Abstract
Infants are sensitive to and converge emotionally with peers’ distress. It is unclear whether these responses extend to positive affect and whether observing peer emotions motivates infants’ behaviors. This study investigates 8-month-olds’ asymmetric frontal EEG during peers’ cry and laughter, and its relation to approach and withdrawal behaviors. Participants observed videos of infant crying or laughing during two separate sessions. Frontal EEG alpha power was recorded during the first, while infants’ behaviors and emotional expressions were recorded during the second session. Facial and vocal expressions of affect suggest that infants converge emotionally with their peers’ distress, and, to a certain extent, with their happiness. At group level, the crying peer elicited right lateralized frontal activity. However, those infants with reduced right and increased left frontal activity in this situation, were more likely to approach their peer. Overall, 8-month-olds did not show asymmetric frontal activity in response to peer laughter. But, those infants who tended to look longer at their happy peer were more likely to respond with left lateralized frontal activity. The link between variations in left frontal activity and simple approach behaviors indicates the presence of a motivational dimension to infants’ responses to distressed peers.
Similar content being viewed by others
Introduction
Toddlers’ relationships with their peers have many positive developmental outcomes in later years, including higher levels of emotional mental health and school success1,2,3,4. Their ability to empathize with their peers, to comfort and to share toys with them increases their chances of becoming friends or preferred play partners. Thus, in order to foster adaptive social development, it is important to understand the development of factors contributing to establishing relations with peers, such as empathy and prosocial behaviors5,6,7. Accumulating evidence suggests that before their first birthday, infants are sensitive and respond to their peers’ emotions, which could represent potential precursors of empathy and prosocial behaviors8,9,10,11. However, the neurocognitive mechanisms underlying these early responses and the relation between them remains unclear. In order to address this gap, the current study investigates infant asymmetric frontal EEG alpha power in response to peers’ affective states and its relation to simple behavioral manifestations of social approach and withdrawal.
Empathy and prosocial behavior are multifaceted constructs. Empathy is an affective response triggered by and congruent with others’ emotions, regulated to a certain extent and accompanied by some minimal implicit distinction between the self and the other12,13. As the definition implies, a multitude of processes are involved in generating empathy, from the perception, evaluation and understanding of others’ emotions, to emotional reactivity and regulation, as well as self-awareness12,13,14. In the same vein, the repertoire of behaviors that can ultimately contribute to the welfare of others are heterogeneous (e.g., comforting, helping, sharing resources) and vary in the extent to which they tap on our cognitive and motor abilities to understand, plan and implement complex sequences of coordinated motor acts15. When empathy is associated with the motivation to act, it can lead to the manifestation of prosocial behaviors16. The processes underlying the many facets of empathy and prosocial behaviors rely on relatively separable neurophysiological systems. From an ontogenetic point of view, this is particularly relevant, because these neuropsychological systems have different developmental trajectories8,10, which could influence the characteristics of different precursors of empathy and prosocial behaviors, and their relation during early development8,10,17,18.
Throughout the first year of life, infants respond emotionally to various cues of their peers’ affective states. At birth, neonates cry when they hear another human neonate crying19,20,21,22,23. These emotionally convergent responses to their peers’ cry persist throughout infancy and toddlerhood, yet with lesser intensity24,25. At the psychophysiological level, changes in arousal can also be observed shortly after the onset of peers’ crying, while overt emotional expression cues are usually observed after prolonged stimulation24,26,27,28,29,30. The more infants are able to use self-soothing behaviors and attentional strategies to regulate their emotions, the more likely they are to down-regulate these negative affect sharing responses26. Around the age of 9-months, infants who have greater abilities to discriminate perceptually between their own body and that of another infant, tend to show less intense emotional responses to their peers’ cry26. Thus, besides being congruent with the observed emotion, these responses are also related to infants’ abilities to regulate their own emotions and to differentiate between self and others, raising the possibility that they could be precursors of empathy development. Although less investigated but equally important, infants also seem tuned to their peers’ positive affect. Watching video recordings of peers laughing was shown to elicit increases in the level of infants’ arousal as indexed by changes in the pupil diameter28,29. Both younger (6-month-old) and older (12- and 15-month-old) infants show such increases in arousal; however, in younger infants these responses appear after longer latencies and are less persistent in time compared to older infants. Moreover, as the infants reach their first birthday, the arousal elicited by peers’ emotions becomes more negatively biased, with negative emotions eliciting a higher arousal compared to the positive ones28,29. Neuroimaging investigations using fMRI and ERP methods indicate that infants’ affective responses to others’ emotions rely on neurocognitive mechanisms which develop throughout infancy in relation to an increasing cortical specialization for processing human faces31 and voices32,33, as well as the activity of brain areas known to be involved in the automatic appraisal of the emotional stimuli and the generation of emotional experiences (e.g., the orbitofrontal cortex, the insula33,34).
Before the age of one, infants do not only react emotionally to their peers’ affective states, but also engage with them in simple forms of interaction involving behaviors within their motor-repertoire. For example, Vandell and Wilson35 showed that the interactions between infants become increasingly reciprocated from 6- to 9-months, as reflected by the presence of turn taking. Looking at the peer with or without associated vocalizations, moving towards the peer, touching the body of the peer or the toy she holds, as well as gesture-like movements were the type of behaviors that efficiently supported the reciprocated social engagement35,36. Interestingly, these behaviors have also been recorded during naturalistic interactions in response to peers’ distress30. The simple social approach behaviors described to support efficient interactions between infants are the foundation for any complex prosocial behaviors. In order to comfort, one needs to approach, to touch, and potentially vocally communicate with those in distress. In toddlerhood and pre-school years, these simple approach behaviors are important dimensions of children’s reactions to their peers’ distress, alongside empathic responses and other motorically-complex prosocial behaviors17,18,37. It is thus possible that the simple social approach behaviors present in infancy are also the precursors of the later developing complex prosocial manifestations. Although some previous studies have explored infants’ emotional responses (e.g., fear, sadness, crying) and attempts to help and comfort their peers, no significant concurrent relations were observed at the age of 8–10-months38. One possible interpretation of these findings is that infants’ emotional responses towards their peer’s affect do not translate into overt behavioral actions at this age. It is also possible that the overreliance on recording the more complex prosocial behaviors and the overt manifestations of emotional responses was not sensitive enough to capture possible precursors of prosocial tendencies. Moreover, observational methods are also opaque in terms of the neurocognitive processes underlying different dimensions of infants’ responses to their peers’ emotions, and, as a consequence, less sensitive in detecting the relation between them39,40. Research with adults has shown that watching images of people expressing positive and negative affect activates both the neural networks involved in experiencing those emotions and the septal area brain region functionally associated with the motivation to act pro-socially16,41,42,43. The level of activation of the septal area while observing others’ emotions, but not other regions of the emotional network, specifically predicted how frequently participants behaved prosocially in daily life. These results support the idea that there is a motivational dimension to empathic responsivity, which is relevant for the occurrence of pro-social behaviors, and that the generic emotional response may not always be indicative of whether someone will behave prosocially. Developmental social neuroscience investigations sensitive to this motivational dimension are thus useful for understanding the origins of infants’ other-oriented behaviors elicited by peers’ emotions.
The study of the asymmetric frontal EEG alpha power in response to peers’ emotions has the potential to give insights into the presence of a motivational correlate to infants’ social approach behaviors. The approach-withdrawal motivational model of frontal asymmetry relies on the assumption that differences in the EEG alpha power between homologous right and left frontal electrodes reflects the activity of the frontal cortex44,45. According to this model, the activity of the left frontal cortex is related to appetitive motivation, the motivation to act towards achieving a goal, and approach-related affect such as happiness, while the activity of the right frontal cortex is associated with withdrawal, behavioral inhibition, and vigilant attention which typically occurs during some negative affective states such as fear and sadness45,46,47,48,49,50. Indeed, infants usually show a left lateralization of the frontal EEG alpha power (i.e., greater activity in the right hemisphere) during social and non-social situations which also elicit facial and vocal expression of negative affect: when an adult stranger approaches while the mother is absent51,52, when infants observe adults’ facial expressions of pain53,54 and sadness54,55, or when confronted with scary toys (e.g., masks, spiders51). Importantly, the right lateralization of the frontal EEG alpha power was also found to significantly predict the manifestation of withdrawal behaviors in these situations, including moving away from the source of distress51. The right lateralization of the frontal EEG alpha power (i.e., greater activity in the left hemisphere) was recorded in infants in response to adult expressions of happiness or the presence of a familiar caregiver52,53,54,55,56, and predicted simple approach behaviors like vocalizations directed towards the other54,57. Considering the importance of developing adaptive relations with peers, the current study aims to investigate the extent to which the pattern of infant asymmetric frontal EEG alpha power recorded previously in response to adult emotional experiences extends to their peers’ affective states, and relates to simple behavioral manifestations of social approach and withdrawal. Given the limited evidence about how infants’ responses towards their peers vary as a function of the valence of the observed emotions, both positive (i.e., laughter) and negative (i.e., crying) emotions were included in the study.
Eight-month-old infants were presented with video recordings of other infants laughing and crying during two separate testing sessions. One session was set-up to facilitate the recording of the EEG. In light of previous findings, we anticipated that observing a peer crying will elicit increased left lateralized EEG alpha power relative to the right hemisphere, while observing a peer laughing the opposite pattern, suggesting increased right and left, respectively, frontal cortex activity. Considering the potential of perceiving another’s distress as motivating social approach, we also expected to observe variations in the degree of lateralization to the right of the frontal EEG alpha power in response to the crying infant video. Individual variability in the lateralization of the frontal EEG alpha power was anticipated to relate significantly to the manifestation of the simple approach behaviors and expressions of emotion. In order to facilitate the manifestation of such responses, infants were presented with video recordings of peers laughing and crying in a separate behavior-only session, in the absence of the constraints required for artifact-free EEG recording, such as reduced body movement. We predicted that those 8-month-old infants who responded with increased right lateralization of the frontal EEG alpha power to their peers’ positive and negative emotions would be more likely to approach them behaviorally. The second session also gave us the opportunity to provide a more detailed account of the types of behavioral responses elicited by peers’ positive emotions in 8-month-old infants.
Methods
Participants
Forty 8-month-old infants participated in this study. Out of this sample, 32 infants (15 females, Mage = 254.16 days, SDage = 9.36 days) provided analyzable data for the EEG recording (Session 1) based on the criteria described below. For the behavioral recording (Session 2), 22 infants (13 females, Mage = 254.45 days, SDage = 9.68 days) provided analyzable data based on the criteria described below. Eighteen infants (12 females, Mage = 252.61 days, SDage = 8.93 days) contributed analyzable data for both EEG and behavioral recordings. From the sample participating in Session 1, 9 participants did not return to the lab for Session 2 because the parents found it difficult to fit another visit into their schedule. More information about attrition rates for each session is presented in the following sections. All participants were recruited from a small urban area in North West England, did not suffer from any neurological or other medical condition, and were observed to have normal vision and audition for their age.
Prior to both sessions, all parents were informed that at the end of the experiment they would receive £10 in order to cover traveling expenses and that the infant will be rewarded with a book for their participation. Informed consent was obtained from all parents prior to the beginning of the procedure. The procedure was carried out in accordance with the ethical standards of the Declaration of Helsinki (BMJ 1991; 302:1194). Ethical approval was granted by the Lancaster University Ethics Committee.
Procedure and measures
The procedure was carried out across two sessions, approximately one week apart from each other (M = 6.52 days; SD = 3.55 days). This strategy was adopted in order to accommodate infants’ reduced attention span and maximize attention to the stimuli. Importantly, the option for two separate sessions minimized the potential carry over effects from one to the other.
EEG recording (Session 1)
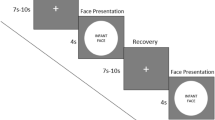
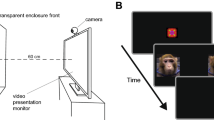
Stimuli and procedure: The stimuli consisted of audio-video recordings of a peer infant crying and of a peer infant laughing, adapted from Geangu et al.29. The infants depicted in the stimuli were 8- to 9-months-old at the time of the recording. Each video recording had an average sound intensity of 70 dB and duration of 90 seconds. Stimuli were presented at a size of 24 × 16 cm on 17-inch CRT computer monitor using MATLAB R2012b (MathWorks, Natick, MA). The order of presentation of the stimuli was counterbalanced across participants. The stimulus presentation began with the display of a dynamic non-social animation for capturing infants’ attention to the screen, which varied in duration from participant to participant depending on how attentive they were. Whenever the experimenter judged that the participants were attentively watching the screen, the first stimulus was presented. Between the first and second stimulus, a non-social animation was always displayed with a duration varying randomly between 30 and 60 secs. If infants became distressed during the stimulus presentation, a maximum of 30-sec was allowed for spontaneous recovery before the procedure was stopped and mothers were invited to comfort their infants. During the entire session, infants sat on their mother’s lap at a distance of approximately 70 cm from the monitor in a dimly lit room. In order to minimize the possibility that mothers could influence infants’ responses to the stimuli, mothers were instructed not to interact with their infant (e.g., talk with, draw attention to the stimuli, display emotional expressions). Cases where these instructions were not followed, were excluded from further analysis. Mothers were told that they can, and should, prevent the infants from grabbing the net/electrodes, and to hold them in a relatively stable position as much as possible throughout the testing session.
EEG recording and analysis: EEG was recorded continuously using a 128-electrode HydroCel Geodesic Sensor Net (Electrical Geodesic Inc., Eugene, OR) and amplified using an EGI NetAmps 300 amplifier. On-line recordings were referenced to the vertex electrode (Cz), and then off-line re-referenced to an average reference. The signal was band-pass filtered at 0.1–100 Hz. EEG data were digitized online at a sampling rate of 500 Hz per channel. Electrode impedances were checked prior to the beginning of the recording and considered acceptable if lower than 50 KΩ, which is a conservative threshold for infants and in accordance with the methodological recommendations for this age group58,59. EEG data were further processed offline using NetStation v4.6.4 (Eugene, OR). EEG data were band-pass filtered (0.3–30 Hz), and segmented according to emotional condition (cry stimulus and laughter stimulus), arising 1.5-min epochs for each task for participant. Next, the segments were checked through visual inspection for eye-movements, eye-blinks and other body movement artifacts. Segments with more than 8 bad channels (besides the 11 marked as bad eye-leads) were manually rejected. For the remaining segments, individual bad channels were replaced using spherical spline interpolation. EEG data were then processed in Matlab R2012b (Mathworks Inc., Natick, MA) for artifact rejection and power analysis. EEG segments showing amplitudes greater than +/−175 µV were marked as bad. The remaining artifact-free segments were analyzed with a Fast Fourier transform (FFT) with a 1-sec Hanning window and 50% overlap with a frequency bin of 0.25 Hz. Participants with less than 10 artifact-free epochs were removed from further analysis to ensure a stable estimate of alpha activity (N = 4). Another 4 participants were removed from further analysis because the EEG recording was stopped early due to their distress, failing to provide sufficient data points. The final sample had an average of 67.53 epochs (min = 10, max = 179) in the laugh and 70.41 epochs (min = 11; max = 156) in the cry conditions. Absolute power spectral density (psd) values for each segment were computed for the 5–7 Hz frequency band for three reasons: (1) majority of the EEG power was represented within this frequency band60; (2) previous studies have associated this frequency with emotion reactivity and emotion regulation during infancy61,62,63; (3) it showed the greatest sensitivity to asymmetry (see Supplementary Information). Alpha power spectral density values were analyzed after being natural log (ln) transformed to normalize the distribution.
Frontal asymmetry scores for each infant in each condition (cry video - ASYMcry; laugh video - ASYMlaugh) were obtained by subtracting the left frontal hemisphere (F3) log-transformed alpha power from the right frontal hemisphere (F4) log-transformed alpha power values (i.e., ln(F4) − ln(F3)). Therefore, positive scores correspond to greater alpha power in the right hemisphere (or increase left activity interpreted as approach-oriented activity) while negative scores correspond to greater alpha power in the left hemisphere (or increased right activity interpreted as withdrawal-oriented activity).
In order to establish that the results for the alpha asymmetry were specific to the frontal locations, alpha power spectral density values were also derived from central (C3/C4) and parietal (P3/P4) scalp locations (see Fig. 1 for the clusters of electrodes corresponding to the scalp locations included in the analysis).
Behavioral recordings (Session 2)
Stimuli and procedure: The stimuli were similar to those used in Session 1, although they differed with respect to length (120 seconds) and the identity of the infant displaying crying and laughing. Again, the infants depicted in the stimuli were 8- to 9-months-old at the time of the recording. The video recordings were sourced from a professional online database (www.istockphoto.com) and were edited to the required duration and an average sound intensity of 70 dB. Each stimulus was displayed at 43 × 27 cm on a 17″ computer monitor. The procedure began with the presentation of a non-social attention grabber, to ensure that the participants were attending to the screen. During the entire procedure, the infant was seated in an age appropriate chair, at the same height and approximately 70 cm away from the screen. The participants’ behavior was recorded by 4 cameras, three located in corners of the room and one placed above the monitor, allowing a close view of the face. Between the stimuli, a 180 seconds break was introduced, during which the experimenter came back to the room and played with the infant. During the break, an animation film was played on the screen. For the entire duration of the session, the mothers were instructed to sit approximately 2 meters behind the infant, reading a magazine, and without engaging through eye contact or voice with the infant.
Behavior coding criteria: We were interested in overt responses indicative of approach behaviors and emotional reactivity. Based on previous research27,64,65,66 and a preliminary inspection of the recordings, the following responses were coded: (a) negative emotional vocalizations; (b) negative facial expressions; (c) positive emotional vocalizations; (d) positive facial expressions; (e) emotionally neutral vocalizations; (f) approach behaviors; (g) withdraw behaviors; and h) looking time. The coding criteria were derived from the Laboratory Temperament Assessment Battery67 (Lab-TAB). In terms of negative facial expressivity, our aim was to capture facial responses which may suggest that infants respond to their peers with emotionally congruent expressions. Thus, we opted for a more generic category, which includes displays of anger, sadness, and fear, as they might be present during infant cry. Table 1 provides a detailed description of the coding criteria for each type of response. A second observer coded 20% of the recordings for reliability (intraclass correlation coefficient for absolute agreement, the reliability coefficients are presented in Table 1).
For coding purposes, all video recordings were divided in 10-sec units. Some of the responses (a–g) were coded as present or absent for each unit. In order to account for variations in stimulus duration length caused by participant’s emotional state, a percentage of units with response present was calculated from the total number of units coded for each participant. For looking time, we coded the duration of visual fixations towards the screen for the entire stimulus presentation. In order to account for variations in stimulus duration, a percentage looking time was calculated out of the entire duration of the stimulus presentation. Datavyu 1.3 free source software (http://datavyu.org) was used for coding. Nine infants were removed from the final dataset for Session 2 due to excessive movement which prevented appropriate face coding (N = 2); technical errors (N = 3); or fussiness at the beginning and throughout the session (N = 4).
Data analysis strategy: The aims of the study were two-fold: (1) to analyze the frontal EEG alpha power (Session 1) and the behavioral responses (Session 2) of 8-month-old infants to their peers’ positive and negative affective states; and (2) to analyze the relation between these responses. In order to ensure that the results for each of these aims rely on the most representative part of our sample, we included in different sections of the analysis all participants who provided analyzable data for that section: Session 1, N = 32; Session 2, N = 22; Session 1 & 2, N = 18. Thus, some of the participants contributing data to either Session 1 or Session 2 analyses, did not contribute data to the analysis of the relation between frontal EEG alpha power and the behavioral responses (Session 1&2).
Results
Frontal EEG asymmetry results (Session 1)
In order to analyze whether peer’s emotions elicited asymmetric frontal EEG activity, separate one-sample t-tests were performed on the frontal EEG asymmetry score obtained during each condition (i.e., laughing and crying). Observing a peer crying elicited an increased left relative to right absolute alpha power (M = −0.081, SD = 0.168), which was significantly different from zero (t(31) = −2.714; p = 0.011). Observing a peer laughing elicited some increased right relative to left absolute alpha power (M = 0.038, SD = 0.232), but the difference from zero did not reach statistical significance (p = 0.361; Fig. 2A).
(A) Means and standard errors for frontal alpha asymmetry scores collected during the two affective conditions. (B) Means and standard errors for the EEG ln alpha power (5–7 Hz) recorded at frontal sensors F3 (left) and F4 (right) during the two affective conditions. (C) Scalp wide alpha power for each condition. Note: EEG power is inversely related to cortical activity - high power reflects lower activity. *p < 0.05.
In order to disentangle the separate contributions of the absolute alpha power recorded from the left and right hemisphere to differences in the frontal asymmetry scores recorded for each condition, a 2 (Condition: laughing, crying) x 2 (Hemisphere: right, left) within-subjects ANOVA was performed on the log-transformed alpha power values. A significant Condition × Hemisphere interaction was obtained (F(1,31) = 11.166; p = 0.002; η2 = 0.265). Post-hoc pairwise comparisons showed that when infants were exposed to a peer crying, higher absolute alpha power was recorded in the left (M = 2.892 μV; SE = 0.084 μV) compared to the right hemisphere (M = 2.811 μV; SE = 0.084 μV), p = 0.011). Moreover, exposure to a laughing peer elicited higher absolute alpha power (M = 2.948 μV; SE = 0.082 μV) in the right hemisphere compared to when participants observed a crying peer (M = 2.811 μV; SE = 0.084 μV), p = 0.007. All other comparisons were not significant (p > 0.361; Fig. 2B).
We further compared both central and parietal regions with a 2 (Condition: laughing, crying) × 2 (Hemisphere: right, left) within-subjects ANOVAs on the log-transformed alpha power values to identify whether there were broader effects of emotional condition on alpha asymmetry. Neither central nor parietal regions showed any significant main effects or interactions (ps > 0.247) with emotional condition (Fig. 2C).
Behavioral results (Session 2)
Table 2 provides an overview of infants’ responses to their peer emotions (N = 22). Repeated measures ANOVAs were conducted in order to analyze the differences between the stimuli in terms of facial and vocal expressivity. Greenhouse-Geisser corrections were applied whenever the assumptions of sphericity were violated. For looking time, approach and withdraw behaviors, repeated measures t-tests were performed. All tests were interpreted at a significance threshold of p = 0.05.
The 2 (Condition: Cry, Laughter) × 2 (Emotion: Positive, Negative) repeated measures ANOVA for facial expressivity revealed a significant interaction between stimulus and emotion, F(1,21) = 16.391; p = 0.001, η2 = 0.438. Post-hoc pairwise comparisons indicated that infants responded with more negative facial expressions to the crying peer than to the laughing one, and with more positive facial expressions to the laughing peer than the crying one (Table 2). Also, infants displayed more positive than negative facial expressions while observing the laughing peer (p = 0.008). No other significant differences were observed (p > 0.304). The 2 (Condition: Cry, Laughter) × 3 (Emotion: Positive, Negative, Neutral) repeated measures ANOVA for vocal expressivity revealed a significant main effect of condition, F(1,21) = 7.727; p = 0.011, η2 = 0.269, which was qualified by a significant interaction with emotion, F(1.31, 21) = 4.724; p = 0.030, η2 = 0.184. Post-hoc pairwise comparisons showed that infants manifested more emotionally negative vocalizations while observing the crying peer than the laughing one. Observing the crying peer also elicited more negative (p = 0.029) and neutral (p = 0.015) vocalizations than the positive ones. In response to the laughing peer, infants manifested more emotionally neutral vocalizations than negative (p = 0.007) and positive (p = 0.021) ones. No other significant differences were observed (p > 0.432).
Infants looked longer at the crying than at the laughing peer, t(21) = 2.449; p = 0.023. No significant differences between the stimuli emerged for the approach and withdraw behaviors.
Relation between frontal EEG asymmetry and behavioral responses to peers’ emotions (Session 1&2)
In order to test our predictions about the relation between individual variability in the lateralization of the frontal EEG alpha power and the behavioral manifestations of approach/withdrawal and emotion expressivity, Pearson’s correlations between the frontal EEG asymmetry scores (Session 1) and the behavioral responses (Session 2) to peers’ emotions were performed (N = 18). The performed correlations were informed by previous research51,52,54,57,68 investigating the link between infant asymmetric frontal alpha power and behavioral responses to emotional events. Thus we did not correct for multiple correlations which might obscure expected results and lead to Type II errors69,70.
Infants’ frontal asymmetry scores recorded in response to the peer crying (i.e., cry lnF4-lnF3 = ASYMcry) were positively correlated with the with the approach behaviors (r = 0.653; p = 0.003) displayed when watching a peer crying in the second session. This indicates that infants who displayed greater degree of left frontal asymmetry when watching a peer crying exhibited more approach behaviors when exposed to the peer crying film in the second session. Infants’ frontal EEG asymmetry scores recorded in response to the laughing peer (i.e., laughter lnF4-lnF3 = ASYMlaugh) were positively correlated with the amount of time infants looked at a happy peer (r = 0.478; p = 0.045) in the second session (Fig. 3B). That is, infants who exhibited more left frontal asymmetry during the laughter film spent more time looking at a peer laughing. Additionally, ASYMlaugh was significantly correlated with the proportion of positive vocalizations (r = 0.519; p = 0.027) and marginally correlated with the neutral vocalizations (r = 0.463; p = 0.053) emitted in response to the sound of a peer laughing. Greater degree of left frontal asymmetry was linked to greater emission of neutral vocalizations during the laughter condition. No other significant correlations between the frontal EEG asymmetry scores (Session 1) and the behavioral responses (Session 2) to peers’ emotions were found (p > 0.145).
Discussion
The current study aimed to investigate the pattern of infant asymmetric frontal EEG alpha power in response to peers’ affective states, and its relation to simple behavioral manifestations of social approach and disengagement. Towards these aims, we presented 8-month-old infants with video recordings of other infants laughing and crying, while we recorded frontal EEG alpha power and examined their behavioral responses during a separate session. The results show that observing peers’ positive and negative affective states elicit distinct patterns of asymmetric EEG alpha power and behavioral manifestations in 8-month-old infants.
At the behavioral level, 8-month-old infants’ emotional responses tended to converge with the affect displayed by their peers. Importantly, this emotional convergence was recorded not only in response to peers’ negative affect as previously shown by several studies24,27,30,38, but also for manifestations of happiness. Observing a distressed infant lead to more displays of negative affect in face and voice than when watching a laughing infant. In turn, peer laughter elicited more facial expressions of happiness. We recorded these behaviors in a separate session from the EEG one, where infants attended independently to the stimuli (i.e., not held by their mothers and not in the immediate proximity of an adult) and had more possibilities to move compared to the EEG session. Certainly, these only represent approximations of their real encounters with peers. Nevertheless, similar video recordings of peer affect were shown to elicit sympathetic arousal in infants as reflected by changes in pupil diameter28,29, suggesting good ecological validity. Moreover, infants in our study appeared to be interested in the stimuli as they engaged visually with them for more than half of their duration. Although they did so for both emotional expressions, they tended to look more at the crying infant.
In line with current theoretical models and previous investigations of infant neural responses to emotional social and non-social stimuli45,46,47,48,49,50,51,52,55, our study shows that 8-month-old infants manifested frontal EEG alpha activity lateralized to the left hemisphere when observing their peers’ negative affect. Since alpha activity has an inhibitory influence on the cortical activity, increased left relative to right frontal alpha power suggests higher activity of the right frontal cortex45,47. This means that infants not only show increased right frontal cortex activity when they encounter a distressed adult52,54,55,71, or when they perceive cues of social threat (i.e., an adult stranger51,72; a social agent blocking the goal achievement of another39), but also when they see a crying peer. According to an approach-withdrawal motivational model of frontal asymmetry45,48,49, these findings suggest that 8-month-old infants are in general more likely to withdraw in the presence of a peer in distress. Nevertheless, variations in the asymmetric frontal EEG alpha power in response to the crying peer were also observed, and these tended to relate to infants’ behavioral responses.
Infants who were more likely to respond with reduced right and greater left frontal asymmetry to crying were also more likely to physically approach their distressed peer. Within the theoretical framework proposing the involvement of the left frontal cortex in an approach system responsible for goal-directed behaviors46,48,49, these results suggest that 8-month-old infants who are more motivated to act are also more likely to approach a peer in distress. Interestingly, the asymmetric frontal activity was not related to the expressions of negative affect when watching a peer crying. These results could partially indicate a dissociation between the motivational and affective dimensions of 8-month-old infants’ responses to their distressed peers, similar to what has been previously reported in adults16,49,73. For example, the activation of the brain regions functionally linked to the motivation to act and not those typically associated with experiencing emotions predicted how much adults behave prosocially during everyday life16. The interactions between infants become increasingly reciprocated from 6- to 9-months, relying on simple motor acts, such as moving towards and touching their peer30,35. Importantly, these simple motor acts continue to be present during toddlerhood and preschool years, when they are related to complex prosocial behaviors, such as helping and comforting, as well as measures of emotional and cognitive dimensions of empathy17,18. Taken together, these findings argue in favour of the need to study infants’ approach behaviors towards others in distress as potential origins of the mature forms of prosocial behaviors. Further longitudinal studies are needed to test this proposal. Concurrent measures of frontal asymmetry, emotional expressivity, and social approach behaviors at multiple time points beginning with infancy could be particularly useful in this respect.
Contrary to some of the previous findings that infants respond with increased right lateralized frontal EEG alpha power to adult facial and vocal expressions of happiness54,55, but in line with others74, at the group level, 8-month-old infants in the current study did not show frontal EEG alpha power asymmetry in response to peer laughter. One possible explanation for these results is that the infants had a generally high degree of positive affect or approach orientation and that the laughter stimuli did not generate any greater left frontal asymmetry from that baseline state. Alternatively, it could be that infants’ ability to process and respond to the communicative value of their peers’ laughter may be insufficiently developed before the age of 12-months11,28,29. Although from an early age infants are able to laugh75, this emotional expression appears to be more frequently associated with the interactions with adults76. As a result, infants may encounter less frequently these specific facial expressions and non-verbal vocalizations when interacting with peers77,78, with consequences for the development of their abilities to extract the corresponding social message. Interestingly, those 8-month-old infants who did respond with a more left lateralized frontal activity to the laughing peer tended to be those who looked more at this stimulus during the behavioral session. As looking behavior can reflect perceptual and cognitive processing of the stimulus79,80, it could be that the left frontal lateralization is more likely to appear in those infants who attend more, and thus are more likely to extract the relevant emotional information from facial and vocal expressions of laughter54. Visual engagement with others could also be regarded as an index of social approach54, and from this perspective our findings would indicate a motivational link between 8-month-olds’ tendency towards left frontal asymmetry and approaching behaviors towards a happy peer. Due to the correlational nature of the analysis, it is, however, difficult to draw conclusions in this respect. The inclusion of a non-emotionally valenced baseline, larger sample size and a wider age range, could allow in the future a more comprehensive analysis of the relation between the cognitive processing of emotional information and frontal asymmetry in response to peers, as well as the meaning of looking behavior during infant interactions.
Another possible explanation for the pattern of frontal asymmetry in response to peer laughter could be that, although observing peers’ happiness leads to some positive affect in 8-month-old infants as shown by their facial expressivity during the behavioral session, this may only reflect limited sympathetic arousal. Indeed, before the age of 12 months the pupillary dilation response to peer laughter is brief and reduced compared to that for crying28,29. In a similar vein, previous studies have shown greater left lateralized frontal activity when 2-month-old infants show intense facial expression of happiness, but not when these were less intense52, while in adults, pleasant pictures tended to elicit increased left frontal activity only if a propensity to experience positive affect was already present81,82. The inclusion of concurrent measures of frontal asymmetry, arousal, and emotional expressivity would be useful for testing this proposal in future research.
Important to note are some limitations to the current study. Although we included a larger sample of participants to begin with, only a subset completed both testing sessions. We are confident, however, that our results are not due to the sample size. First, our findings converge in several ways with those previously reported in studies using similar paradigms24,26,54,55,72 and show medium-large to large effects. Second, the correlations fall within the 95% CI (rs = 0.30 to 0.52) of the correlation coefficients reported in previous studies that investigated the relation between infant frontal asymmetry and behavioral responses similar to those investigated here54,57,68,83. This suggests that our study is sufficiently powered to detect correlations between brain and behavior84,85. The recording of the behavioral and frontal EEG during separate testing sessions may also limit the interpretation of our findings. The greater freedom of movement allowed during the behavioral session could have caused too much noise in the EEG data and was the main reason behind our procedural decision. Although recent studies show that the patterns of frontal alpha power asymmetry in response to emotional events are stable across measurements at different time points in infancy and toddlerhood56, it would be important in the future to test the possibility of using concurrent behavioral and brain activity measures, in order to assess the generalizability of our results. Furthermore, the delineation between the frontal alpha and the theta band for the lower frequencies in infants younger than 10-months appears to be still open for discussion and further validation. For example, while some previous studies include some of the lower frequencies (e.g., 4 Hz) in the frontal alpha band61,62,63, others do not86. Based on preliminary analysis of our results (see Supplementary Information), as well as based on previous studies using similar age groups and experimental paradigms61,62,63, we considered the 5 to 7 Hz band is most representative of the frontal alpha. Nevertheless, further validation using emotional stimuli is needed60.
In summary, the results show that observing other infants crying or laughing elicits in 8-month-old infants distinct patterns of asymmetric frontal activity, as well as overt responses suggesting the presence of convergent emotional responses and social approach behaviors. These findings add valuable information to a limited body of knowledge about the potential early origins of empathy and pro-social behaviors, and their underlying neurocognitive mechanisms. The specific link between approach behaviors and variations in left frontal activity indicates the presence of a motivational dimension to infants’ responses to distressed peers and emphasizes the importance of investigating the role of these simple behaviors in the ontogeny of prosocial abilities.
Data Availability
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
References
Ladd, G. W. & Price, J. M. Predicting children’s social and school adjustment following the transition from preschool to kindergarten. Child Dev. 58, 1168–1189 (1987).
Tomada, G., Schneider, B. H., De Domini, P., Greenman, P. S. & Fonzi, A. Friendship as a predictor of adjustment following a transition to formal academic instruction and evaluation. Int. J. Behav. Dev. 29, 314–322 (2005).
Taylor, A. R. & Machida, S. The contribution of parent and peer support to head start children’s early school adjustment. Early Child. Res. Q. 9, 387–405 (1994).
Vandell, D. L. Parents, peer groups, and other socializing influences. Dev. Psychol. 36, 699–710 (2000).
Sebanc, A. M., Kearns, K. T., Hernandez, M. D. & Galvin, K. B. Predicting Having a Best Friend in Young Children: Individual Characteristics and Friendship Features. J. Genet. Psychol. 168, 81–95 (2007).
Howes, C. & Phillipsen, L. Gender and friendship: relationships within peer groups of young children. Soc. Dev. 1, 230–242 (1992).
Vandell, D. L., Wilson, K. S. & Buchanan, N. R. Peer interaction in the first year of life: an examination of its structure, content, and sensitivity to toys. Child Dev. 51, 481–488 (1980).
Decety, J. & Svetlova, M. Putting together phylogenetic and ontogenetic perspectives on empathy. Dev. Cogn. Neurosci. 2, 1–24 (2012).
Geangu, E. In International Encyclopedia of the Social and Behavioral Sciences (ed. Wright, J. D.) 7, 549–553 (Elsevier, 2015).
Decety, J. & Michalska, K. J. Neurodevelopmental changes in the circuits underlying empathy and sympathy from childhood to adulthood. Dev. Sci. 13, 886–99 (2010).
Crespo-Llado, M. M., Vanderwert, R. E. & Geangu, E. Individual differences in infants’ neural responses to their peers’ cry and laughter. Biol. Psychol. 135, 117–127 (2018).
De Vignemont, F. & Singer, T. The empathic brain: how, when and why? Trends Cogn. Sci. 10, 435–41 (2006).
Mccall, C. & Singer, T. In Understanding other minds. Perspectives from developmental social neuroscience (eds Baron-Cohen, S., Tager-Flusberg, H. & Lombardo, M. V.) 195–209 (Oxford University Press, 2013).
Decety, J. & Jackson, P. L. A Social-Neuroscience Perspective on Empathy. Current Directions in Psychological Science 15, 54–58 (2006).
Svetlova, M., Nichols, S. R. & Brownell, C. A. Toddlers’ prosocial behavior: from instrumental to empathic to altruistic helping. Emotion 81, 1814–27 (2007).
Morelli, S. A., Rameson, L. T. & Lieberman, M. D. The neural components of empathy: Predicting daily prosocial behavior. Soc. Cogn. Affect. Neurosci. https://doi.org/10.1093/scan/nss088 (2012).
Huang, H., Su, Y. & Jin, J. Empathy-Related Responding in Chinese Toddlers: Factorial Structure and Cognitive Contributors. Infant Child Dev. 26, 1–18 (2017).
Lin, H. & Grisham, M. Infant Behavior and Development Distressed yet empathically sensitive: Preschoolers’ responses to infant crying. Infant Behav. Dev. 49, 46–49 (2017).
Dondi, M., Simion, F. & Caltran, G. Can Newborns Discriminate Between Their Own Cry and the Cry of Another Newborn Infant? Dev. Psychol. 35, 418–426 (1999).
Field, T. et al. Depressed mothers’ infants show less negative affect during non-contingent interactions. Infant Behav. Dev. 28, 426–430 (2005).
Sagi, A. & Hoffman, M. L. Empathic distress in the newborn. Dev. Psychol. 12, 175–176 (1976).
Simner, M. L. Newborn’s response to the cry of another infant. Dev. Psychol. 5, 136–150 (1971).
Martin, G. B. & Clark, R. D. III. Distress crying in neonates: species and peer specificity. Dev. Psychol. 18, 3–9 (1982).
Nichols, S. R., Svetlova, M. & Brownell, C. A. Toddlers’ Responses to Infants’ Negative Emotions. Infancy 20, 70–97 (2015).
Nichols, S. R., Svetlova, M. & Brownell, C. A. The role of social understanding and empathic disposition in young children’s responsiveness to distress in parents and peers. Cogn. Brain. Behav. An Interdiscip. J. XIII, 449–478 (2009).
Geangu, E., Benga, O., Stahl, D. & Striano, T. Individual Differences in Infants’ Emotional Resonance to a Peer in Distress: Self-Other Awareness and Emotion Regulation. Soc. Dev. 20, 450–470 (2011).
Geangu, E., Benga, O., Stahl, D. & Striano, T. Contagious crying beyond the first days of life. Infant Behav. Dev. https://doi.org/10.1016/j.infbeh.2010.03.004 (2010).
Upshaw, M. B., Kaiser, C. R., Sommerville, J. A., Ford, R. & Perlman, S. B. Parents’ empathic perspective taking and altruistic behavior predicts infants’ arousal to others’ emotions. 6, 1–11 (2015).
Geangu, E., Hauf, P., Bhardwaj, R. & Bentz, W. Infant pupil diameter changes in response to others’ positive and negative emotions. PLoS One 6, e27132 (2011).
Hay, D. F., Nash, A. & Pedersen, J. Response of six-month-old infants to the distress of their peers. Child Dev. 52, 1071–1075 (1981).
Johnson, M. H. Interactive Specialization: A domain-general framework for human functional brain development? Dev. Cogn. Neurosci. 1, 7–21 (2011).
Missana, M., Altvater-mackensen, N. & Grossmann, T. Neural correlates of infants’ sensitivity to vocal expressions of peers. Dev. Cogn. Neurosci.. https://doi.org/10.1016/j.dcn.2017.04.003 (2017).
Blasi, A. et al. Early specialization for voice and emotion processing in the infant brain. Curr. Biol. 21, 1220–1224 (2011).
Minagawa-Kawai, Y., Mori, K., Hebden, J. C., Dupoux, E. & Royal, B. P. Optical imaging of infants’ neurocognitive development: recent advances and perspectives. Dev. Neurobiol. 68, 712–28 (2008).
Vandell, D. L. & Wilson, K. S. Infants’ Interactions with Mother, Sibling, and Peer: Contrasts and Relations between Interaction Systems. Child Dev. 58, 176–186 (1987).
Hay, D. F., Nash, A. & Pedersen, J. Interaction between six-month-old peers. Child Dev. 54, 557–562 (1983).
Rhee, S. H. et al. Early concern and disregard for others as predictors of antisocial behavior. J. Child Psychol. Psychiatry 54, 157–166 (2013).
Roth-Hanania, R., Davidov, M. & Zahn-Waxler, C. Empathy development from 8 to 16 months: early signs of concern for others. Infant Behav. Dev. 34, 447–58 (2011).
Cowell, J. M. & Decety, J. Precursors to morality in development as a complex interplay between neural, socioenvironmental, and behavioral facets. Proc. Natl. Acad. Sci. 112, 201508832 (2015).
Paulus, M. The emergence of prosocial behavior: Why do infants and toddlers help, comfort, and share? Child Dev. Perspect. 8, 77–81 (2014).
Moll, J. et al. Human fronto – mesolimbic networks guide decisions about charitable donation. Proc. Natl. Acad. Sci. 103, 15623–15628 (2006).
Moll, J. et al. Impairment of prosocial sentiments is associated with frontopolar and septal damage in frontotemporal dementia. Neuroimage 54, 1735–1742 (2011).
Inagaki, T. K. & Eisenberger, N. I. Giving support to others reduces sympathetic nervous system-related responses to stress. Psychophysiology 53, 427–435 (2016).
Coan, J. A. & Allen, J. J. B. The state and trait nature of frontal EEG asymmetry in emotion. The Asymmetrical Brain 565–615 (2003).
Coan, J. A. & Allen, J. J. B. Frontal EEG asymmetry as a moderator and mediator of emotion. Biol. Psychol. 67, 7–49 (2004).
Davidson, R. J. & Irwin, W. The functional neuroanatomy of emotion and affective style. Trends Cogn. Sci. 3, 84–94 (1999).
Reznik, S. J. & Allen, J. J. B. Frontal asymmetry as a mediator and moderator of emotion: An updated review. Psychophysiology 1–32 https://doi.org/10.1111/psyp.12965 (2017).
Harmon-Jones, E. & Allen, J. J. B. Anger and frontal brain activity: EEG asymmetry consistent with approach motivation despite negative affective valence. J. Pers. Soc. Psychol. 74, 1310–1316 (1998).
Hewig, J. Intentionality in frontal asymmetry research. Psychophysiology 55, e12852, https://doi.org/10.1111/psyp.12852 (2018).
Harmon-Jones, E. Clarifying the emotive functions of asymmetrical frontal cortical activity. Psychophysiology 40, 838–848 (2003).
Diaz, A. & Bell, M. A. Frontal EEG asymmetry and fear reactivity in different contexts at 10 months. Dev. Psychobiol. 54, 536–45 (2012).
Fox, N. A. & Davidson, R. J. Patterns of brain electrical activity during facial signs of emotion in 10-month-old infants. Dev. Psychol. 24, 230–236 (1988).
Missana, M., Grigutsch, M. & Grossmann, T. Developmental and Individual Differences in the Neural Processing of Dynamic Expressions of Pain and Anger. PLoS One 9, e93728 (2014).
Field, T., Pickens, J., Fox, N. A., Gonzalez, J. & Nawrocki, T. Facial expression and EEG responses to happy and sad faces/voices by 3-month-old infants of depressed mothers. Br. J. Dev. Psychol. 16, 485–494 (1998).
Davidson, R. J. & Fox, N. A. Asymmetrical brain activity discriminates between positive and negative afffect stimuli in human infants. Science (80-.). 218, 1235–1237 (1982).
Brooker, R. J., Canen, M. J., Davidson, R. J. & Goldsmith, H. H. Short- and long-term stability of alpha asymmetry in infants: Baseline and affective measures. Psychophysiology 54, 1100–1109 (2017).
Fox, N. A. & Davidson, R. J. Electroencephalogram asymmetry in response to the approach of a stranger and maternal separation in 10-month-old infants. Dev. Psychol. 23, 233–240 (1987).
Johnson, M. H. et al. Recording and analyzing high-density event-related potentials with infants using the Geodesic Sensor Net. Dev. Neuropsychol. 19, 295–323 (2001).
Ferree, T. C., Luu, P., Russell, G. S. & Tucker, D. M. Scalp electrode impedance, infection risk, and EEG data quality. Clin. Neurophysiol. 112, 536–544 (2001).
Marshall, P. J., Bar-haim, Y. & Fox, N. A. Development of the EEG from 5 months to 4 years of age. Clin. Neurophysiol. 113, 1199–1208 (2002).
Calkins, S. D., Fox, N. A. & Marshall, T. R. Behavioral and physiological antecedents of inhibited and uninhibited behavior. Child Dev. 67, 523–540 (1996).
Santesso, D. L., Schmidt, L. A. & Trainor, L. J. Frontal brain electrical activity (EEG) and heart rate in response to affective infant-directed (ID) speech in 9-month-old infants. Brain Cogn. 65, 14–21 (2007).
Schmidt, L. A. Patterns of second-by-second resting frontal brain (EEG) asymmetry and their relation to heart rate and temperament in 9-month-old human infants. Pers. Individ. Dif. 44, 216–225 (2008).
Gill, K. L. & Calkins, S. D. Do aggressive/destructive toddlers lack concern for others? Behavioral and physiological indicators of empathic responding in 2-year-old children. Dev. Psychopathol. 15, 55–71 (2003).
Markova, G., Markova, G. & Legerstee, M. Contingency, Imitation, and Affect Sharing: Foundations of Infants’ Social Awareness. Dev. Psychol. 42, 132–141 (2006).
Spinrad, T. L. & Stifter, C. A. Toddlers’ Empathy-Related Responding to Distress: Predictions From Negative Emotionality and Maternal Behavior in Infancy. Infancy 10, 97–121 (2006).
Goldsmith, H. H., Reilly, J., Lemery, K. S., Longley, S. & Prescott, A. The laboratory temperament assessment battery. Unpubl. Man. 1–124 (2001).
Diego, M. A. et al. EEG responses to mock facial expressions by infants of depressed mothers. Infant Behav. Dev. 27, 150–162 (2004).
Rothman, K. J. Six persistent research misconceptions. J. Gen. Intern. Med. 29, 1060–1064 (2014).
Rothman, K. J. No adjustments are needed for multiple comparisons. Epidemiology 1, 43–46 (1990).
Fox, N. A. If It’s Not Left, It’s Right. 863–872 (1989).
Davidson, R. J. & Fox, N. A. Frontal brain asymmetry predicts infants’ response to maternal separation. J. Abnorm. Psychol. 98, 127–131 (1989).
Harmon-Jones, E., Gable, P. A. & Peterson, C. K. The role of asymmetric frontal cortical activity in emotion-related phenomena: a review and update. Biol. Psychol. 84, 451–62 (2010).
Jones, N. A., Field, T., Fox, N. A., Davalos, M. & Gomez, C. EEG during different emotions in 10-month-old infants of depressed mothers. J. Reprod. Infant Psychol. 19, 295–312 (2001).
Nwokah, E. E., Hsu, H. C., Dobrowolska, O. & Fogel, A. The development of laughter in mother-infant communication: Timing parameters and temporal sequences. Infant Behav. Dev. 17, 23–35 (1994).
Mireault, G. C. et al. Laughing matter: infant humor in the context of parental affect. J. Exp. Child Psychol. 136, 30–41 (2015).
Rubenstein, J. & Howes, C. The effects of peers on toddler interaction with mother and toys. Child Dev. 47, 597–605 (1976).
Vandell, D. L. & Mueller, E. C. In Friendship and social relations in children (eds Foot, H. C., Chapman, A. J. & Smith, J. R.) 181–208 (John Wiley & Sons, Ltd., 1980).
Colombo, J. & Mitchell, D. W. Infant visual habituation. Neurobiol. Learn. Mem. 92, 225–34 (2009).
Oakes, L. M. Using habituation of looking time to assess mental processes in infancy. J. Cogn. Dev. 11, 1–10 (2010).
Harmon-Jones, E. & Gable, P. A. Neural Activity Underlying the Effect of Approach-Motivated Positive Affect on Narrowed Attention. Psychol. Sci. 20, 406–409 (2009).
Gable, P. A. & Harmon-jones, E. Approach-Motivated Positive Affect Reduces Breadth of Attention. Psychol. Sci. 19, 476–482 (2008).
Dawson, G. et al. Frontal electrical brain activity in infants of depressed mothers: Relations to variations in infant behaviour. Dev. Psychopathol. 11, 589–605 (1999).
Field, A. P. & Gillet, R. How to do a meta-analysis. Br. J. Math. Stat. Psychol. 63, 665–694 (2010).
Hedges, L. V. & Olkin, L. Statistical methods for meta-analysis (Academic Press, 1985).
Bazhenova, O. V., Stroganova, T. A., Doussard-Roosevelt, J. A., Posikera, I. A. & Porges, S. W. Physiological responses of 5-month-old infants to smiling and blank faces. Int. J. Psychophysiol. 63, 64–76 (2007).
Acknowledgements
We would like to express our gratitude to all families who dedicated their time to participate in this research project with their children. Without their continuous interest in our work and desire to help, these findings would have not been possible. The authors would also like to thank Dr. Alissa Westerlund and Dr. Quoc Vuong for their invaluable support and constructive comments. The work for this paper was partly supported by an EPSRC IAA grant (University of York) awarded to Dr. Elena Geangu.
Author information
Authors and Affiliations
Contributions
E.G. and M.M.C.L. designed the study. M.M.C.L. collected the data. M.M.C.L. and E.R. coded the behavioral responses. E.G., M.M.C.L., and R.V. analyzed the data. E.G., M.M.C.L., R.V., and E.R. wrote the paper. All authors reviewed the manuscript.
Corresponding author
Ethics declarations
Competing Interests
The authors declare no competing interests.
Additional information
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Crespo-Llado, M.M., Vanderwert, R., Roberti, E. et al. Eight-month-old infants’ behavioral responses to peers’ emotions as related to the asymmetric frontal cortex activity. Sci Rep 8, 17152 (2018). https://doi.org/10.1038/s41598-018-35219-4
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-018-35219-4
Keywords
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.