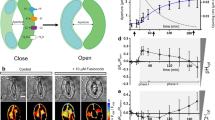
Abstract
In plants, stomata regulate water loss through transpiration for plant growth and survival in response to various environmental stressors; and simple methods to assess stomatal dynamics are needed for physiological studies. Herein, we report a fluorescence-imaging-based method using fluorescein diacetate tagged with Hoechst 33342, a nuclear staining chemical probe (HoeAc2Fl) for the qualitative assessment of stomatal dynamics. In our method, the stomatal movement is inferred by simple monitoring of the fluorescence intensity in the nucleus of the stomata.
Similar content being viewed by others
Introduction
Stomatal dynamics influence plant transpiration, gas exchange, drought tolerance, and defense1,2; and stomatal movement takes place in response to several environmental stimuli, such as blue light, red light, low CO2, and chemicals. The signaling mechanisms that underly stomatal movement have attracted the attention of plant physiologists3,4,5, and there has been a great deal of research interest into the development of chemical tools for the study of stomatal movements6,7,8,9,10,11,12. For example, in pioneering work, Cutler et al. reported pyrabactin, a synthetic agonist of abscisic acid (ABA) receptors, which led to their identification and the development of stomatal closing agents6,13; and Kinoshita et al. identified several small molecules that affect stomatal movements from a chemical library9. However, to speed the development of molecules capable of affecting stomatal movements, simple and high-throughput methods for chemical screening are urgently needed. Stomatal movements are currently evaluated by either the direct monitoring of stomatal aperture under the microscope, or analyses of thermal images, that reflect the degree of transpiration from stomata14,15,16. Although these methods are robust and reliable, the former is time-consuming and low throughput, the latter requires the special equipment17.
Herein, we report a simple and convenient method for the assessment of stomatal closing/opening in Arabidopsis thaliana based on fluorescent live imaging by Hoechst-tagged acetylfluorescein (HoeAc2Fl, Fig. 1a). Our method enables objective assessment of stomatal dynamics by simple monitoring of the fluorescence intensity of HoeAc2Fl in the nucleus of the stomata.
Results and Discussion
HoeAc2Fl is a fluorescent stain comprising Hoechst 33342 and fluorescein diacetate moieties, and was originally developed for nuclear staining of mammalian cells18,19. We discovered the guard cells of closed stomata can be selectively stained by application of HoeAc2Fl to the plant (Fig. 1b). Therefore, we proposed that HoeAc2Fl could constitute a useful chemical tool for the assessment of stomatal dynamics by simple monitoring of the intensity of the fluorescence of the nuclei of the guard cells.
Our studies commenced with the evaluation of the exact intracellular localization of HoeAc2Fl using the stomata of red fluorescent protein-fused histone protein-overexpressing plants (P35S::H2B-tdTomato), wherein the red fluorescence of H2B-tdTomato is localized in the nuclei20. After closure of the stomata by leaving the plant in the dark, the stomata were stained with HoeAc2Fl. The characteristic green fluorescence of HoeAc2Fl was observed from the nuclei of almost all of the closed stomata, co-localizing with H2B-tdTomato (Fig. 2a). In contrast, when the stomata were treated in the light, almost no fluorescence was observed from HoeAc2Fl-stained stomata (Fig. 2b). The exact correlation between fluorescence intensity and stomatal aperture showed that the visible fluorescence was only observed from stomata with an aperture of less than about 2.5–3 µm (Fig. 2c, three representative images of closed or open stomata are shown in Fig. S1, and fluorescence images of various stomata having different apertures are shown in Fig. S2). This threshold of the stomatal aperture is very close to the previously reported standard of aperture for determining opened/closed stomata, wherein stomata with an aperture of 1–3 µm are said to be closed; and those with an aperture of 2–6 µm are said to be open5. The mean fluorescence intensity of the nucleus in guard cells in the dark conditions was significantly higher than that in the light conditions (Fig. 2d), clearly demonstrating that HoeAc2Fl can only stain closed stomata. From the time course of the staining process with HoeAc2Fl, 60–90 min of incubation in the dark is enough to obtain the robust fluorescence intensity (Fig. 3a,b). After staining, the fluorescence intensity in nucleus was gradually decreased in the light condition according with gradual opening of stomata (Fig. 3a,b). In contrast, after incubation in the light condition, the fluorescence intensity increased in accordance with the stomatal closure under the dark. That is, the stomata gradually closed from 90 min (210 min in total, Fig. 3c) and completely closed around 150 min (270 min in total, Fig. 3c). Concurrently, the fluorescence intensity increased from 90 min (210 min in total, Fig. 3d) and reached plateau around 150 min (270 min in total, Fig. 3d). These results clearly demonstrated that the nuclear staining by HoeAc2Fl is reversible and depends on the stomatal dynamics. Similar results were obtained using Hoechst 33342, which was also found to stain closed but not open stomata, although some nonspecific staining was also observed at the edge of the stomata (Fig. 4a). This result strongly suggests that the unique localization property of HoeAc2Fl can be attributed to the Hoechst 33342 moiety. In contrast, almost no staining was observed by 4′,6-diamidino-2-phenylindole (DAPI)21,22, a conventional nucleus-staining fluorescent reagent10 (Fig. 4b). These results establish HoeAc2Fl as a practical chemical tool for the rapid identification of open/closed stomata; and its superior photochemical properties (such as longer excitation and emission wavelengths, and higher quantum yield) compared to Hoechst 33342 make it more useful.
(a,b) Differential interference images (DIC) and fluorescent (tdTomato or Fluorescein) microscopic images of HoeAc2Fl-stained stomata of P35S ::H2B-tdTomato35S::H2B-tdTomato in the dark (a) or light (b) conditions; only the closed stomata were stained with HoeAc2Fl (the stomatal aperture was 2.15 µm), whereas opened stomata were not (the stomatal aperture was 4.70 µm) (see images in the fluorescein channel). The scale bars, 10 µm. (c) Relationship between stomatal apertures and fluorescent intensity of the nucleus of HoeAc2Fl-stained guard cells in the dark (black circle) or light condition (blue diamond). (d) Dotted plot of the fluorescence intensity of HoeAc2Fl-stained stomata in the dark (black circle) or light (blue diamond) conditions. Bars represent mean fluorescence intensity (n = 30). Significant differences were evaluated by one-way ANOVA/Tukey HSD post hoc test (p < 0.01).
(a) Stomatal aperture of Col-0 during the staining process with HoeAc2Fl in the dark (0–90 min) and stomatal opening process in the light condition (total incubation time was 120-210 min, which is incubated for 30–120 min in the light condition). Error bars represent mean and SD (n = 25). (b) Dotted plot of the fluorescence intensity of staining process in the dark (0–90 min) and stomatal opening process in the light condition (total incubation time was 120–210 min, which is incubated for 30–120 min in the light condition) of HoeAc2Fl. Bars represent mean fluorescence intensity (n = 25). (c) Stomatal aperture of Col-0 during the staining process with HoeAc2Fl in the light (0–120 min) and stomatal closing process in the dark condition (total incubation time was 150–300 min, which is incubated for 30–180 min in the dark condition). Error bars represent mean and SD (n = 25). (d) Dotted plot of the fluorescence intensity of staining process in the light (0–120 min) and stomatal closing process in the dark condition (total incubation time was 150–300 min, which is incubated for 30–180 min in the dark condition) of HoeAc2Fl. Bars represent mean fluorescence intensity (n = 25). Significant differences were evaluated by one-way ANOVA/Tukey HSD post hoc test (p < 0.01).
Next, we sought to account for the selectivity of HoeAc2Fl for closed over open stomata. Our hypothesis was the efflux of HoeAc2Fl from open stomata precludes their staining. Accordingly, we carried out the staining experiment at a lower temperature, to suppress the transport activity. However, nuclear staining was unaffected, in spite of the significant decrease in the stomatal aperture under low temperature (Fig. S3). This result indicates that no transporter is involved in the efflux of HoeAc2Fl from the opened stomata. A mechanistic explanation for the selective nuclear localization of HoeAc2Fl is therefore unclear, and remains to be clarified.
We next used HoeAc2Fl to study chemically-triggered stomata dynamics23. Treatment of plants with abscisic acid (ABA) in the light has been previously reported to close stomata24. The peeled epidermis was stained by HoeAc2Fl followed by treatment of ABA in the light condition, fluorescence was observed from the nucleus of their guard cells (Fig. 5a–d). In contrast, treatment with auxin (IAA)25, coronatine (COR)2,26,27, and fusicoccin (FC)28,29 opened most of the stomata, and almost no fluorescence was observed in the nucleus of their guard cells (Fig. 5e–i). Similar to the light-triggered stomatal dynamics, the correlations between fluorescent intensity and stomatal aperture also demonstrated that fluorescence was only observed from closed stomata (Fig. 5i). Remarkable differences in the mean fluorescence intensities of ABA-treated stomata and IAA/COR/FC-treated stomata (Fig. 5d,j) were observed, confirming the applicability of HoeAc2Fl to the assessment of chemically induced stomatal dynamics. It is already well known that aperture lengths for open (2–6 µm) and closed (1–3 µm) stomata partly overlap in 2–3 µm5, and this marginal aperture length often cause difficulties to determine the results in stomatal bioassays. We examine the reliability of our method among this marginal region of aperture (2–3 µm). In the dose-dependent addition of COR, 0.3 µM of COR cause the stomatal aperture of the marginal length (2–3.5 µm) (Fig. S4a). Under the same condition, the mean fluorescent intensity of HoeAc2Fl was very close to that of open stomata treated by>1 µM of COR and significantly lower than that in the mock condition (Fig. S4b). Similar results were also obtained by the dose-dependency of FC (Fig. S5) and light intensity (Fig. S6). These results clearly demonstrated that our method judged that stomata of marginal aperture length belongs to the ‘open stomata’ and enabled clear decision of the results.
(a) Fluorescent microscopic images of HoeAc2Fl-stained stomata of Col-0 treated with ABA (10 µM) in the light. The scale bars, 10 µm. (b) Stomatal aperture of Col-0 treated without or with ABA (10 µM). Error bars represent mean and SD (n = 25). (c) Relationship between stomatal apertures and fluorescence intensity of the nucleus of HoeAc2Fl-stained guard cells in the mock condition (black circle), in the absence (red cross) or the presence of ABA (blue diamond). (d) Dotted plot of the fluorescence intensity of HoeAc2Fl-stained stomata in the mock condition (black circle), in the absence (black cross) or the presence of ABA (black diamond). (e–g) Fluorescent microscopic images of HoeAc2Fl-stained stomata of Col-0 treated with IAA (10 µM, e), COR (10 µM, f), or FC (10 µM, g) in the dark. The scale bars, 10 µm. (h) Stomatal aperture of Col-0 treated without or with various chemicals (IAA, COR, and FC, 10 µM). Error bars represent mean and SD (n = 25). Significant differences were evaluated by one-way ANOVA/Tukey HSD post hoc test (p < 0.01). (i) Relationship between stomatal apertures and fluorescence intensity of the nucleus of HoeAc2Fl-stained guard cells in the mock condition (black circle) or treated with IAA (green square), COR (blue triangle), or FC (red cross). (j) Dotted plot of the fluorescence intensity of HoeAc2Fl-stained stomata in the mock condition (black circle) or treated with IAA (green square), COR (blue triangle), or FC (red cross). Bars represent mean fluorescence intensity (n = 25). Significant differences were evaluated by one-way ANOVA/Tukey HSD post hoc test (p < 0.01).
Conclusion
HoeAc2Fl is proposed as a tool to easily and quickly assess whether plant stomata are open or closed based on its selectivity for the guard cells of closed stomata. The mechanistic basis for this selectivity is unknown. When the stomata were stained by HoeAc2Fl, the fluorescence was observed only from closed stomata. The clear threshold of the fluorescence provides objective criteria for the assessment of stomatal dynamics, although it is not quantitative. Instant determination of stomatal dynamics by measuring the fluorescence of HoeAc2Fl with objective analyses is expected to enable high-throughput screening of chemical libraries, which may lead to the discovery of novel chemical probes that can improve our understanding of plant responses to changes in their environments, and ultimately lead to improved crop production.
References
Kim, T. H., Bohmer, M., Hu, H., Nishimura, N. & Schroeder, J. I. Guard cell signal transduction network: advances in understanding abscisic acid, CO2, and Ca2+ signaling. Annual Review of Plant Biology 61, 561–591 (2010).
Melotto, M., Underwood, W., Koczan, J., Nomura, K. & He, S. Y. Plant stomata function in innate immunity against bacterial invasion. Cell 126, 969–980 (2006).
Munemasa, S. et al. Mechanisms of abscisic acid-mediated control of stomatal aperture. Curr Opin Plant Biol 28, 154–162 (2015).
Assmann, S. M. & Jegla, T. Guard cell sensory systems: recent insights on stomatal responses to light, abscisic acid, and CO2. Current Opinion in Plant Biology 33, 157–167 (2016).
Jezek, M. & Blatt, M. R. The Membrane Transport System of the Guard Cell and Its Integration for Stomatal Dynamics. Plant Physiol 174, 487–519 (2017).
Park, S.-Y. et al. Abscisic acid inhibits type 2C protein phosphatases via the PYR/PYL family of START proteins. Science 324, 1068–1071 (2009).
Okamoto, M. et al. Activation of dimeric ABA receptors elicits guard cell closure, ABA-regulated gene expression, and drought tolerance. Proceedings of the National Academy of Sciences of the United States of America 110, 12132–12137 (2013).
Vaidya, A. S. et al. A Rationally Designed Agonist Defines Subfamily IIIA Abscisic Acid Receptors As Critical Targets for Manipulating Transpiration. ACS Chem Biol 12, 2842–2848 (2017).
Toh, S. et al. Identification and Characterization of Compounds that Affect Stomatal Movements. Plant Cell Physiol 59, 1568–1580 (2018).
Jens, F. et al. Insights into the in Vitro and in Vivo SAR of Abscisic Acid – Exploring Unprecedented Variations of the Side Chain via Cross‐Coupling‐Mediated Syntheses. European Journal of Organic Chemistry 2018, 1403–1415 (2018).
Jens, F. et al. Potent Analogues of Abscisic Acid – Identifying Cyano‐Cyclopropyl Moieties as Promising Replacements for the Cyclohexenone Headgroup. European Journal of Organic Chemistry 2018, 1416–1425 (2018).
Benson, C. L. et al. Abscisic acid analogs as chemical probes for dissection of abscisic acid responses in Arabidopsis thaliana. Phytochemistry 113, 96–107 (2015).
Zhao, Y. et al. Chemical genetic interrogation of natural variation uncovers a molecule that is glycoactivated. Nature Chemical Biology 3, 716–721 (2007).
Jones, H. G. et al. Use of infrared thermography for monitoring stomatal closure in the field: application to grapevine. Journal of Experimental Botany 53, 2249–2260 (2002).
Merlot, S. et al. Use of infrared thermal imaging to isolate Arabidopsis mutants defective in stomatal regulation. The Plant Journal 30, 601–609 (2002).
Hashimoto, M. et al. Arabidopsis HT1 kinase controls stomatal movements in response to CO2. Nature cell biology 8, 391–397 (2006).
Toda, Y. et al. DeepStomata: Facial Recognition Technology for Automated Stomatal Aperture Measurement. bioRxiv, 365098 (2018).
Nakamura, A. et al. Hoechst tagging: a modular strategy to design synthetic fluorescent probes for live-cell nucleus imaging. Chemical Communications 50, 6149–6152 (2014).
Nakamura, A. & Tsukiji, S. Ratiometric fluorescence imaging of nuclear pH in living cells using Hoechst-tagged fluorescein. Bioorganic & Medicinal Chemistry Letters 27, 3127–3130 (2017).
Adachi, S. et al. Programmed induction of endoreduplication by DNA double-strand breaks in Arabidopsis. Proc Natl Acad Sci USA 108, 10004–10009 (2011).
Coleman, A. W., Maguire, M. J. & Coleman, J. R. Mithramycin- and 4’-6-diamidino-2-phenylindole (DAPI)-DNA staining for fluorescence microspectrophotometric measurement of DNA in nuclei, plastids, and virus particles. Journal of Histochemistry & Cytochemistry 29, 959–968 (1981).
Monda, K. et al. Enhanced Stomatal Conductance by a Spontaneous Arabidopsis Tetraploid, Me-0, Results from Increased Stomatal Size and Greater Stomatal Aperture. Plant Physiology 170, 1435–1444 (2016).
Acharya, B. R. & Assmann, S. M. Hormone interactions in stomatal function. Plant Molecular Biology 69, 451–462 (2009).
Luan, S., Li, W., Rusnak, F., Assmann, S. M. & Schreiber, S. L. Immunosuppressants implicate protein phosphatase regulation of K+ channels in guard cells. Proceedings of the National Academy of Sciences of the United States of America 90, 2202–2206 (1993).
Blatt, M. R. & Thiel, G. K+ channels of stomatal guard cells: bimodal control of the K+ inward-rectifier evoked by auxin. The Plant Journal 5, 55–68 (1994).
Okada, M. et al. Total syntheses of coronatines by exo-selective Diels-Alder reaction and their biological activities on stomatal opening. Organic & biomolecular chemistry 7, 3065–3073 (2009).
Ueda, M. et al. Noncanonical Function of a Small-Molecular Virulence Factor Coronatine against Plant Immunity: An In Vivo Raman Imaging Approach. ACS Central Science 3, 462–472 (2017).
Squire, G. R. & Mansfield, T. A. The action of fusicoccin on stomatal guard cells and subsidiary cells. New Phytologist 73, 433–440 (1974).
Turner, N. C. & Graniti, A. Fusicoccin: a Fungal Toxin that opens Stomata. Nature 223, 1070–1071 (1969).
Acknowledgements
P35S::H2B-tdTomato plants of the Col-0 ecotype were kindly provided by Prof. Taku Demura and Dr. Misato Ohtani (Nara Institute of Science and Technology, Japan). We also thank Dr. Yasuhiro Ishimaru (Tohoku University) for technical advice. This work was supported by a Grant-in-Aid for Scientific Research for MU from MEXT, Japan (no. 26282207, 17H06407, and no. 17H00885), for ST (nos. 15H05949 “Resonance Bio” and 18H04546 “Chemistry for Multimolecular Crowding Biosystems”), for YT (no. 18H02101, no. 19H05283), JSPS A3 Foresight Program (MU), and JSPS Core-to-Core Program Asian Chemical Biology Initiative (MU), and JST (JPMJPR16QR to YT).
Author information
Authors and Affiliations
Contributions
M.U. conceived, designed and coordinated the research project. Y.T. and S.M. designed and performed all experiments. A.N. and S.T. synthesized chemical probe. Y.T., S.M., A.N., S.T. and M.U. analyzed data. S.E. discovered the original phenomena. M.U., Y.T. and S.T. wrote the manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Takaoka, Y., Miyagawa, S., Nakamura, A. et al. Hoechst-tagged Fluorescein Diacetate for the Fluorescence Imaging-based Assessment of Stomatal Dynamics in Arabidopsis thaliana. Sci Rep 10, 5333 (2020). https://doi.org/10.1038/s41598-020-62239-w
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-020-62239-w
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.