellonella. Thus, Mcl strains that produce larger asexual sporangiospores are more virulent in the wax moth host than strains that produce small sporangiospores. The existence of strains with different virulence offers an opportunity to identify the genes behind the ability to infect humans.
Several models have been developed to study mucormycosis in invertebrates and vertebrates, both in vitro and in vivo9,10,11,12,13,14. Mammalian models would represent the best model due to its proximity to humans, but large-scale studies are not possible because of ethical considerations, logistic restraints associated with their use and difficulties in studying the dynamics of host-pathogen interaction in vivo. In recent years, the zebrafish (Danio rerio) has become an accepted model because its immune system is similar to the human15. A zebrafish larval infection model has been recently established to study early innate immune response to M. circinelloides in a whole-animal system and in real-time in vivo12. Despite that this model has been used with an Mcl strain, which is avirulent in a diabetic murine model and shows reduced virulence in wax moth host, it mimics a range of aspects of human mucormycosis showing that macrophages and neutrophils were readily recruited to the site of infection. Although sporangiospore phagocytosis was observed, they hardly activated gene expression of pro-inflammatory cytokines12. This work extends the zebrafish model for mucormycosis to adult fish because of the difficulties to use strains that produce large sporangiospores in larvae, since they get frequently stuck in the narrow needles used for infection, and to have the possibility of analyzing both innate and adaptive immune responses. Our results confirm the link between sporangiospore size and virulence previously in wax moth9, larval zebrafish12 and murine14 models, but reveal a robust inflammatory response elicited after sporangiospore germination and mycelial tissue invasion. This host inflammatory response is characterized by neutrophil and macrophages mobilization from the kidney, the main adult fish hematopoietic organ, to the infection site, and the modulation of 857 genes related to immune response and iron metabolism, among others. Importantly, the results also uncover a previously unappreciated ability of mucormycosis to induce the apoptosis of recruited macrophages, what might represent a key virulence mechanism of this fungal infection providing a novel target for therapeutic intervention.
Methods
Ethics statement
The experiments complied with the Guidelines of the European Union Council (Directive 2010/63/EU) and the Spanish RD 53/2013. Experiments and procedures were performed as approved by the Consejería de Agua, Agricultura, Ganadería y Pesca de la CARM (authorization number #A13170801).
Zebrafish husbandry
Zebrafish (Danio rerio H.) were obtained from the Zebrafish International Resource Center and mated, staged, raised and processed as described16.
Mucor circinelloides strains and growth conditions
The leucine auxotroph R7B, derived from the (−) mating type M. circinelloides f. lusitanicus CBS 277.49, was used as the wild-type strain. The M. circinelloides f. lusitanicus strain of the (+) mating type NRRL3631 was used in virulence assays as an avirulent control. Both strains used in this study R7B and NRRL3631 (NRRL for simplicity) were grown at 26 °C in complete medium YPG, minimal YNB medium or semi-complex MMC medium17. Media were supplemented with L-leucine (20 μg/ml) or uridine (200 μg/ml), when required. The pH was adjusted to 4.5 for mycelial growth. Sporangiospores were inactivated by ultraviolet irradiation during 30 min at 254 nm (UV crosslinker, Hoefer).
Zebrafish infection assays
Spores were collected from mycelium grown in YPG during five days, washed in distilled water and filtered using a cell strainer of 70 μm nylon mesh to eliminate rests of mycelium. These spores were kept in distilled water and used in the virulence assays during the next five days. Spore viability was assessed by plating on YPG medium, and no differences in viability were observed between R7B and NRRL. Zebrafish were intraperitoneally (i.p.) injected with live or UV-inactivated R7B (2.5 × 105) or NRRL (2.5 × 105 or 107) sporangiospores/fish in groups of twenty specimens at 26 °C in 10-liter tanks. After the challenge, the fish were monitored every 12 h over a 7-day period for clinical signs of disease and mortality. At 16 hours post-infection (hpi), samples of the kidney and the abdominal organs were collected and processed for analysis of gene expression, while complete specimens were processed for histology (see below).
Mouse macrophage cultures and treatments
The mouse macrophage cell line J774 was obtained from the European Collection of Authenticated Cell Cultures (ECACC) and grown in DMEM-high glucose supplemented with 10% heat-inactivated FCS, 100 U/mL penicillin and 100 µg/mL streptomycin at 37 °C in 5% CO2. Cells were incubated with 1:1 live or UV-killed sporangiospores from R7B and NRRL strains alone or in combination with 10 ng/ml lipopolysaccharide (LPS) from Escherichia coli 0111.B4 (Sigma-Aldrich) for 3 and 16 h, then washed twice with PBS and processed for RT-qPCR analysis as indicated below.
Histology
Zebrafish were fixed in AZF solution (3% zinc chloride, 5.6% formol, 1% acetic acid) for four days at 4 °C, and descalcificated in EDTA (0.25 M, pH 8) for three days at 4 °C. Afterward, they were dehydrated, embedded in Paraplast Plus and sectioned at 5 µm. Serial sections were stained with periodic acid-Schiff stain (PAS) and specific antibodies to zebrafish myeloperoxidase (Mpx, #GTX128379, GeneTex), to zebrafish lymphocyte cytosolic protein 1 (Lcp1, #GTX124420, GeneTex) and to a fully conserved epitope of active caspase-3 (#AF835, R&D Systems) by using standard procedures18,19.
The quantification of Lcp1+, Mpx+, and Casp3+ cells was calculated as the mean ± SEM of the immunostained area/total area of 4 randomly distributed optical regions from 4 fish at ×200 magnification. The stained areas were measured by image analysis using a Nikon Eclipse E600 light microscope, an Olympus SC30 digital camera (Olympus Soft Imaging Solutions), and Leica Qwin software (Leica Microsystems).
Library preparation and sequencing
Total RNA was extracted from the abdominal organs using TRIzol® Plus RNA Purification System (Thermo Fisher Scientific) following the manufacturer’s instructions. To maximize target coverage, equal amounts of total RNA from three biological replicates of each strain were pooled for RNA-seq library construction. Both library preparation and sequencing was performed at Baseclear (Leiden, The Netherlands). The cDNA library was sequenced using Illumina HiSeq 2500 sequencer with one lane, 50 cycles, single-read sequencing strategy.
RNA-seq data analysis
Quality control of the reads was carried out using FastQC v0.11.220. Reads were trimmed to remove adapter sequences; the first 14 bases were removed from each read. Trimmed reads were mapped to the Ensembl (Flicek et al., 2014) Zebrafish genome release 75, using Bowtie2 version 2.1.021 with “very-sensitive” and “end-to-end” parameters, then short reads with more than one hit were eliminated. The number of reads per gene generated was determined with HTSeq22, using the default “union” counting option. In order to identify the low expression genes, the number of reads per gene was normalized to counts per million (CPM), a simple measure of read abundance independent of the mean expressed transcript length and thus more comparable across different samples23. Only those genes with at least 2 CPM in at least one sample were considered as transcriptionally active and used for subsequent analyses. Differential expression of genes in fish inoculated with R7B spores (RDRZ) versus controls inoculated with PBS (PBS2) was estimated with the package edgeR (R version: 3.0.1, edgeR version: 3.2.4,24); using statistical methods based on pairwise comparison with common dispersion used to estimate variance between samples. For this analysis without biological replicates, the common dispersion was estimated of a conservative way from a list of putative housekeeping genes (determined for these samples, with a maximum fold change below 1.99 and transcriptionally expressed) and using the script: estimateDisp(dge,robust = TRUE,winsor.tail = c(0.05,0.2). The common dispersion calculated was 0.0840. Genes that exhibited an FDR ≤ 0.01 and a log2FoldChange > 1 (log2FC) were considered differentially expressed (DEG). A combination of David annotation system (http://david.abcc.ncifcrf.gov/), gene ontology (GO) database (www.geneontology.org) and manual curation were used to obtain the functional classification of the DEG. Enrichment analyses were performed using Blast2GO25, with an FDR ≤ 0.05. The enrichment ratio for each GO term was obtained by dividing the percentage of sequences of the test set versus the percentage of sequences of the reference set, where the reference set was the predicted genes in the genome, and the test sets were the up-regulated and down-regulated genes. The function to most specific terms was used to reduce the result-set of over-represented GO terms. The list of genes encoding proteins with transcription factor functions was downloaded from AnimalTFDB 2.026. The gplots R library was used to generate the gene expression heatmaps (http://cran.r-project.org/web/packages/gplots/index.html).
RT-qPCR
Total RNA extracted from zebrafish kidney and abdominal organs and mouse J774 macrophages as indicated above was treated with DNase I, Amplification grade (1 unit/μg RNA, Invitrogen) and the SuperScript III RNase H− ReverseTranscriptase (Thermo Fisher Scientific) was then used to synthesize the first strand of cDNA with an oligo-dT18 primer from 1 μg of total RNA at 50 °C for 50 min. Real-time PCR was performed with an ABI PRISM 7500 instrument (Applied Biosystems) using SYBR Green PCR Core Reagents (Applied Biosystems). Reaction mixtures were incubated for 10 min at 95 °C, followed by 40 cycles of 15 s at 95 °C, 1 min at 60 °C, and finally, 15 s at 95 °C, 1 min at 60 °C and 15 s at 95 °C. For each mRNA, the gene expression was normalized to the ribosomal proteins S11 content in each sample using the Pfaffl method (30). Table 1 shows the primers used. In all cases, each PCR was performed with triplicate samples and repeated with at least three independent samples.
Statistical analysis
Error bars indicate standard error of the mean (SEM). For gene expression experiments data are shown as mean ± SEM of three separate experiments. The differences between experimental treatments and control (PBS) were analyzed by Student’s t test. Log-rank (Mantel–Cox) test was used for the survival curves.
Results
An adult zebrafish model recapitulates the linkage between sporangiospore size dimorphism and virulence of M. circinelloides
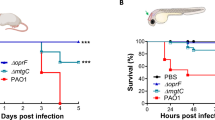
Adult fish were i.p. infected with different doses of sporangiospores from the R7B and NRRL strains, which produce large and small sporangiospores, respectively. The results showed that 2.5 × 105 sporangiospores consistently resulted in 100% mortality of zebrafish infected with R7B as early as 24 hpi and no mortality with the same dose of NRRL sporangiospores (Fig. 1A). This clear difference in virulence between strains that produce big and small sporangiospores was previously observed in infections of Galleria mellonela larvae9. However, a much higher dose of NRRL sporangiospores (107) was able to kill zebrafish as effective as 2.5 × 105 sporangiospores of R7B, the effect being dependent on sporangiospores viability (Fig. 1A). Moribund fish showed redness of the abdomen and petechial hemorrhaging, and the mycelium was observed invading different abdominal organs (Fig. 1B). Histological examination confirmed germination of R7B sporangiospores and hyphal invasion, and frequently vacuolization and disorganization, of various organs, including muscle, liver, intestine, ovarium, and testis (Fig. 1B,C). Although similar results were found when using the high dose of sporangiospores of the NRRL strain, abundant sporangiospores remained inside the peritoneal cavity and did not germinate (Fig. 1C).
M. circinelloides R7B is highly pathogenic for zebrafish. Zebrafish were i.p. injected with 2.5 × 105 or 107 (high dose: HD) live or UV-killed sporangiospores of the R7B or NRRL strains. (A) The survival rates of infected zebrafish are shown. Each experimental group contained 20 zebrafish, and each experiment was performed in duplicate. *P < 0.05; **P < 0.01; ***P < 0.001. (B) Gross morphological examinations of adult zebrafish infected with M. circinelloides R7B and NRRL were imaged at 16 hpi. (C) Histological analysis of M circinelloides-infected zebrafish. Transverse sections were obtained from zebrafish injected with 2.5 × 105 R7B spores/fish or 107 NRRL spores/fish at 16 hpi. Note that the mycelium (black arrows) can invade several organs and promotes a strong leukocyte infiltration (red arrows). L, liver; G, gut; M, muscle; O, ovary; T, testis. Scale bars 10 µm
Zebrafish transcriptome analysis in response to M. circinelloides infection reveals genes with putative functions in mucormycosis
To determine zebrafish transcriptomic response to infection with M. circinelloides R7B strain, we prepared and sequenced two RNA libraries from zebrafish inoculated with a 2.5 × 105 sporangiospores of the R7B strain (RDRZ) or buffer (PBS2). We obtained about of 15 and 14 million short reads of a length of 50 nt and an average quality score (Phred) of 39.19 and 39.22 for PBS2 and RDRZ libraries, respectively (Table S1). After trimming, the total of reads with a length mean of 36 nt for each sample were mapped to the zebrafish reference genome. After eliminating ambiguous reads (with more than one hit to the genome), we obtained 9,372,491 reads (64.5%) and 9,644,092 reads (62%) for RDRZ and PBS2 libraries, respectively (Table S1). Overall transcriptional activity, using normalized reads with at least 2 CPM in at least one sample, indicated that 16,071 genes are transcriptionally active, representing about 60.7% of predicted genes in the D. rerio genome.
To study the zebrafish transcriptional response to infection with M. circinelloides R7B strain, we compared the RDRZ sample versus the control (PBS2). A total of 857 differentially expressed genes were detected, from which 712 were up-regulated and 145 down-regulated (Fig. 2A and Additional file 1). A combination of David annotation system and GO database allowed to associate a functional annotation and/or molecular function to 83.9% of differentially expressed genes (Additional file 1 and Fig. S1).
Differential expression analysis. (A) Heat-map shows the 857 differentially expressed genes clustered by their expression levels in response to M. circinelloides R7B strain infection. Roman numbers and left column colors indicate independent clusters. Color key indicates the log2 fold change values. (B) The graph shows the most specific biological process enriched. (C) The graph shows the most specific molecular function enriched. In (B,C), X-axis indicates the enrichment ratio obtained by dividing the sequences percentage of the test set versus the sequences percentage of the reference set (see methods section). Positive and negative values, respectively represent biological processes and molecular function enriched in up-regulated, and down-regulated genes. AMC, aminoglycan catabolic process; ATA, ATPase activity; BCU, blood coagulation; CBI, calcium ion binding; CRE, cellular response to estrogen stimulus; CTT, carbohydrate transmembrane transport; DRB, defense response to bacterium; EIA, endopeptidase inhibitor activity; HEB, heme binding; HOG, hydrolase activity, hydrolyzing O-glycosyl compounds; INB, iron ion binding; IRE, immune response; LPT, lipid transport; MOM, monocarboxylic acid metabolic process; ORP, oxidation-reduction process; OXI, oxidoreductase activity; PAC, protein activation cascade; PEC, peptidoglycan catabolic process; PRO, proteolysis; RIR, regulation of immune system process; ROC, response to organic cyclic compound; SAR, sarcomere organization; SEP, serine-type endopeptidase activity; SMC, small molecule catabolic process; STT, sodium ion transmembrane transporter activity; TAC, transporter activity; TTA, active transmembrane transporter activity; UTT, urea transmembrane transport; VCM, ventricular cardiac myofibril assembly. Asterisks indicate the statistical significance level, FDR ≤ 0.001 (***), FDR ≤ 0.01 (**) and FDR ≤ 0.05 (*).
To identify the biological process and molecular functions involved in the Zebrafish-M. circinelloides interaction, we performed a GO enrichment analysis using Blast2GO. The GO terms with an FDR ≤ 0.05 were considered enriched. Our analysis showed enrichment of 52 biological processes and 29 molecular functions in the up-regulated genes and only two biological processes and three molecular functions in the down-regulated genes (Additional file 2). Subsequently, the function of most specific terms was used to reduce the over-represented GO terms. This analysis indicated that the up-regulated genes were enriched in biological processes and molecular functions related to peptidoglycan catabolic, protein activation cascade, defense response to bacterium, blood coagulation, immune response, regulation of immune response, urea transport activity, oxidoreductase activity, endopeptidase inhibitor, lipid transporter activity, serine-type endopeptidase activity, transmembrane transporter activity, and calcium ion binding among others. In contrast, the down-regulated genes were enriched only in biological process and molecular functions related to lipid transport activity (Fig. 2B,C and Additional file 2).
The clustering by expression levels of the 857 genes grouped them in 6 clusters (Fig. 2A). Most of the transcriptional activity in response to infection by the R7B strain was displayed on the up-regulated genes (clusters I, II, III). Cluster I comprised 57 genes strongly induced related to iron homeostasis, cytoskeleton and muscle contraction. Like cluster I, cluster II included 99 induced genes related mainly to cytoskeleton and muscle contraction. Interestingly, the biggest cluster III was constituted by 556 slightly induced genes involved in protein activation cascade, regulation of immune response and transport. On the other hand, the down-regulated genes were grouped in the clusters IV, V and VI. Custer IV included 80 slightly repressed genes related to oxidoreductase activity. Cluster V comprised 41 repressed genes involved mainly in lipid transport. Cluster VI formed by 24 strongly repressed, however, their functions were not clear (Fig. 2A and Additional file 1). In addition, an enrichment analysis of each cluster showed GO terms enriched only for the clusters III and V. Cluster III the most enriched categories were related to protein activation cascade, coagulation, response to estrogen, regulation of immune response, response to wounding, transport activity, oxidoreductase activity, endopeptidase inhibitor activity, enzyme inhibitor activity and lipid transport activity among others. Also, cluster V was enriched in genes encoding proteins involved in lipid transport, response to estradiol and single-organism transport (Additional file 1).
Of the 857 differentially expressed genes, 26 were enriched in GO terms related to immune response and/or regulation of immune system response. As shown in Table 2 this group included up-regulated genes related to chemokine signal transduction, complement system pathway, major histocompatibility complex, cytokines system, proteoglycan metabolism, pathogen recognition, activation of innate immunity and Lck/Yes novel (LYN) tyrosine kinase. Interestingly, our enrichment analysis showed a genes group enriched for a GO term related to defense response to bacterium. This group comprised six genes encoding proteins with functions connected to interleukin system, peptidoglycan recognition, ribonuclease, pathogen recognition and activation of innate immunity through toll-like receptor 5b, antimicrobial peptide, and iron homeostasis. Another of the biological processes enriched in the infection by M. circinelloides was the protein activation cascade process, which encompassed four induced genes mainly related to the complement system including the complement C2, complement factor B and the mannan-binding lectin serine peptidase 2 genes (Table 2).
Sporangiospore germination and host infection induce a strong inflammatory response
RT-qPCR analyses confirmed the local strong immune response to the infection and validated the transcriptomic analysis (Fig. 3). Thus, the R7B strain drastically increased the transcript levels of interleukin-1β (il1b), tumor necrosis factor α (tnfa) and il22 genes, which encodes major pro-inflammatory cytokines. Interestingly, sporangiospores germination and host infection was required to promote a local inflammatory response, since challenge with live NRRL and UV-killed R7B and NRRL sporangiospores failed to increase the mRNA levels of il1b, tnfa, and il22, while a higher dose of NRRL sporangiospores, which could kill the fish, promoted a similar effect than a lower dose of highly virulent R7B. These results were confirmed in mouse J774 macrophages, where live R7B sporangiospores induced higher Il1b transcript levels than NRRL independently of costimulation with LPS (Fig. 4).
R7B is more potent than NRRL inducing immune-related gene expression in zebrafish. Zebrafish were i.p injected with 2.5 × 105 or 107 (high dose: HD) live or UV-inactivated sporangiospores of the R7B or NRRL strains. At 16 hpi, the expression of the indicated immune-related genes was analyzed by RT-qPCR in the infection site and compared to those of control fish injected with PBS (n = 4). *P < 0.05; ***P < 0.001. ns, nonsignificant.
M. circinelloides promotes myeloid cell depletion in the kidney
The above results prompted us to examine the impact of infection at systemic levels. Therefore, we analyze the gene expression levels of genes encoding major pro-inflammatory mediators in the kidney, which is the principal hematopoietic organ in adult zebrafish. Infection with both strains resulted in dramatically reduced mRNA levels of il1b, tnfa, prostaglandin-endoperoxide synthase 2a (ptgs2a) and ptgs2b compared with fish injected with PBS (Fig. 5). Notably, the transcript levels of genes encoding neutrophil (myeloperoxidase, Mpx)27 and macrophage (macrophage expressed 1, Mpeg1)28 markers, also robustly declined (Fig. 5), suggesting the depletion of both cell types in the kidney. This was confirmed by staining neutrophils and macrophages with antibodies to zebrafish Lcp1 and Mpx that consistently revealed a drastic reduction of macrophages (Lcp1+/Mpx−) and neutrophils (Lcp1+/Mpx+) at 16 hpi in the kidney of R7B infected fish (Fig. 6A,B).
M. circinelloides inhibits the expression of immune-related genes in the head kidney of zebrafish. The mRNA levels of the genes coding for the indicated pro-inflammatory and neutrophil (mpx) and macrophage (mpeg1) markers were determined by RT-qPCR at 16 hpi in the head kidney from zebrafish challenged with 2.5 × 105 R7B spores/fish or 107 NRRL spores/fish (n = 8). *P < 0.05; **P < 0.01; ***P < 0.001. ns, nonsignificant.
M. circinelloides promotes neutrophils and macrophages depletion in the head kidney. (A) Representative sections of zebrafish head kidneys infected with 2.5 × 105 R7B spores/fish at 16 hpi and immunostained with anti-Lcp1 (pan-leukocyte marker) and anti-Mpx (neutrophil marker) antibodies. Note the robust depletion of myeloid cells. Scale bars 20 µm. (B) Quantification of Lcp1+ and Mpx+ cells is shown as the mean ± SEM of the immunostained area/total area of 4 randomly distributed optical areas from 4 fish at ×200 magnification. *P<0.05; **P<0.01; ***P<0.001.
M. circinelloides infection induces apoptosis of recruited macrophages in the infection site
We then analyzed the expression of the genes encoding Mpx and Mpeg1 in the infection site. Strikingly, the transcript levels of mpx increased in infected fish with both live and UV-killed sporangiospores from both strains (Fig. 7A), whereas those of mpeg1 robustly declined in fish infected with R7B and, to some extent, with the high dose of NRRL (Fig. 7B); the two conditions that resulted in high fish mortality. This result might suggest that infection promoted macrophage killing. Histological analysis using the anti-Lcp1 and anti-Mpx antibodies revealed that numerous macrophages (Lcp1+/Mpx−), but not neutrophils (Lcp1+/Mpx+), were apoptotic, i.e. Casp3+, in fish infected with the R7B strain and with the high dose of the NRRL strain (Fig. 8A,B), confirming the gene expression analysis.
M. circinelloides altered the expression profile of gene encoding neutrophil and macrophages markers in the infection site. The mRNA levels of the genes coding for neutrophil (mpx) (A) and macrophages (mpeg1) (B) markers were determined by RT-qPCR at 16 hpi in the head kidney of zebrafish challenged with live or UV-killed 2.5×105 R7B spores/fish and 2.5×105 or 107 (high dose, HD) NRRL spores/fish (n=4). *P<0.05; ***P<0.001. ns, nonsignificant.
M. circinelloides promotes the recruitment and apoptosis of macrophages in the infection site. (A) Representative serial sections of zebrafish head kidneys challenged with PBS, 2.5×105 R7B spores/fish and 107 (high dose, HD) NRRL spores/fish at 16 hpi and immunostained with anti-Lcp1, anti-Mpx, and anti-active Casp3 antibodies. Infiltrated leukocytes (black arrows) at 16 hpi were PAS+, Lcp1+ and most of them Casp3+ in the fish infected with the virulent strain (R7B) and the HD of the avirulent NRRL strain. Scale bars 20 µm. (B) Quantification of Lcp1+, Mpx+, and Casp3+ cells is shown as the mean ± SEM of the immunostained area/total area of 4 randomly distributed optical regions from 4 fish at ×200 magnification. *P<0.05.
Discussion
Although mucormycosis has historically been considered a rare disease, at present is the third most prevalent fungal infection29. Although both in vitro and in vivo models in invertebrates and vertebrates have been established, additional models able to shed light into the early stage of infection, when the transition to filamentous growth occurs, will help to design appropriate prophylactic in targeted populations. We have developed here an adult zebrafish model of mucormycosis by i.p. injection of sporangiospores that recapitulates the link between sporangiospore size and virulence previously reported in wax moth9 and larval zebrafish30 models. This observation confirms the relevance of identifying therapeutic targets to inhibit fungal germination and potentiate protective immunity to sporangiospores before the transition to mycelial growth31.
Our model also revealed a robust inflammatory response at the infection site characterized by the upregulation of genes encoding major pro-inflammatory cytokines, such as Il1b, Tnfa, Il22, several chemokines and chemokine receptors, complement factors, and pattern recognition receptors (PRR). It is known that invasive filamentous growth induces IL1B and TNFA release by human neutrophils through a TLR2-signaling pathway32, while human monocyte-derived dendritic cells release IL1B, TNFA and IL23 via dectin-1 signaling33. However, hyphae are highly resistant to oxidative damage32. Our transcriptomic analysis revealed the induction of genes encoding complement factors, including complement factor B (Cfb) and mannan-binding lectin serine peptidase 2 (Masp2), which are involved in the activation of the alternative and lectin pathways, respectively. This observation, together with the ability of M. circinelloides sporangiospores to activate such pathways34, suggests a role of complement activation on the control of M. circinelloides germination and mycelial growth. However, functional studies will be required to demonstrate a role for complement in the control of mucormycosis. Furthermore, genes encoding two peptidoglycan recognition proteins (PGRP), were also induced in infected fish. Although these proteins are involved in bacterial clearance, a recent report showed that Candida albicans infection induces PGRP2 in human corneal epithelial cells though dectin-1/NF-κB signaling and, more importantly, that PGRP2 suppress colony-forming units of C. albicans in vitro35. Finally, several genes related to iron homeostasis, including the antimicrobial peptide hepcidin, were highly induced by infection, further confirming the critical role of iron acquisition by Mucorales in the fate of the infection36.
Our results also showed a robust neutrophil and macrophage mobilization from the kidney, the main adult fish hematopoietic organ, to the infection site. Curiously, while the induction of the potent inflammatory response required sporangiospores germination and mycelia colonization of host tissues, neutrophil mobilization was similarly elicited by both strains as well as by alive and UV-killed sporangiospores. Similarly, recruitment of macrophages and neutrophils to viable and UV-killed was also reported at real-time using a larval zebrafish model, although both phagocyte types showed clustering exclusively around viable spores, what may inhibit spore dissemination30. In contrast, spores from other fungi, such as Rhizopus oryzae and Aspergillus fumigatus, are unable to promote neutrophil chemotaxis in vitro37. Although these differences may be related to the particular fungal pathogens, the ex vivo systems are unable to model the critical interactions between macrophages and neutrophils that occur in vivo. For example, in a mouse model of pulmonary A. fumigatus infection, it has been shown that neutrophil recruitment depends on the release of IL-1α and CXCL1 by CCR2+ monocytes38, highlighting the need of in vivo fungal infection models.
The most important observation of our study is the previously unappreciated ability of mucormycosis to induce the apoptosis of recruited macrophages and the robust depletion of both macrophages and neutrophils from the hematopoietic compartment. Notably, the apoptosis of recruited macrophages was caused by the virulent R7B strain but also by a high dose of the less pathogenic NRRL, suggesting that germination is required for induction of macrophage apoptosis. Similarly, manipulation of neutrophils and macrophages apoptosis and myelopoiesis has been shown to be critical for the in vivo clearance of A. fumigatus39 and Pneumocystis40 lung infections, and C. albicans renal infection41. Also, Cryptococcus neoformans can induce macrophage apoptosis in a capsule-dependent manner, suggesting the presence of molecules in the capsule that trigger apoptosis42. Collectively, these results suggest that induction of macrophage apoptosis might represent a key virulence mechanism of mucormycosis and, therefore, provides a novel target for therapeutic intervention.
In summary, we have established an adult zebrafish mucormycosis model highly complementary to the mouse one which allows reconstituting the complex interactions between macrophages and neutrophils in response to fungal infections and, with its amenable genetic and pharmacological manipulation, will contribute to understanding the mechanisms involved in the manipulation of innate immunity by Mucorales. Using this model, we have confirmed the link between sporangiospore size and virulence, revealed a strong inflammatory response elicited after sporangiospore germination and mycelial tissue invasion, which is characterized by neutrophil and macrophages recruitment, and the modulation of 857 genes related to immune response and iron metabolism, and uncovered the ability of mucormycosis to induce the apoptosis of recruited macrophages.
Data availability
All data generated or analyzed during this study are included in this published article and its Supplementary Information files. Illumina RNA sequencing data have been deposited in the NCBI Sequence Read Archive (SRA) under accession number SRP132190.
References
Mendoza, L. et al. Human Fungal Pathogens of Mucorales and Entomophthorales. Cold Spring Harbor perspectives in medicine 5, https://doi.org/10.1101/cshperspect.a019562 (2015).
Petrikkos, G. et al. Epidemiology and clinical manifestations of mucormycosis. Clin Infect Dis 54(Suppl 1), 34, https://doi.org/10.1093/cid/cir866 (2012).
Ribes, J. A., Vanover-Sams, C. L. & Baker, D. J. Zygomycetes in human disease. Clin Microbiol Rev 13, 236–301, https://doi.org/10.1128/CMR.13.2.236-301.2000 (2000).
Roden, M. M. et al. Epidemiology and outcome of zygomycosis: a review of 929 reported cases. Clinical infectious diseases: an official publication of the Infect Dis Soc Amer 41, 634–653, https://doi.org/10.1086/432579 (2005).
Lanternier, F. et al. Mucormycosis in organ and stem cell transplant recipients. Clin Infect Dis 54, 1629–1636, https://doi.org/10.1093/cid/cis195 (2012).
Chayakulkeeree, M., Ghannoum, M. A. & Perfect, J. R. Zygomycosis: the re-emerging fungal infection. Eur J Clin Microbiol Infect Dis 25, 215–229, https://doi.org/10.1007/s10096-006-0107-1 (2006).
Fanfair, R. et al. Necrotizing Cutaneous Mucormycosis after a Tornado in Joplin, Missouri, in 2011. New Eng J Med 367, https://doi.org/10.1056/NEJMoa1204781.
Torres-Martínez, S. et al. Molecular tools for carotenogenesis analysis in the zygomycete Mucor circinelloides. Meth Mol Biol 898, 85–107, https://doi.org/10.1007/978-1-61779-918-1_5 (2012).
Li, C. H. et al. Sporangiospore size dimorphism is linked to virulence of Mucor circinelloides. PLoS Pathog 7, e1002086, https://doi.org/10.1371/journal.ppat.1002086 (2011).
Ibrahim, A. S., Spellberg, B., Avanessian, V., Fu, Y. & Edwards, J. E. Rhizopus oryzae adheres to, is phagocytosed by, and damages endothelial cells in vitro. Infect Immun 73, 778–783, https://doi.org/10.1128/IAI.73.2.778-783.2005 (2005).
Chamilos, G. et al. Drosophila melanogaster as a model host to dissect the immunopathogenesis of zygomycosis. Proc Natl Acad Sci USA 105, 9367–9372, https://doi.org/10.1073/pnas.0709578105 (2008).
Voelz, K., Gratacap, R. L. & Wheeler, R. T. A zebrafish larval model reveals early tissue-specific innate immune responses to Mucor circinelloides. Dis Mod Mech 8, https://doi.org/10.1242/dmm.019992 (2015).
Waldorf, A. R., Ruderman, N. & Diamond, R. D. Specific susceptibility to mucormycosis in murine diabetes and bronchoalveolar macrophage defense against Rhizopus. J Clin Invest 74, 150–160, https://doi.org/10.1172/JCI111395 (1984).
Trieu, T. A. et al. RNAi-Based Functional Genomics Identifies New Virulence Determinants in Mucormycosis. PLoS Pathog 13, e1006150, https://doi.org/10.1371/journal.ppat.1006150 (2017).
Tobin, D. M., May, R. C. & Wheeler, R. T. Zebrafish: A See-Through Host and a Fluorescent Toolbox to Probe Host–Pathogen Interaction. PLoS Pathog 8, https://doi.org/10.1371/journal.ppat.1002349 (2012).
Westerfield, M. The zebrafish book. A guide for the laboratory use of zebrafish Danio (Brachydanio) rerio. 4th edn, (University of Oregon Press, 2000).
Nicolas, F. E. et al. The RNAi machinery controls distinct responses to environmental signals in the basal fungus Mucor circinelloides. BMC Genomics 16, 237, https://doi.org/10.1186/s12864-015-1443-2 (2015).
Mulero, I., Sepulcre, M. P., Meseguer, J., Garcia-Ayala, A. & Mulero, V. Histamine is stored in mast cells of most evolutionarily advanced fish and regulates the fish inflammatory response. Proc Natl Acad Sci USA 104, 19434–19439, https://doi.org/10.1073/pnas.0704535104 (2007).
Roca, F. J. et al. Evolution of the inflammatory response in vertebrates: fish TNF-alpha is a powerful activator of endothelial cells but hardly activates phagocytes. J Immunol 181, 5071–5081, https://doi.org/181/7/5071 (2008).
Andrews, S. FastQC: A quality control tool for high throughput sequence data. (2010).
Langmead, B. & Salzberg, S. L. Fast gapped-read alignment with Bowtie 2. Nature methods 9, 357–359, https://doi.org/10.1038/nmeth.1923 (2012).
Anders, S., Pyl, P. T. & Huber, W. HTSeq–a Python framework to work with high-throughput sequencing data. Bioinformatics 31, 166–169, https://doi.org/10.1093/bioinformatics/btu638 (2015).
Li, B., Ruotti, V., Stewart, R. M., Thomson, J. A. & Dewey, C. N. RNA-Seq gene expression estimation with read mapping uncertainty. Bioinformatics 26, 493–500, https://doi.org/10.1093/bioinformatics/btp692 (2010).
Robinson, M. D., McCarthy, D. J. & Smyth, G. K. edgeR: a Bioconductor package for differential expression analysis of digital gene expression data. Bioinformatics 26, 139–140, https://doi.org/10.1093/bioinformatics/btp616 (2010).
Conesa, A. et al. Blast2GO: a universal tool for annotation, visualization and analysis in functional genomics research. Bioinformatics 21, 3674–3676, https://doi.org/10.1093/bioinformatics/bti610 (2005).
Zhang, H. M. et al. AnimalTFDB 2.0: a resource for expression, prediction and functional study of animal transcription factors. Nuc Acids Res 43, D76–81, https://doi.org/10.1093/nar/gku887 (2015).
Renshaw, S. A. et al. A transgenic zebrafish model of neutrophilic inflammation. Blood 108, 3976–3978, https://doi.org/blood-2006-05-024075 (2006).
Ellett, F., Pase, L., Hayman, J. W., Andrianopoulos, A. & Lieschke, G. J. mpeg1 promoter transgenes direct macrophage-lineage expression in zebrafish. Blood 117, e49-56, https://doi.org/blood-2010-10-314120 (2011).
Ibrahim, A. S., Edwards, J. E. J. & Filler, S. G. Zygomycosis. 241–251 (Oxford University Press;, 2003).
Voelz, K., Gratacap, R. L. & Wheeler, R. T. A zebrafish larval model reveals early tissue-specific innate immune responses to Mucor circinelloides. Dis Model Mech 8, 1375–1388, https://doi.org/10.1242/dmm.019992 (2015).
Brown, G. D. et al. Hidden killers: human fungal infections. Sci Transl Med 4, 165rv113, https://doi.org/10.1126/scitranslmed.3004404 (2012).
Chamilos, G., Lewis, R. E., Lamaris, G., Walsh, T. J. & Kontoyiannis, D. P. Zygomycetes hyphae trigger an early, robust proinflammatory response in human polymorphonuclear neutrophils through toll-like receptor 2 induction but display relative resistance to oxidative damage. Antimicrob Agents Chemother 52, 722–724, https://doi.org/10.1128/AAC.01136-07 (2008).
Chamilos, G. et al. Generation of IL-23 producing dendritic cells (DCs) by airborne fungi regulates fungal pathogenicity via the induction of T(H)-17 responses. PLoS One 5, e12955, https://doi.org/10.1371/journal.pone.0012955 (2010).
Granja, L. F. et al. Spores of Mucor ramosissimus, Mucor plumbeus and Mucor circinelloides and their ability to activate human complement system in vitro. Med Mycol 48, 278–284, https://doi.org/10.3109/13693780903096669 (2010).
Hua, X. et al. A Novel Innate Response of Human Corneal Epithelium to Heat-killed Candida albicans by Producing Peptidoglycan Recognition Proteins. PLoS One 10, e0128039, https://doi.org/10.1371/journal.pone.0128039 (2015).
Baldin, C. & Ibrahim, A. S. Molecular mechanisms of mucormycosis-The bitter and the sweet. PLoS pathogens 13, e1006408, https://doi.org/10.1371/journal.ppat.1006408 (2017).
Waldorf, A. R. & Diamond, R. D. Neutrophil chemotactic responses induced by fresh and swollen Rhizopus oryzae spores and Aspergillus fumigatus conidia. Infect Immun 48, 458–463 (1985).
Caffrey, A. K. et al. IL-1alpha signaling is critical for leukocyte recruitment after pulmonary Aspergillus fumigatus challenge. PLoS Pathog 11, e1004625, https://doi.org/10.1371/journal.ppat.1004625 (2015).
Shepardson, K. M. et al. Myeloid derived hypoxia inducible factor 1-alpha is required for protection against pulmonary Aspergillus fumigatus infection. PLoS Pathog 10, e1004378, https://doi.org/10.1371/journal.ppat.1004378 (2014).
Taylor, D., Wilkison, M., Voyich, J. & Meissner, N. Prevention of bone marrow cell apoptosis and regulation of hematopoiesis by type I IFNs during systemic responses to pneumocystis lung infection. J Immunol 186, 5956–5967, https://doi.org/10.4049/jimmunol.1003558 (2011).
Lionakis, M. S. et al. CX3CR1-dependent renal macrophage survival promotes Candida control and host survival. J Clin Invest 123, 5035–5051, https://doi.org/10.1172/JCI71307 (2013).
Ben-Abdallah, M. et al. Fungal-induced cell cycle impairment, chromosome instability and apoptosis via differential activation of NF-kappaB. PLoS pathogens 8, e1002555, https://doi.org/10.1371/journal.ppat.1002555 (2012).
Acknowledgements
We thank I. Fuentes and P. Martínez for their excellent technical assistance. This work was supported by the Spanish Ministry of Economy and Competitiveness (grants BIO2011-23400 and BIO2014-52655-R to VM and BFU2015- 65501-P to VG), all co-funded with Fondos Europeos de Desarrollo Regional/European Regional Development Funds), and Fundación Séneca-Agencia de Ciencia y Tecnología de la Región de Murcia, Spain to VG (19339/PI/14). MINM was funded by the Spanish Ministry of Education, Culture and Sport (FPU-14/01832) and FEN was funded by Spanish Ministry of Economy and Competitiveness (RYC-2014-15844). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Author information
Authors and Affiliations
Contributions
V.M. and V.G. conceived the study; A.L.-M., F.E.N., S.T.-M., R.M.R.-V., V.G. and V.M. designed research; A.L.-M., F.E.N., D.G.M., A.B.P.O., M.I.N.-M., M.A.H.-O. and A.H.-E. performed research; A.L.-M., F.E.N., D.G.M., A.B.P.O., M.I.N.-M., M.A.H.-O., A.H.-E., S.T.-M., R.M.R.-V., V.G. and V.M. analyzed data; and V.G. and V.M. wrote the manuscript with minor contribution from other authors.
Corresponding authors
Ethics declarations
Competing Interests
The authors declare no competing interests.
Additional information
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
López-Muñoz, A., Nicolás, F.E., García-Moreno, D. et al. An Adult Zebrafish Model Reveals that Mucormycosis Induces Apoptosis of Infected Macrophages. Sci Rep 8, 12802 (2018). https://doi.org/10.1038/s41598-018-30754-6
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-018-30754-6
This article is cited by
-
H3K4 methylation regulates development, DNA repair, and virulence in Mucorales
IMA Fungus (2024)
-
Comparative genomics applied to Mucor species with different lifestyles
BMC Genomics (2020)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.