Abstract
T-2 toxin (T-2), a naturally occurring mycotoxin that often accumulates in aquatic animals via contaminated feed, is toxic to animals, including humans. In this study, six groups of shrimp (n = 30 shrimps/group) were given T-2 in feed at concentrations of 0–12.2 mg/kg for 20 days. T-2 accumulation, intestinal histopathology, digestive enzyme activities and subsequent effects on shrimp are reported. Compared to the control, T-2 significantly reduced weight gain, specific growth rate, and survival. The histopathology of shrimp intestine showed concentration-dependent degenerative and necrotic changes in response to dietary T-2. Progressive damage to the microstructures of shrimp intestine occurred with increasing dietary T-2 concentrations, with initial inflammation of the mucosal tissue at T-2 concentrations of 0.5 and 1.2 mg/kg, progressing to disappearance of intestinal villi and degeneration and necrosis of the submucosa at 12.2 mg/kg. Intestinal amylase and protease activities increased at low T-2 concentrations but showed significant inhibition at high concentrations; however, the opposite trend occurred for lipase activity. Collectively, these results indicate that digestive enzyme activities and mucosal structures are markedly affected by exposure to T-2, and these may have contributed to the lower survival rate of shrimp.
Similar content being viewed by others
Introduction
With the rapid development of aquaculture, Litopenaeus vannamei has become one of the largest shrimp exports from China to the world1,2. In the past decade, the production of shrimp in aquaculture has intensified and the industry expanded extensively. At the same time, mycotoxin contamination of aquatic feed has increased because more cereal types with high protein are now incorporated into these feeds, replacing animal proteins to reduce feed costs3,4.
T-2 toxin (T-2) is among the most toxic of the trichothecene mycotoxins, a large group of compounds produced by several Fusarium species that occur in mold-damaged foods around the world5,6. T-2 is rapidly absorbed by aquatic animals and causes a wide range of toxic effects7. Ingestion of T-2 by aquatic organisms has been found to damage the stomach, hepatopancreas and intestinal mucosa, and reduce feed intake and growth8,9.
As a food safety measure, the residues of T-2 in food and feeds are closely monitored10. Extensive research has explored the mechanisms of T-2 toxicity in humans and animals, with inhibition of protein synthesis, damage to digestive tract and reduction in immunity being the main mechanisms found. For example, in ducks fed T-2 containing feed for three weeks, the rate of weight gain was significantly reduced, and the digestive tract was severely damaged11. On exposure of catfish to T-2 at 1.0 mg/kg in the diet, intestinal immunity declined, and mortality increased up to 84%12. In Litopenaeus vannamei and Penaeus monodon given a diet containing T-2 at 1.0–2.0 mg/kg for up to 10 weeks, the digestive tract mucosa was severely inflamed13.
Shrimp intestine is not only a digestive organ, but also an important part of the immune system14. Several animal studies have evaluated the effects of a range of nutrients on intestinal structure15,16,17. However, relatively few studies have explored the influence of mycotoxins (especially T-2) on intestinal histopathology. Furthermore, it was found that the effects of T-2 on shrimp intestinal histopathology have not been reported. Intestinal digestive enzyme activities during shrimp growth have been studied18,19,20. Protease, amylase and lipase play a key role in food digestion and nutrient absorption from the intestine21,22. Effects of T-2 on shrimp digestive enzymes have not been reported.
In our previous research, we have found that T-2 damaged the microstructure of shrimp hepatopancreas in a concentration-dependent manner and had a significant effect on alkaline phosphatase (AKP), glutamic-oxaloacetic transaminase (GOT) and glutamic-pyruvic transaminase (GPT) activities23. And the effects of T-2 on the survival rate of shrimp weighing 3.5 ± 0.5 g and 8.5 ± 0.5 g were significantly different23,24. In this study, shrimp with a body weight of 5.0 ± 0.5 g were studied. Growth parameters, intestinal histopathology and digestive enzyme activities were analyzed to better understand the toxic effects of T-2 in shrimp.
Results
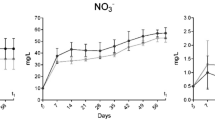
Growth parameters of shrimp exposed to T-2 toxin
Growth parameters of shrimp (n = 30/group) exposed to T-2 are presented in Fig. 1. Compared to the control group, all growth parameters of T-2 dosed shrimp declined significantly. As the concentration of T-2 increased, the weight gain rate and specific growth rate of shrimp gradually decreased. The survival rate showed a highly significant difference between treatments and control (P < 0.05). The worst survival rate was observed in the group given 1.2 mg/kg. However, the survival rate began to rise gradually with higher T-2 concentrations in the feed.
Effects of T-2 toxin on shrimp intestinal histopathology
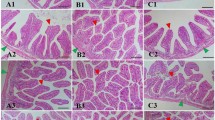
The criteria for evaluation of the extent of damage caused by T-2 toxin on shrimp intestinal histopathology are shown in Table 1. According to the criteria, the degree of damage was divided into 5 levels: normal (−), minimal (+), mild (++), moderate (+++) and severe (++++). The microstructures of shrimp intestine in the control and test groups (n = 5) are shown in Fig. 2. The shrimp intestine in the control group exhibited a well-defined striated border, complete mucosal folds and a clear organelle structure (Fig. 2-a). In the 0.5 and 1.2 mg/kg T-2 concentration groups, the shrimp intestine appeared inflamed with enlarged striated edge and shorter mucosal folds (Fig. 2-b,c). In the 2.4 mg/kg group, the intestinal submucosal space was increased, the villi were almost non-existent, and the mucosal folds were much shorter (Fig. 2-d). In the 4.8 mg/kg group, the intestine was swollen, the striated border was missing, and the intercellular space was even larger, so that the layers were separated (Fig. 2-e). In the highest T-2 concentration group (12.2 mg/kg), the intestine was severely damaged, the intestinal villi had disappeared, mucosal folds were extremely short, and the submucosa had undergone marked degeneration and necrosis (Fig. 2-f).
Effects of T-2 toxin on shrimp digestive enzyme activities
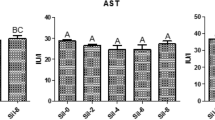
The effects of different T-2 concentrations on shrimp intestinal digestive enzymes are shown in Fig. 3. With increasing concentrations of T-2, the activity of intestinal protease and amylase decreased and this would have reduced protein and carbohydrate digestion. The reason for the reduction in protease activity may be that T-2 inhibited the expression of protein. Significant differences (P < 0.5) were observed in intestinal lipase activity. It was highest with T-2 at 1.2 mg/kg of feed. As T-2 concentrations increased to higher levels, step-wise reductions in lipase activity were noted, but only the highest (12.2 mg/kg) concentration was associated with activity that was significantly below that of the control.
Concentration-response relationships between T-2 toxin and digestive enzyme activities
Concentration-response correlations illustrate the relationships between intestinal enzyme activities expressed as the ratio between the test and control groups in the y-axis and T-2 concentrations in feed (mg/kg) in the x-axis (Figs 4–6). The activities of intestinal protease, amylase and lipase were consistent when analyzed using Allometric25, LogNormal26 and GaussAmp models27, respectively. The concentration-response correlations between the T-2 concentration and digestive enzyme activities were high (R2 = 0.8976 to 0.9891), and variance analysis showed that the differences were significant. Intestinal amylase exhibited the minimal EC50 induced by T-2. In contrast to protease and amylase, at concentrations between 0.5 and 3.2 mg/kg, T-2 had a stimulating effect on lipase activity, and the highest lipase activity occurred at the T-2 concentration of 1.52 mg/kg.
Discussion
Contamination of feed ingredients with toxic concentrations of mycotoxins has been so serious in recent years, that it seems likely to limit the expansion of aquaculture in Asia28,29. It has been shown that dietary aflatoxin B1 caused poor growth performance and deformities of juvenile grass carp30. Dietary mixtures of aflatoxin B1 and fumonisin B1 resulted in reduction in growth performance in juvenile catfish31. Weight gain and survival of shrimp were impaired by dietary deoxynivalenol after five weeks of exposure32. It is clear that mycotoxins can inhibit the growth of shrimp. In this study, shrimp (5.0 ± 0.5 g) were exposed to different T-2 concentrations in feed for 20 days, and it had a major impact on the weight gain rate and specific growth rate of shrimp after 20 d of exposure. Both of these parameters declined in shrimp given T-2 at any concentration, responses that reflect toxicity. However, it was very interesting that the survival rate of shrimp was not consistent with the theoretical speculation. The group exposed to the lowest T-2 concentration was associated with the most severe impact on survival. This might have been due to the higher concentrations of T-2 inducing damage to the intestine that was severe enough to reduce further T-2 absorption and thus systemic toxicity. By contrast, in our previous research with shrimp weighing 8.5 + 0.5 g, there was a modest decline in survival rate with T-2 in feed at concentrations of 1.2 mg/kg and greater24. In our various studies with dietary T-2, survival was the lowest (72%) in shrimp that weighed 3.5 + 0.5 g that were given the toxin at 1.2 mg/kg of feed23. In addition, Bundit et al.33 also discovered that T-2 inhibited black tiger shrimp (average weight = 4.7 g) survival rate even at 0.1 mg/kg. Combined with the results of this study, it seems that low T-2 concentrations have a marked inhibitory effect on the survival rate of juvenile shrimp (average weight ~ 5.0 g).
Shrimp intestine is a digestive organ and an important part of the immune system that plays a major role in nutrient and also toxin absorption14,34. Because the gastrointestinal mucosa is the first barrier between the body and orally ingested exogenous compounds, it has developed various mechanisms to limit absorption of toxins35. Studies have shown that T-2 not only reduces the shrimp growth but also can cause marked changes to the digestive system structure9. Supamattaya et al.13 have shown that feeding Litopenaeus vannamei and Penaeus monodon a diet containing T-2 at 1.0–2.0 mg/kg for 8 and 10 weeks can cause serious degeneration and atrophic changes in the intestines. T-2 can induce gross and histologic changes in the intestine of rats36. T-2 altered intestinal morphology in turkeys with resultant shorter and thinner villi37. In our study, marked intestinal tissue damage was evident as T-2 concentration increased. In the high T-2 concentration group, the shrimp intestinal tract was severely damaged, where almost all of the intestinal villi were detached or non-existent, the mucosal structure was loose, and the submucosa had partially undergone dissolution. Such drastic damage will affect shrimp health by reducing nutrient digestion and absorption. It is inferred that there was a direct relationship between the damage to the intestinal structure and shrimp survival rate.
There were changes in the activities of three key enzymes, protease, lipase and amylase, which could alter shrimp nutrient metabolism38,39. Studies have shown that low concentrations of certain mycotoxins in feed can stimulate the activity of digestive enzymes in experimental animals. For example, aflatoxin B1 at 2.5 mg/kg in the diet of chickens increased amylase activity, and 40 μg/kg increased both amylase and protease activity40,41. Digestive enzymes of broiler chickens were also influenced by T-242. These results are similar to the trend of intestinal digestive enzymes of shrimp observed in the low T-2 concentration groups of this study. The activities of the three digestive enzymes increased slightly at T-2 concentrations of 0.5 and 1.2 mg/kg with lipase reaching a maximum of 66.42 ± 3.60 U/mg protein in the shrimp fed a diet containing T-2 at 1.2 mg/kg. We postulate that shrimp were stressed by the two lower concentrations of T-2 in a manner that increased lipase secretion. However, protease and amylase activities were decreased in response to higher concentrations of T-2, and those changes might be attributable to reduced protein synthesis.
Based on the concentration-response relationship between T-2 and digestive enzyme activities, the correlation coefficients of the concentration-response curves were high (R2 = 0.8976 to 0.9891), indicating that the concentration-response curves fitted the experimental data well. The NOAEL values of intestinal protease and amylase activities were ~0.50 mg/kg, indicating that T-2 can inhibit protease and amylase at fairly low concentrations43. Comparing the curve equation of the digestive enzymes, the EC50 for T-2 toxin-induced intestinal amylase activity was the lowest. This means that as a biomarker of T-2 intoxication, amylase would be more sensitive than intestinal protease and lipase of shrimp.
Rotter et al.44 and Awad et al.45 found that deoxynivalenol, a type B trichothecene, can cause necrosis of the digestive tract mucosa, which would then seriously impact on the healthy growth of animals. When digestive enzymes are induced by exogenous compounds and thus stimulate the activity of the host’s natural digestive enzyms, it can lead to a change in shrimp growth38,46. Some mycotoxins can decrease digestive enzyme activity as well as other digestive functions in animals, and thereby inhibit their growth and development47,48. The survival rate of shrimp sharply decreased in the T-2 high concentration groups. This appears to suggest that intestinal tract inflammation, intestinal wall thinning, shrinkage of intestinal villi and folds, and inhibition of key digestive enzymes collectively caused significant changes in shrimp digestive function, resulting in reduced survival of the organism.
Methods
Animals and chemicals
Litopenaeus vannamei (5.0 ± 0.5 g) were purchased from East Island (Zhanjiang, China). T-2 (purity ≥98%) was purchased from Enzo (USA). All other chemicals (Analytical reagents) used in the study were obtained from Qiyun Biological Technology (Guangzhou, China).
Shrimp toxicity study
T-2 mixed shrimp feed was prepared according to Dai et al.49. Shrimp were divided into six groups (30 shrimps/group) and placed in six water tanks (75 × 60 × 50 cm; water volume: 150 L) for 7 d for them to adapt to the conditions (pH: 7.5 ± 0.1; water temperature: 25 ± 1 °C; salinity: 10‰; dissolved oxygen: 7.0–7.5 mg/L). According to the increasing concentration grouping paradigm of 20 d accumulation toxicity test, the concentrations of T-2 used were 0 (control), and 1/50, 1/20, 1/10, 1/5 and 1/2 LC50 (LC50 = 24.4 mg T-2/kg feed)50, thus the concentrations were 0 (control), 0.5, 1.2, 2.4, 4.8 and 12.2 mg T-2/kg feed respectively. Shrimp were fed three times a day (total daily feed intake ~ 5% of body weight) for 20 days10,24. One-third of the water in the tank was replaced with fresh water every morning. On day 21, the total weight of all shrimp in each tank was determined. Shrimp were anesthetized with ice, killed, and the midgut was removed and stored at −70 °C until required.
Growth parameters of shrimp
The weight gain rate, survival rate and specific growth rate of shrimp (n = 30/group) in different treatment groups were calculated51,52 as follows:
where Wf and Wi are the final and initial average body weights on day 21 and 1 respectively. Nf and Ni are the final and initial (=50) number of shrimp in each group.
Histopathology of intestine
The midgut of fresh shrimp intestine (n = 5) from each group was fixed in 10% neutral-buffered formalin for 24 h and dehydrated with a gradient of alcohol (50% to 95%). Next, intestines were embedded, sectioned using a microtome and stained as described by Qiu et al.23. Histopathologic changes in the intestine were observed using a light microscope (Olympus CKX41, Tokyo, Japan).
Digestive enzyme analyses
Midguts (n = 5) of shrimp intestine from each group were homogenized (IKAT 25, Staufen, Germany) for 1 min (5000 × g) in cold distilled water and centrifuged (Himac CS150GXII, Hitachi, Tokyo) for 20 min (8,000 × g) at 4 °C. The supernatant was used to measure the digestive enzyme activities. Protease activity was determined by the casein-hydrolysis method of Furne et al.53. Amylase activity was determined by the starch-hydrolysis method of Zokaeifar et al.54. Lipase activity was determined according to the method of Muralisankar et al.19 by degrading triacylglycerol to free fatty acids. Digestive enzyme activities are expressed as U/mg of protein.
The concentration-response curves between T-2 and digestive enzyme activities in shrimp intestine were constructed using Origin 8.5. The curves were drawn with T-2 concentration (mg/kg) as the x-axis, and the ratio between the value of the experimental group and the control group (relative coefficient) as the y-axis. The NOAEL (no observable adverse effect level, concentration of T-2 when the ratio of enzyme activity was 1), MEC (maximal effect concentration of T-2, concentration of T-2 when the ratio of enzyme activity was maximum) and EC50 (concentration for 50% of maximal effect, concentration of T-2 when the ratio of enzyme activity was 0.5) were calculated by GraphPad Prism 7 (GraphPad Software, La Jolla, CA).
Statistical analyses
Data are presented as the mean ± standard deviation (SD). All statistical analyses were conducted using GraphPad Prism 7, SPSS 22.0 (IBM, Chicago, USA) and Origin 8.5 (OriginLab, Massachusetts, USA). One-way ANOVA was performed and Duncan’ s multiple range test at a significant level of 0.05 was used to determine differences among groups.
References
Chang, Z. W., Chiang, P. C., Cheng, W. & Chang, C. C. Impact of ammonia exposure on coagulation in white shrimp, Litopenaeus vannamei. Ecotox. Environ. Safe. 118, 98–102, https://doi.org/10.1016/j.ecoenv.2015.04.019 (2015).
Facanha, F. N., Oliveira-Neto, A. R., Figueiredo-Silva, C. & Nunes, A. J. P. Effect of shrimp stocking density and graded levels of dietary methionine over the growth performance of Litopenaeus vannamei reared in a green-water system. Aquaculture. 463, 16–21, https://doi.org/10.1016/j.aquaculture.2016.05.024 (2016).
Mateo, J. J., Mateo, R. & Jimenez, M. Accumulation of type A trichothecenes in maize, wheat and rice by Fusarium sporotrichioides isolates under diverse culture conditions. Int. J. Food. Microbiol. 72, 115–123, https://doi.org/10.1016/S0168-1605(01)00625-0 (2002).
Marijani, E. et al. Mycoflora and mycotoxins in finished fish feed and feed ingredients from smallholder farms in East Africa. Egypt. J. Aquat. Res. 43, 169–176, https://doi.org/10.1016/j.ejar.2017.07.001 (2017).
Krska, R., Welzig, E. & Boudra, H. Analysis of Fusarium toxins in feed. Anim. Feed. sci. Technol. 137, 241–264, https://doi.org/10.1016/j.anifeedsci.2007.06.004 (2007).
Meister, U. Analysis of T-2 and HT-2 toxins in oats and other cereals by means of HPLC with fluorescence detection. Mycotoxin. Res. 24, 31–39, https://doi.org/10.1007/BF02985268 (2008).
Sudakin, D. L. Trichothecenes in the environment: relevance to human health. Toxicol. Lett. 143, 97–107, https://doi.org/10.1016/S0378-4274(03)00116-4 (2003).
Eriksen, G. S. & Petterson, H. Toxicological Evaluation of Trichothecenes in Animal Feed. Anim. Feed sci. Technol. 114, 205–239, https://doi.org/10.1016/j.anifeedsci.2003.08.008 (2004).
Meissonnier, G. M. et al. Subclinical doses of T-2 toxin impair acquired immune response and liver cytochrome P450 in pigs. Toxicol. 247, 46–54, https://doi.org/10.1016/j.tox.2008.02.003 (2008).
Lu, P. L. et al. A Sensitive and Validated Method for Determination of T-2 and HT-2 Toxin Residues in Shrimp Tissues by LC-MS/MS. Food. Anal. Method. 9, 1580–1594, https://doi.org/10.1007/s12161-015-0336-y (2016).
Young, J. C., Zhou, T., Yu, H., Zhu, H. H. & Gong, J. H. Degradation of trichothecene mycotoxins by chicken intestinal microbes. Food. Chem. Toxicol. 45, 136–143, https://doi.org/10.1016/j.fct.2006.07.028 (2007).
Manning, B. B. et al. Exposure to feedbone mycotoxins T-2 toxin or ochratoxin a causes increased mortality of channel catfish challenged with Edwardsiella ictaluri. J. Aquat. Anim. Health. 17, 147–152, https://doi.org/10.1577/H03-063.1 (2005).
Supamattaya, K., Bundit, O., Boonyarapatlin, M. & Schatzmayr, G. Effects of mycotoxins T-2 and Zearalenone on growth performance immuno-ohysiological parameters and histological changes in Black tiger shrimp (Penaeus monodon) and white shrimp (Litopenaeus vannamei). XII International Symposium of Fish Nutrition & Feeding. 41, 218–221 (2006).
Duan, Y. F. et al. Effect of dietary Clostridium butyricum on growth, intestine health status and resistance to ammonia stress in Pacific white shrimp Litopenaeus vannamei. Fish. Shellfish. Immun. 65, 25–33, https://doi.org/10.1016/j.fsi.2017.03.048 (2017).
Li, G., Shinozuka, J., Uetuka, K., Nakayama, H. & Doi, K. T-2 toxin-induced apoptosis in intestinal crypts epithelial cells of mice. Exp. Toxicol. Pathol. 49, 447–450, https://doi.org/10.1016/S0940-2993(97)80132-7 (1997).
Jiang, R. et al. Dietary plasma protein reduces small intestinal growth and lamina propria cell density in early weaned pigs. J. Nutr. 130, 21–26, https://doi.org/10.1093/jn/130.1.21 (2000).
Samanta, A. K., Natasha, J., Senani, S., Kolte, A. P. & Manpal, S. Prebiotic inulin: Useful dietary adjuncts to manipulate the livestock gut microflora. Braz. J. Microbiol. 44, 1–14, https://doi.org/10.1590/S1517-83822013005000023 (2013).
Zhou, X. X., Wang, Y. B. & Li, W. F. Effect of probiotic on larvae shrimp (Penaeus vannamei) based on water quality, survival rate and digestive enzyme activities. Aquaculture. 287, 349–353, https://doi.org/10.1016/j.aquaculture.2008.10.046 (2009).
Muralisankar, T. et al. Effects of dietary zinc on the growth, digestive enzyme activities, muscle biochemical compositions, and antioxidant status of the giant freshwater prawn Macrobrachium rosenbergii. Aquaculture. 448, 98–104, https://doi.org/10.1016/j.aquaculture.2015.05.045 (2015).
Shao, J. C. et al. Evaluation of biofloc meal as an ingredient in diets for white shrimp Litopenaeus vannamei under practical conditions: Effect on growth performance, digestive enzymes and TOR signaling pathway. Aquaculture. 479, 516–521, https://doi.org/10.1016/j.aquaculture.2017.06.034 (2017).
Silveira, A. S. et al. An immune-related gene expression atlas of the shrimp digestive system in response to two major pathogens brings insights into the involvement of hemocytes in gut immunity. Dev. Comp. Immunol. 79, 44–50, https://doi.org/10.1016/j.dci.2017.10.005 (2018).
Thongprajukaew, K., Kovitvadhi, U., Kovitvadhi, S., Somsueb, P. & Rungruangsak-Torrissen, K. Effects of different modified diets on growth, digestive enzyme activities and muscle compositions in juvenile Siamese fighting fish (Betta splendens Regan, 1910). Aquaculture. 322–323, 1–9, https://doi.org/10.1016/j.aquaculture.2011.10.006 (2011).
Qiu, M. et al. Effects of T-2 toxin on growth, immune function and hepatopancreas microstructure of shrimp (Litopenaeus vannamei). Aquaculture. 462, 35–39, https://doi.org/10.1016/j.aquaculture.2016.04.032 (2016).
Deng, Y. J. et al. Effects of T-2 Toxin on Pacific White Shrimp Litopenaeus vannamei: Growth, and Antioxidant Defenses and Capacity and Histopathology in the Hepatopancreas. J. Aquat. Anim. Health. 29, 15–25, https://doi.org/10.1080/08997659.2016.1249577 (2017).
Giacomo, M. D. & Farina, R. A. Allometric models in paleoecology: Trophic relationships among Pleistocene mammals. Palaeogeogr. Palaeocl. 471, 15–30, https://doi.org/10.1016/j.palaeo.2017.01.040 (2017).
Forrest, R. E., McAllister, M. K., Martell, S. J. D. & Walters, C. J. Modelling the effects of density-dependent mortality in juvenile red snapper caught as bycatch in Gulf of Mexico shrimp fisheries: Implications for management. Fish. Res. 146, 102–120, https://doi.org/10.1016/j.fishres.2013.04.002 (2013).
Wang, W. C., Natelson, R. H., Stikeleather, L. F. & Roberts, W. L. Product sampling during transient continuous countercurrent hydrolysis of canola oil and development of a kinetic model. Comput. Chem. Eng. 58, 144–155, https://doi.org/10.1016/j.compchemeng.2013.06.003 (2013).
Huang, Z. R. et al. Effect of T-2 toxin injected shrimp muscle extracts on mouse macrophage cells (RAW264.7). Drug. Chem. Toxicol. 41, 16–21, https://doi.org/10.1080/01480545.2016.1278227 (2018).
Pleadin, J. et al. Survey of T-2/HT-2 toxins in unprocessed cereals, food and feed coming from Croatia and Bosnia & Herzegovina. Food. Chem. 224, 153–159, https://doi.org/10.1016/j.foodchem.2016.12.063 (2017).
Zeng, Z. Z. et al. Dietary aflatoxin B1 decreases growth performance and damages the structural integrity of immune organs in juvenile grass carp (Ctenopharyngodon idella). Aquaculture. 500, 1–17, https://doi.org/10.1016/j.aquaculture.2018.09.064 (2019).
Adeyemo, B. T., Tiamiyu, L. O. & Ayuba, V. O. M, S. & Odo, J. Effects of dietary mixed aflatoxin B1 and fumonisin B1 on growth performance and haematology of juvenile Clarias gariepinus catfish. Aquaculture. 491, 190–196, https://doi.org/10.1016/j.aquaculture.2018.03.026 (2018).
Xie, S. W. et al. Effect of deoxynivalenol on growth performance, histological morphology, anti-oxidative ability and immune response of juvenile Pacific white shrimp, Litopenaeus vannamei. Fish. Shellfish. Immun. 82, 442–452, https://doi.org/10.1016/j.fsi.2018.08.053 (2018).
Bundit, O., Kanghae, H., Phromkunthong, W. & Supamattaya, K. Effects of mycotoxin T-2 and zearalenone on histopathological changes in black tiger shrimp (Penaeus monodon Fabricius). Songklanakarin J. Sci. Technol. 28, 937–949 (In Thailand) (2006).
Maresca, M. From the Gut to the Brain: Journey and Pathophysiological Effects of the Food-Associated Trichothecene Mycotoxin Deoxynivalenol. Toxins. 5, 784–820, https://doi.org/10.2527/jas2013-6427 (2013).
Bouhet, S. & Oswald, I. P. The effects of mycotoxins, fungal food contaminants, on the intestinal epithelial cell-derived innate immune response. Vet. Immunol. Immunopathol. 108, 199–209, https://doi.org/10.1016/j.vetimm.2005.08.010 (2005).
Jacevic, V. et al. Pathohistological changes in small and large intestine of rats acutely intoxicated by T-2 toxin. Archive of Oncology. 9, 40–41 (2001).
Sklan, D. et al. The effect of chronic feeding of diacetoxyscirpenol and T-2 toxin on performance, health, small intestinal physiology and antibody production in turkey poults. Brit. Poul. Sci. 44, 46–52, https://doi.org/10.1080/0007166031000085373 (2003).
Wang, Y. B. Effect of probiotics on growth performance and digestive enzyme activity of the shrimp Penaeus vannamei. Aquaculture. 269, 259–264, https://doi.org/10.1016/j.aquaculture.2007.05.035 (2007).
Anand, P. S. S. et al. Effect of dietary supplementation of periphyton on growth performance and digestive enzyme activities in Penaeus monodon. Aquaculture. 392–395, 59–68, https://doi.org/10.1016/j.aquaculture.2013.01.029 (2013).
Han, X. Y., Huang, Q. C., Li, W. F., Jiang, J. F. & Xu, Z. R. Changes in growth performance, digestive enzyme activities and nutrient digestibility of cherry valley ducks in response to aflatoxin B1 levels. Livestock. Sci. 119, 216–222, https://doi.org/10.1016/j.livsci.2008.04.006 (2008).
Applegate, T. J., Schatzmayr, G., Pricket, K., Troche, C. & Jiang, Z. Effect of aflatoxin culture on intestinal function and nutrient loss in laying hens. Poultry. Sci. 88, 1235–1241, https://doi.org/10.3382/ps.2008-00494 (2009).
Osselaere, A. et al. Toxic effects of dietary exposure to T-2 toxin on intestinal and hepatic biotransformation enzymes and drug transporter systems in broiler chickens. Food. Chem. Toxicol. 55, 150–155, https://doi.org/10.1016/j.fct.2012.12.055 (2013).
Ravenzwaaya, B. V. et al. The sensitivity of metabolomics versus classical regulatory toxicology from a NOAEL perspective. Toxicol. Lett. 16, 20–28, https://doi.org/10.1016/j.toxlet.2014.03.004 (2014).
Rotter, B. A. Invited review: Toxicology of deoxynivalenol (Vomi-toxin). J. Toxicol. Env. Heal. 48, 1–34, https://doi.org/10.1080/009841096161447 (1996).
Awad, W. A. et al. In vitro effects of deoxynivalenol on small intestinal D-glucose uptake and absorption of deoxynivalenol across the isolated jejunal epithelium of laying hens. Poultry. Sci. 86, 15–20, https://doi.org/10.1093/ps/86.1.15 (2007).
Liu, C. H., Chiu, C. S., Ho, P. L. & Wang, S. W. Improvement in the growth performance of white shrimp, Litopenaeus vannamei, by a protease-producing probiotic, Bacillus subtilis E20, from natto. J. App. Microbiol. 107, 1031–1041, https://doi.org/10.1111/j.1365-2672.2009.04284.x (2009).
Muneer, M. A., Farah, I. O., Newman, J. A. & Goyal, S. M. Immunosuppression in animals. British Veterinary Journal. 144, 288–301, https://doi.org/10.1016/0007-1935(88)90116-9 (1988).
Stanley, V. G., Ojo, R., Woldesenbet, S., Hutchinson, D. H. & Kubena, L. F. The Use of Saccharomyces cerevisiae to Suppress the Effects of Aflatoxicosis in Broiler Chicks. Poultry. Sci. 72, 1867–1872, https://doi.org/10.3382/ps.0721867 (1993).
Dai, Z. et al. Preparation and assessment of T-2 toxic feed of shrimp by microcapsule technology. Guangdong Agricultural Sciences. 40, 92–94, https://doi.org/10.3969/j.issn.1004-874X.2013.11.027 (2013). (In China)
Wang, Y. L. et al. Acute oral toxicity of T-2 toxin on Litopenaeus vannamei. Modern Food Science and Technology. 31, 221–226, https://doi.org/10.13982/j.mfst.1673-9078.2015.1.009 (2015). (In China)
Tian, L. X., Liu, Y. J., Yang, H. J., Liang, G. Y. & Niu, J. Effects of different dietary wheat starch levels on growth, feed efficiency and digestibility in grass carp (Ctenopharyngodon idella). Aquacult. Int. 20, 283–293, https://doi.org/10.1007/s10499-011-9456-6 (2012).
Yang, Q. H., Tan, B. P., Dong, X. H., Chi, S. Y. & Liu, H. Y. Effects of different levels of Yucca schidigera extract on the growth and nonspecific immunity of Pacific white shrimp (Litopenaeus vannamei) and on culture water quality. Aquaculture. 439, 39–44, https://doi.org/10.1016/j.aquaculture.2014.11.029 (2015).
Furne, M. et al. Digestive enzyme activities in Adriatic sturgeon Acipenser naccarii and rainbow trout Oncorhynchus mykiss. A comparative study. Aquaculture. 250, 391–398, https://doi.org/10.1016/j.aquaculture.2005.05.017 (2005).
Zokaeifar, H. et al. Effects of Bacillus subtilis on the growth performance, digestive enzymes, immune gene expression and disease resistance of white shrimp, Litopenaeus vannamei. Fish. Shellfish. Immun. 33, 516–521, https://doi.org/10.1016/j.fsi.2012.05.027 (2012).
Acknowledgements
This study was financially supported by the National Natural Science Foundation of China (NSFC) (No. 31371777), Science and Technology Planning Project of Guangdong Province (No. 2014B020205006), Graduate Education Innovation Program of Guangdong Ocean University (No. 201831), Technology Research Program of Shenzhen (No. KYTDPT20160316161010).
Author information
Authors and Affiliations
Contributions
Z.H., Y.W., J.Z. and R.G. had the major responsibility for preparing the paper; Z.H. and M.Q. collected and analyzed the data; Z.H., Y.W. and L.S. performed the measurements; Y.D., X.W. and S.B. contributed to the conception of the study.
Corresponding author
Ethics declarations
Competing Interests
The authors declare no competing interests.
Additional information
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Huang, Z., Wang, Y., Qiu, M. et al. Effects of T-2 toxin on digestive enzyme activity, intestinal histopathology and growth in shrimp Litopenaeus vannamei. Sci Rep 9, 13175 (2019). https://doi.org/10.1038/s41598-019-49004-4
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-019-49004-4
This article is cited by
-
Digestive enzyme activities, intestinal histology, and gut microbiota of Pacific white shrimp Litopenaeus vannamei fed with turmeric, curcumin, and nanomicelle curcumin
Aquaculture International (2023)
-
A Mixture of Multi-Strain Probiotics (Lactobacillus Rhamnosus, Lactobacillus Helveticus, and Lactobacillus Casei) had Anti-Inflammatory, Anti-Apoptotic, and Anti-Oxidative Effects in Oxidative Injuries Induced By Cadmium in Small Intestine and Lung
Probiotics and Antimicrobial Proteins (2023)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.