Abstract
Calotropis gigantea (C. gigantea) extract with an ecofriendly nanotechnology approach could provide promising antimicrobial activity against skin pathogens. This study investigates the antimicrobial capability of green synthesized binary ZnO–CuO nanocomposites from C. gigantea against non-MDR (Staphylococcus aureus and Escherichia coli) and MDR (Klebsiella pneumoniae, Pseudomonas aeruginosa and methicillin-resistant S. aureus) skin pathogens. Scanning electron microscopy and transmission electron microscopy revealed the size and shape of B3Z1C sample. Results of X-ray powder diffraction, energy-dispersive spectroscopy, FTIR and UV–Vis spectroscopy analyses confirmed the presence of mixed nanoparticles (i.e., zinc oxide, copper oxide, carbon and calcium) and the stabilising phytochemical agents of plant (i.e., phenol and carbonyl). Antimicrobial results showed that carbon and calcium decorated binary ZnO–CuO nanocomposites with compositions of 75 wt% of ZnO and 25 wt% CuO (B3Z1C) was a strong bactericidal agent with the MBC/MIC ratio of ≤ 4 and ≤ 2 for non-MDR and MDR pathogens, respectively. A significant non-MDR zone of inhibitions were observed for BZC by Kirby–Bauer disc-diffusion test. Further time-kill observation revealed significant fourfold reduction in non-MDR pathogen viable count after 12 h study period. Further molecular studies are needed to explain the biocidal mechanism underlying B3Z1C potential.
Similar content being viewed by others
Introduction
Ulcerative skin infections arising from the colonisation and development of Gram-positive bacteria, Gram-negative bacteria, and multidrug-resistant bacteria are significant health-care problems that seriously affect human skin. A prospective quantitative study reported that the prevalence rates of skin pressure ulcers (PUs) are 15.5% in Kuala Lumpur, Malaysia (2013)1, 33% in Palestine (2017)2, and 16% in Bandung, Indonesia (2017)3. Skin infection has been found in 60 (74.0%) of the collected samples from PUs of hospitalised patients, and these PUs primarily comprise Enterobacteriaceae strains (49.0%), such as Escherichia coli (E. coli), Klebsiella pneumoniae (K. pneumoniae), Enterobacter spp., and Proteus spp.; followed by Staphylococcus aureus (S. aureus) (28.0%) and nonfermenting GNB (23.0%), mostly Pseudomonas aeruginosa (P. aeruginosa), Acinetobacter spp., and methicillin-resistant S. aureus (MRSA)4,5,6,7. PUs are open infected wounds that develop on the skin as result of pressure on one spot of the body for too long or from friction on the skin. Some studies have found that new inorganic oxide antimicrobial agents synthesised from natural plants can be remarkable alternatives for infectious skin treatments of PUs because they are rich in numerous varieties of metal oxides that release ions and in reactive oxygen species (ROS), such as hydroxyl radical (·OH−) and superoxide (·O2−) which cause increased cell permeability, rupture, and death in microorganisms8,9.
The incorporation of inorganic metal and metal oxides in sponges10, hydrogels11,12, and bandages13,14 has become a research hotspot because of these materials’ advantages as antimicrobial agents for treating locally infected skin ulcers. Mixed inorganic metal and metal oxides are effective disinfectants because of their relatively nontoxicity, chemical stability, and efficient antibacterial activity (Table 1). The use of binary antimicrobial agents (e.g., CuO, ZnO, and Ag–ZnO) has been highlighted over single antimicrobial agents given the stronger synergic effect of the former in eliminating bacterial colonies at low concentrations10,25,39, more pronounced wound-healing ability10, lower cytotoxicity10, better biocompatibility25, and improved cell viability which indicates safe human application 25. The combined use of binary antimicrobial agents could reduce the cytotoxicity but not the antimicrobial effect10,25. Furthermore, several studies have shown that the incorporation of antimicrobial agents such as CuO40, CuSO441, ZnO42, ZnO-SiO243, and Re-ZnO44 into biopolymers can effectively combat Gram-positive and Gram-negative bacteria in a concentration-dependent manner. However, binary ZnO/CuO nanocomposites prepared from Calotropis gigantea (C. gigantea) leaves in the current work were found to exert a strong antimicrobial effect on multi-drug resistant (MDR) pathogens such as P. aeruginosa and MRSA compared with other previously reported antimicrobial binary inorganic oxides nanocomposites (Table 2). It can effectively work against MDR pathogens at a very low minimum bactericidal concentration (MBC) of about 0.3125 mg/mL.
Accordingly, the present study focused on the preparation of green synthesised binary ZnO-CuO nanocomposites using C. gigantea leaf extract. The microbial activity of these nanocomposites was investigated by culturing with skin ulcer pathogens such as E. coli, K. pneumoniae, S. aureus, P. aeruginosa, and MRSA. Furthermore, the effects of different compositions on ZnO-CuO nanocomposites were explored with respect to their prospective antimicrobial application.
Materials and methods
Preparation of leaf extract and binary inorganic oxides
Whole C. gigantea plant was collected from Perai Pulau Pinang, Malaysia and identified by an expert from the Unit Herbarium, Pusat Pengajian Sains Kajihayat USM Pulau Pinang (Herbarium No.: 11843). C. gigantea leaves were extracted using deionised water and boiled using hot plate47,48. Then, the filtered leaf extracts were taken and boiled with a stirrer–heater. Binary ZnO–CuO nanocomposites were prepared by adding copper (II) nitrate trihydrate and zinc nitrate hexahydrate into the extract solutions simultaneously and then boiled until they were reduced to pastes. These pastes were calcined in an air-heated furnace47,48. Notably, the mixing composition of copper (II) nitrate trihydrate and zinc nitrate hexahydrate was varied with constant rotation speed and calcination temperatures (Table 3). The samples prepared at weight percentages of 25 wt%, 50 wt%, and 75 wt% of zinc nitrate hexahydrate were denoted as B1Z3C, B1Z1C, and B3Z1C, respectively. Commercial B3Z1C sample was prepared by mixing ZnO (< 100 nm; Aldrich) and CuO (< 10 µm; Sigma–Aldrich) with an agate mortar (Table 3).
Physicochemical characterisation
The crystal phases of BZC nanocomposites were studied by X-ray diffraction (XRD; Bruker D8 powder diffractometer) operated in reflection mode with a Cu Kα radiation (40 kV, 30 mA) diffracted beam monochromator. The step scan mode with a step size of 0.030° within the range of 10° to 90° was used. Scanning electron microscopy (SEM; Fei Quanta FEG 650) was used for morphology and microstructure observations of BZC nanocomposites. The purity of BZC was identified by energy-dispersive X-ray (EDAX) spectroscopy which was equipped with SEM. Detailed morphology of B3Z1C nanocomposites was further confirmed by transmission electron microscopy (TEM; FEI TECHNAI F20 G2).The characteristic optical properties of BZC nanocomposites were studied using a UV–Vis spectrophotometer (Varian) at room temperature within the range of 200–900 nm. FTIR spectroscopy (Perkin Elmer) was recorded within the range of 4000–400 cm−1 through the KBr pellet method to observe the functional groups involved in the natural-plant green synthesis and stabilization of B3Z1C nanocomposites.
Minimum inhibitory concentration (MIC)/MBC determination and tolerance level
Antibacterial activity of BZC nanocomposites against S. aureus 29213, E. coli 25922, P. aeruginosa 27853, K. pneumoniae 700603, and MRSA 38591 were assessed using broth-dilution method on 96-well plates as described by Harun et al46. Absorbance was read at 980 nm wavelength46. High wavelength was selected because of BZC nanoparticle deposition. The bactericidal and bacteriostatic capacity of the samples was determined by the tolerance level46.
Time-kill assay
The antibacterial activity of BZC nanocomposites against time was performed using time-kill assay as illustrated in a previous protocol46. S. aureus bacterial suspension adjusted to 0.5 McFarland standard turbidity was used and diluted with sample solution to a final concentration of 2.5 mg/mL.
Kirby–Bauer disc-diffusion test
The antibacterial activity of BZC nanocomposites against S. aureus was further evaluated using Kirby–Bauer disc-diffusion test49. BZC nanocomposite solutions (2.5 and 10 mg/mL) were prepared and used further for antibacterial studies. About 20 µL of BZC nanocomposite solution, negative control (10% DMSO + distilled water), and C. gigantea leaf extract were loaded into 6 mm sterile filter papers, and the solution was allowed to be diffused within 15–30 min. Then, all discs were properly placed on agar which was already previously spread with bacterial culture. A standard antibiotic comprising 10 µg of Oxoid streptomycin antimicrobial susceptibility discs served as a positive control. After 24 h of incubation at 37 °C, the different levels of zone of inhibition were measured.
Results and discussion
Surface morphology of binary ZnO–CuO nanocomposites
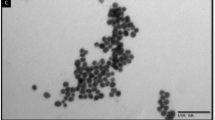
The SEM images of BZC nanocomposites are shown in Fig. 1. B1Z1C had a porous nature (Fig. 1c) with few irregular rod-shaped particles (inset in Fig. 1c). Meanwhile, B1Z3C (Fig. 1a) and B3Z1C (Fig. 1e) had porous honeycomb structures with agglomerated morphology (inset in Fig. 1a,e). The large porous honeycomb structures further increased the available surface area for antimicrobial activity26. These uniform pores were produced during green synthesis owing to the escape of gases at high temperatures26. The EDAX profile of the green synthesised B3Z1C nanocomposites confirmed the presence of Zn, Cu, and O, which were about 49.97 wt%, 20.34 wt%, and 21.32 wt%, respectively. Some weak signals for C, Mg, S, Cl, K, Na, and Ca atoms were found for all BZC nanocomposites (Fig. 1b,d,f). Similar results have been reported for green nanoparticles derived from Artemisia haussknechtii leaf extract50, aqueous Artemisia haussknechtii flower extract9, Protoparmeliopsis muralis lichen51, Ochradenus baccatus leaves52, and Jatropha curcas L. leaf53. The presence of elements such as C, Mg, S, Cl, K, Na, and Ca in small amounts indicated the participation of plant phytochemical groups in reducing and capping the green synthesised BZC nanocomposites9,50,51,52,53. Meanwhile, the TEM image of B3Z1C nanocomposites revealed irregular oval and quasi-spherical shape with an average length of 8.126 nm and diameter of 7.515 nm in size (Fig. 1g). These structures could increase the available surface area for reaction. The magnified TEM image of the B3Z1C nanocomposites along with the lattice fringes with an interfringe distance of 0.248 and 0.254 nm belonged to ZnO and CuO, respectively (Fig. 1h).
Morphology of BZC nanocomposites; (a) SEM image of B1Z3C (10.00 µm), (b) EDAX of B1Z3C, (c) SEM image of B1Z1C (10.00 µm), (d) EDAX of B1Z1C, (e) SEM image of B3Z1C (10.00 µm), (f) EDAX of B3Z1C, (g) TEM image of B3Z1C (10 nm) and (h) Magnified TEM image of B3Z1C nanocomposites along with lattice fringes (2 nm).
Crystal analysis of binary ZnO–CuO nanocomposites
Prominent diffractive peaks on the differential ratio of binary ZnO–CuO nanocomposites were indexed by comparing the green ZnO and CuO diffraction angle 2θ with ICDD ZnO 01-089-0510 and ICDD CuO 01-089-5897, as presented in Fig. 2. Green CuO was observed to have 12 characteristic peaks at 32.32°, 35.50°, 38.71°, 45.01°, 48.37°, 53.29°, 58.15°, 61.09°, 65.56°, 67.90°, 72.16°, and 75.13°, which corresponded to the crystal surfaces (110), (− 111), (111), (202), (− 202), (020), (202), (− 113), (− 311), (220), (311), and (004), respectively. It had the following lattice parameters: a = 4.686486, b = 3.421156, c = 5.129263, α = 90°, β = 99.413°, γ = 90°, and d-spacing of 2.52761 Å with a monoclinic crystalline structure. Green ZnO was observed to have 12 characteristic peaks at 31.87°, 34.57°, 36.37°, 47.62°, 56.68°, 62.92°, 66.43°, 68.02°, 72.28°, 76.87°, 81.04°, and 89.44°, which corresponded to the crystal surfaces (100), (002), (101), (102), (110), (103), (200), (201), (004), (202), (104) and (203), respectively. It had the following lattice parameters: a = 3.252352, b = 3.252352, c = 5.209155, α = 90°, β = 90°, γ = 120°, and d-spacing of 2.47193 Å with a hexagonal wurtzite crystalline structure.
XRD diffraction peaks of BZC nanocomposites prepared at different composition. (a) C. gigantea leaves powder, (b) Green ZnO, (c) Green CuO, (d) B1Z3C, (e) B1Z1C and (f) B3Z1C [open circle: C. gigantea leaves, filled balck circle: ZnO, filled black rhombus: CuO, open red rhombus: additional peaks after green synthesis].
Meanwhile, six characteristic peaks of ZnO for sample B3Z1C were identified at 31.72°, 34.45°, 36.25°, 47.35°, 56.41°, and 62.71° and deemed to correspond to the (100), (002), (101), (102), (110), and (103) crystal surfaces, respectively. Two other characteristic peaks of CuO at 38.62° and 67.78° were found and deemed to correspond to the (111) and (220) crystal surfaces, respectively. For sample B1Z3C, the peaks at 31.72°, 34.45°, 36.25°, 47.35°, 56.41°, 62.71°, and 68.05° belonged to the (100), (002), (101), (102), (110), (103), and (201) indices of ZnO nanoparticles, respectively. The diffractive peaks of CuO detected at 35.68°, 38.62°, 58.33°, 61.27°, and 65.80° corresponded to the (–111), (111), (202), (–113), and (–311) crystal surfaces, respectively. All 2θ values of ZnO and CuO for BZC nanocomposites slightly shifted, indicating that some modifications of ZnO with CuO occurred and a strong crosslinking framework structure of Zn–O–Cu atoms formed. Moreover, the binary mixing of CuO and ZnO resulted in decreased crystallinity of BZC nanocomposites. The peak intensity drastically increased with increased amount of ZnO or CuO in the BZC nanocomposites (Fig. 2), thereby indicating the variation in composition (25 wt%, 50 wt%, and 75 wt% of ZnO) during green synthesis. A few additional peaks were observed at 23.65°, 25.69°, 27.73°, 29.47°, and 40.78° (Fig. 2). This finding was possibly due to the presence of the phytochemical element of C. gigantea leaves as a capping and reducing agent47. The XRD patterns of powdered C. gigantea leaves successfully revealed trace natural elements such as calcium and carbon (Fig. 2). C. gigantea natural plant is rich in calcium and carbon elements. Calcium was observed to have six characteristic peaks at 28.80°, 50.47°, 58.89°, 66.70°, 67.70°, and 73.92°. The additional peaks detected at 31.53° and 40.94° were attributed to the natural graphene-like carbon present in the BZC nanocomposites54 as carbon is the main phytochemical element in the leaves of the C. gigantea medicinal plant55.
The main novelty of this study was the detection of pythochemical elements such natural calcium56 and carbon54,57 in leaf extract, which could further boost the antimicrobial activity of BZC nanocomposites. Calcium and carbon elements have never been reported before in the studies of Sharma et al., Gawade et al., and C R Rajith Kumar et al. performed on the same C. gigantea medicinal plant24,47,48.
FT-IR analysis of binary ZnO–CuO nanocomposites
The FTIR spectra of B3Z1C nanocomposites and C. gigantea leaves are shown in Fig. 3. The presence of capping and stabilization agents such as flavonoids, polyphenolics, and terpenoids can be confirmed from this analysis. The weak absorption band at 447 cm–1 was characteristic of the ZnO functional group58,59. However, the CuO functional group was not visible owing to its low composition in the B3Z1C nanocomposite binary system. The spectra further showed a very intense band at 3438 cm−1 associated with the O–H stretching polyphenols (flavonoids) present in the plant extract. The characteristic peaks at 1633 and 1765 cm−1 can be attributed to C=C (carbonyl group) and C=O stretching, respectively. The absorption band between 1110 and 1115 cm−1 could be attributed to C–O stretching owing to the biomolecules of C. gigantea leaves. The broad absorption band at 1385 cm−1 was observed owing to the O–C–O stretching modes of vibration of esters. The absorption band observed at 680 cm−1 belonged to primary amines, indicating proteins. Therefore, the presence of phenolic and carbonyl compounds of C. gigantea leaves played vital roles in the stabilisation of green B3Z1C nanocomposite formation and antimicrobial activity15.
UV–Vis spectroscopy analysis of binary ZnO–CuO nanocomposites
The UV–Vis diffuse reflectance spectra of C. gigantea extract and B3Z1C nanocomposites are shown in Fig. 4. The appearance of a small broad peak at approximately 317 nm indicated the formation of irregular oval and quasi-spherical B3Z1C nanocomposites. Absorption peaks at 206 nm could be attributed to various chromophores, including the C=C bond of various compounds, the C=O bond of carbonyl compounds, and the benzene ring, whereas the absorption peak at 269 nm may be related to the various aromatic compounds, such as phenolics60. A sharp distinct peak was found at 233 nm owing to the formation of natural graphene-like carbon which played an important role in antimicrobial efficacy against MDR strains61.
Antimicrobial properties of binary ZnO–CuO nanocomposites
About 37% of patients with skin-ulcer disease are infected with Gram-positive S. aureus pathogen62. The antimicrobial characterisation BZC nanocomposites with different ratios is presented in Fig. S1 and Table 4. The MICs of B1Z3C, B1Z1C, and B3Z1C were 5, 2.5, and 0.625 mg/mL for S. aureus, respectively. Similar to the MIC values, B1Z3C and B1Z1C had MBCs of 20 mg/mL, and the counterpart for B3Z1C was 2.5 mg/mL for S. aureus. B3Z1C exerted a higher bactericidal effect against the S. aureus strain at the lowest MIC/MBC values (0.625 mg/mL/2.5 mg/mL). Antimicrobial activity was further enhanced by increasing the amount of ZnO nanoparticles in the binary compound (ZnO-CuO). This finding can be explained by the fact that the binary B3Z1C nanocomposites were highly diffusible and able to generate more Zn2+ ions19. Moreover, Cu2+ ions bound the cell wall of host cells through surface proteins and entered the cell19. Subsequently, the change in cell metabolism led to the microbe’s cell death19. Commercial B3Z1C was also prepared and tested against S. aureus for comparison. Results showed that commercial B3Z1C was a bacteriostatic agent because the MBC/MIC ratio was ≥ 1646 (Table 4 and Fig. S1). However, the green B3Z1C was labelled as a strong bactericidal agent because the tolerance ratio was ≤ 4.
Further antimicrobial analysis of B3Z1C nanocomposites was conducted on selected skin-ulcer pathogens, and results are shown in Table 5. These pathogens are commonly associated with skin-ulcer disease4,5,6,7. Also, the inhibitory activities of binary antimicrobial agents on bacterial colonies highly depend on the antimicrobial efficacy of dual-ionic systems and types of microbial pathogens, such as non-MDR Gram-positive bacteria (S. aureus), Gram-negative bacteria (E. coli) and MDR bacteria (P. aeruginosa, K. pneumoniae, and MRSA). The MIC amounts for B3Z1C were 0.625, 0.15625, 0.625, and 0.15625 mg/mL for E. coli, P. aeruginosa, K. pneumoniae, and MRSA, respectively. MBC values with 2.5, 0.3125, 1.25, and 0.3125 mg/mL were also observed for this green binary inorganic oxide sample. Table 5 indicates that for all tested microbes, the tolerance levels for B3Z1C were less than 4, indicating that the sample was a strong bactericidal agent. Binary B3Z1C has strong antimicrobial activity against Gram-negative bacteria (E. coli). Table 5 is the evidence for this finding. Clearly, B3Z1C showed very promising results against all tested MDR microbes such as P. aeruginosa, K. pneumoniae, and MRSA. This outcome may be due to the B3Z1C nanoparticles’ larger surface-to-volume ratio and the cell-membrane penetration of the bacteria by its ions. Some studies have reported that the antimicrobial effectiveness of green synthesised inorganic oxide nanoparticles depends on high particle dosage and small nanoparticle size, which could explain the higher antimicrobial activities of B3Z1C. The antimicrobial activity of B3Z1C was due to the electrostatic interaction between positively charged zinc and copper ions (Zn2+ and Cu2+) and negatively charged microbial cell membranes21. The antimicrobial activity of B3Z1C nanocomposites relied on the generation of ROS as well17,19. Moreover, free ions from natural organic carbon and calcium derived from C. gigantea leaf extract played an important role in exerting the synergic effect that killed MDR microbes at very low concentrations54,56.
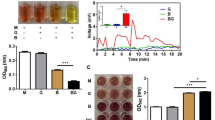
Results of time-kill assay were presented in terms of the changes in log10 CFU/mL of viable S. aureus colonies, as shown in Fig. S2. The green synthesised B3Z1C nanocomposites were found to have significant bactericidal activity. Figure 5 presents the time-kill curve graph for the strain. Generally, bacterial growth includes a log or exponential phase in which bacterial-cell doubling occur and their biomass increases from day 1 to day 263,64. A reduction in viable count from 4.3 log10 to 3.4 log10 was observed after 6 h of incubation for S. aureus. By 12 h, only 1.3 log10 of bacterial colonies were found. At 24 h, the bacteria were completely killed. Thus, Gram-positive S. aureus bacteria were effectively controlled by the synergistic combination of 75 wt% of ZnO and 25 wt% of CuO nanoparticles in the presence of natural graphene-like carbon, calcium, and phytochemical constituents such as cardiac glycosides, tannins, saponins, terpenes, flavonoids, and phenolics in C. gigantea leaf extract54,56,65,66,67,68.
Furthermore, Kirby–Bauer disc-diffusion method was used to evaluate the antimicrobial activity of BZC nanocomposites against Gram-positive S. aureus. The cultures exposed to negative control sample did not show any inhibition zones around the filters, indicating that they did not have any antibacterial properties. However, B3Z1C exhibited a wider zone of inhibition (ZOI) than other BZC samples possibly because of the nanoparticle size and the fast diffusion of metal ions into agar medium (Fig. S3 and Table 6). The antimicrobial activity of all green BZC samples further improved with increased concentration. C. gigantea extract also exhibited a slight ZOI toward S. aureus which could be attributed to bioactive compounds such as carbonyl and phenolic groups. The antibiotic streptomycin serving as a positive control exhibited a larger ZOI, as shown in Fig. S3 and Table 6.
Conclusions
Binary B3Z1C nanocomposites prepared at compositions of 75 wt% of ZnO and 25 wt% CuO demonstrated significant antimicrobial property against non-MDR and MDR pathogens with tolerance ratio of ≤ 4 and ≤ 2, respectively. Besides, promising antimicrobial effect of B3Z1C sample towards non-MDR bacteria (S. aureus) were seen from disc diffusion assay and time kill analysis. The mechanisms underlying the biocidal activity of B3Z1C nanocomposites may involve the presence of natural carbon, free ions (i.e., Cu2+, Zn2+ and Ca2+), and ROS. Further In vitro and In vivo toxicity studies are needed to understand B3Z1C efficiency in treating PU infections.
Data availability
The datasets generated and/or analysed during the current study are not publicly available due to the patent application for methods of making and using and compositions of binary nanocomposites formed by green synthesis but are available from the corresponding author on reasonable request.
References
Khor, H. M. et al. Determinants of mortality among older adults with pressure ulcers. Arch. Gerontol. Geriatr. 59, 536–541 (2014).
Qaddumi, J. A. S. & Almahmoud, O. Pressure ulcers prevalence and potential risk factors among intensive care unit patients in governmental hospitals in Palestine: A cross-sectional study. Open Public Health J. 12, 121–126 (2019).
Sari, S. P. et al. The prevalence of pressure ulcers in community-dwelling older adults: A study in an Indonesian city. Int. Wound J. 16, 534–541 (2019).
Park-Lee, E. & Caffrey, C. Pressure Ulcers Among Nursing Home Residents: United States, 2004. NCHS Data Brief. 14, 1–8 (2009).
Braga, I. A., Brito, C. S., Filho, A. D., Filho, P. P. G. & Ribas, R. M. Pressure ulcer as a reservoir of multiresistant Gram-negative bacilli: Risk factors for colonization and development of bacteremia. Braz. J. Infect. Dis. 21(2), 171–175 (2017).
Dana, A., N. & Bauman, W. A. Review bacteriology of pressure ulcers in individuals with spinal cord injury: What we know and what we should know. J. Spinal Cord Med. 38(2), 147–160 (2015).
El-Toraei, I. & Chung, B. The management of pressure sores. J. Dermatol. Surg. Oncol. 3(5), 507 (1977).
Sumbal, Nadeem, A., Naz, S., Ali, J. S., Mannan, A. & Zia, M. Synthesis, characterization and biological activities of monometallic and bimetallic nanoparticles using Mirabilis jalapa leaf extract. Biotechnol. Rep. 24, e00338 (2019).
Alavi, M. & Karim, N. Antiplanktonic, antibiofilm, antiswarming motility and antiquorum sensing activities of green synthesized Ag–TiO2, TiO2–Ag, Ag–Cu and Cu–Ag nanocomposites against multi-drug-resistant bacteria. Artif. Cells Nanomed. Biotechnol. 46(3), S399–S413 (2018).
Lu, Z. et al. Enhanced antibacterial and wound healing activities of microporous chitosan-Ag/ZnO composite dressing. Carbohydr. Polym. 156, 460–469 (2017).
Nguyen, T. D. et al. In vivo study of the antibacterial chitosan/polyvinyl alcohol loaded with silver nanoparticle hydrogel for wound healing applications. Hindawi Int. J. Polym. Sci. 2019, 7382717 (2019).
Kamoun, E. A., Kenawy, E. R., Tamer, T. M., El-Meligy, M. A. & Eldin, M. S. Poly (vinyl alcohol)-alginate physically crosslinked hydrogel membranes for wound dressing applications: Characterization and bio-evaluation. Arab. J. Chem. 8, 38–47 (2015).
Kumar, P. T. S. et al. Flexible and microporous chitosan hydrogel/nano ZnO composite bandages for wound dressing: In vitro and in vivo evaluation. ACS Appl. Mater. Interfaces. 4, 2618–2629 (2012).
Arshad, R. et al. ZnO-NPs embedded biodegradable thiolated bandage for postoperative surgical site infection: In vitro and in vivo evaluation (2019).
Yulizar, Y., Bakri, R., Apriandanu, D. O. B. & TaufikHidayat, T. ZnO/CuO nanocomposite prepared in one-pot green synthesis using seed bark extract of Theobroma cacao. Nano-Struct. Nano-Objects. 16, 300–305 (2018).
Dobrucka, R., Kaczmarek, M., Lagiedo, M., Kielan, A. & Dlugaszewska, J. Evaluation of biologically synthesized Au-CuO and CuO-ZnO nanoparticles against glioma cells and microorganisms. Saudi Pharm. J. 27, 373–383 (2019).
Khan, S. A., Noreen, F., Kanwal, S., Iqbal, A. & Hussain, G. Green synthesis of ZnO and Cu-doped ZnO nanoparticles from leaf extracts of Abutilon indicum, Clerodendruminfortunatum, Clerodendruminerme and investigation of their biological and photocatalytic activities. Mater. Sci. Eng. C. 82(C), 46–59 (2017).
Aloucheh, R. M., Yangjeh, A. H., Bayrami, A., Navid, S. L. & Asadi, A. Green synthesis of ZnO and ZnO/CuO nanocomposites in Mentha longifolialeaf extract: Characterization and their application as antibacterial agents. J. Mater. Sci. Mater. Electron. 29(16), 13596–13605 (2018).
Widiarti, N., Sae, J. K. & Wahyuni, S. Synthesis CuO-ZnO nanocomposite and its application as an antibacterial agent. IOP Conf. Series Mater. Sci. Eng. 172, 012036 (2017).
Hassan, I. A., Sathasivam, S., Nair, S. P. & Carmalt, C. J. Antimicrobial properties of copper-doped ZnO coatings under darkness and white light illumination. ACS Omega. 2, 4556–4562 (2017).
Alswat, A. A., Ahmad, M. B. & Saleh, T. A. Preparation and characterization of zeolite\zinc oxide-copper oxide nanocomposite: Antibacterial activities. Colloid Interface Sci. Commun. 16, 19–24 (2017).
Qiu, S. et al. Synthesis, characterization, and comparison of antibacterial effects and elucidating the mechanism of ZnO, CuO and CuZnO nanoparticles supported on mesoporous silica SBA-3. RSC Adv. 10, 2767–2785 (2020).
Al-Dhabaan, F. A., Shoala, T., Ali, A. A. M., Alaa, M. & Abd-Elsalam, K. Chemically-produced copper, zinc nanoparticles and chitosan-bimetallic nanocomposites and their antifungal activity against three phytopathogenic fungi. Int. J. Agric. Technol. 13(5), 753–769 (2017).
Kumar, C. R. R. et al. One-pot green synthesis of ZnO–CuO nanocomposite and their enhanced photocatalytic and antibacterial activity. Adv. Nat. Sci. Nanosci. Nanotechnol. 11, 015009 (2020).
Hu, M. et al. Zinc oxide/silver bimetallic nanoencapsulated in PVP/PCL nanofibres for improved antibacterial activity. Artif. Cells Nanomed. Biotechnol. 46(6), 1248–1257 (2018).
Basavalingiah, K. R., Harishkumar, S., Nagaraju, G. & Rangappa, D. Highly porous, honeycomb like Ag–ZnO nanomaterials for enhanced photocatalytic and photoluminescence studies: Green synthesis using Azadirachtaindicagum. SN Appl. Sci. 1, 935 (2019).
Bazant, P. et al. Hybrid nanostructured Ag/ZnO decorated powder cellulose fillers for medical plastics with enhanced surface antibacterial activity. J. Mater. Sci. Mater. Med. 25(11), 2501–2512 (2014).
Motshekga, S. C., Ray, S. S., Onyango, M. S. & Momba, M. N. B. Preparation and antibacterial activity of chitosan-based nanocomposites containing bentonite-supported silver and zinc oxide nanoparticles for water disinfection. Appl. Clay Sci. 114, 330–339 (2015).
Yang, S. et al. Antibacterial and mechanical properties of honeycomb ceramic materials incorporated with silver and zinc. Mater. Des. 59, 461–465 (2014).
Sharma, M., Hazra, S. & Basu, S. Synthesis of heterogeneous Ag-Cu bimetallic monolith with different mass ratios and their performances for catalysis and antibacterial activity. Adv. Powder Technol. 28, 3085–3094 (2017).
Paszkiewicz, M. et al. The antibacterial and antifungal textile properties functionalized by bimetallic nanoparticles of Ag/Cu with different structures. J. Nanomater. 2016, 6056980 (2016).
Joshi, B., Regmi, C., Dhakal, D., Gyawali, G. & Lee, S. W. Efficient inactivation of Staphylococcus aureus by silver and copper loaded photocatalytic titanate nanotubes. Prog. Nat. Sci. Mater. Int. 28, 15–23 (2018).
Azizi-Lalabadi, M., Ehsani, A., Divband, B. & Alizadeh-Sani, M. Antimicrobial activity of titanium dioxide and zinc oxide nanoparticles supported in 4A zeolite and evaluation the morphological characteristic. Sci. Rep. 9, 1743 (2019).
Daou, I., Moukrad, N., Zegaoui, O. & Filali, F. R. Antimicrobial activity of ZnO-TiO2 nanomaterials synthesized from three different precursors of ZnO: Influence of ZnO/TiO2 weight ratio. Water Sci. Technol. 77(5–6), 1238–1249 (2018).
Shadmehri, A. A., Namvar, F., Miri, H., Yaghmaei, P. & Moghaddam, M. N. Assessment of antioxidant and antibacterial activities of zinc oxide nanoparticles, graphene and graphene decorated by zinc oxide nanoparticles. Int. J. Nano Dimens. 10(4), 350–358 (2019).
Jaiswal, A. K., Gangwar, M., Nath, G. & Yadav, R. R. Antimicrobial activity of bimetallic Cu/Pd nanofluids. J. Adv. Chem. Eng. 6(2), 151 (2016).
Al-Asfar, A., Zaheer, Z. & Aazam, E. S. Eco-friendly green synthesis of Ag@Fe bimetallic nanoparticles: Antioxidant, antimicrobial and photocatalytic degradation of bromothymol blue. J. Photochem. Photobiol. B Biol 185, 143–152 (2017).
Vasile, B. S. et al. Synthesis and characterization of a novel controlled release zinc oxide/gentamicin–chitosan composite with potential applications in wounds care. Int. J. Pharm. 463(2), 161–169 (2014).
Chabala, L. F. G., Cuartas, C. E. E. & Lopez, M. E. L. Release behavior and antibacterial activity of chitosan/alginate blends with Aloe vera and silver nanoparticles. Mar. Drugs. 15, 328 (2017).
Youssef, A. M. et al. Synthesis and evaluation of eco-friendly carboxymethyl cellulose/polyvinyl alcohol/CuO bionanocomposites and their use in coating processed cheese. RSC Adv. 10, 37857 (2020).
Abd El-Aziz, M. E. et al. Preparation and characterization of chitosan/polyacrylic acid/copper nanocomposites and their impact on onion production. Int. J. Biol. Macromol. 123, 856–865 (2018).
Youssef, A., EL-Nagar, I., El-Torky, A. & Abd El-Hakim, A. E. Preparation and characterization of PMMA nanocomposites based on ZnO-NPs for antibacterial packaging applications. In Proceedings of the 5th World Congress on New Technologies (NewTech'19) Lisbon, Portugal. Paper No. ICNFA 105. (2019).
Al-Tayyar, N. A., Youssef, A. M. & Al-Hindi, R. R. Antimicrobial packaging efficiency of ZnO-SiO2 nanocomposites infused into PVA/CS film for enhancing the shelf life of food products. Food Packaging Shelf Life. 25, 100523 (2020).
El-Sayed, S. M., El-Sayed, H. S., Ibrahim, O. A. & Youssef, A. M. Rational design of chitosan/guar gum/zinc oxide bionanocomposites based on Roselle calyx extract for Ras cheese coating. Carbohydr. Polym. 239, 116234 (2020).
Jafari, A., Ghane, M., Sarabi, M. & Siyavoshifar, F. Synthesis and antibacterial properties of zinc oxide combined with copper oxide nanocrystales. Orient. J. Chem. 27(3), 811–822 (2011).
Harun, N. H. et al. Bactericidal capacity of a heterogeneous TiO2/ZnO nanocomposite against multidrug-resistant and non-multidrug-resistant bacterial strains associated with nosocomial infections. ACS Omega. 5, 12027–12034 (2020).
Sharma, J. K., Akhtar, M., Ameen, S., Srivastava, P. & Singh, G. Green synthesis of CuO nanoparticles with leaf extract of Calotropis gigantea and its dye-sensitized solar cells applications. J. Alloys Compounds. 632, 321–325 (2015).
Gawade, V. V. et al. Green synthesis of ZnO nanoparticles by using Calotropis proceraleaves for the photodegradation of methyl orange. J Mater Sci Mater Electron. 28(18), 14033–14039 (2017).
El-Kased, R. F., Amer, R. I., Attia, D. & Elmazar, M. M. Honey-based hydrogel: In vitro and comparative in vivo evaluation for burn wound healing. Sci. Rep. 7, 9692 (2017).
Alavi, M. & Karimi, N. Characterization, antibacterial, total antioxidant, scavenging, reducing power and ion chelating activities of green synthesized silver, copper and titanium dioxide nanoparticles using Artemisia haussknechtii leaf extract. Artif. Cells Nanomed. Biotechnol. 46(8), 2066–2081 (2017).
Alavi, M., Karimi, N. & Valadbeigi, T. Antibacterial, antibiofilm, antiquorum sensing, antimotility, and antioxidant activities of green fabricated Ag, Cu, TiO2, ZnO, and Fe3O4 NPs via Protoparmeliopsis muralis Lichen Aqueous Extract Against Multi-Drug-Resistant Bacteria. ACS Biomater. Sci. Eng. 5, 4228–4243 (2019).
Al-Shabib, N. A. et al. Biofabrication of zinc oxide nanoparticle from Ochradenus baccatus leaves: Broad-spectrum antibiofilm activity, protein binding studies, and in vivo toxicity and stress studies. J. Nanomater. 2018, 8612158 (2018).
Goutam, S. P. et al. Green synthesis of TiO2 nanoparticles using leaf extract of Jatropha curcas L. for photocatalytic degradation of tannery wastewater. Chem. Eng. J. 336, 386–396 (2018).
Bhavyasree, P. G. & Xavier, T. S. Green synthesis of copper oxide/carbon nanocomposites using the leaf extract of Adhatoda vasica Nees, their characterization and antimicrobial activity. Heliyon. 6, e03323 (2020).
Kumar, P. S., Chezhian, A., Raja, P. S. & Sathiyapriya, J. Computational selections of terpenes present in the plant Calotropis gigantea as mosquito larvicide’s by blocking thesterol carrying protein, AeSCP-2. Bangladesh J Pharmacol. 7, 1–5 (2012).
Roy, A., Gauri, S. S., Bhattacharya, M. & Bhattacharya, J. Antimicrobial activity of CaO Nanoparticles. J. Biomed. Nanotechnol. 9, 1–8 (2013).
Kumar, S. R. K. et al. Highly efficient multipurpose graphene oxide embedded with copper oxide nanohybrid for electrochemical sensors and biomedical applications. J. Sci. Adv. Mater. Devices. 2(4), 493–500 (2017).
Elumalai, K., Velmurugan, S., Ravi, S., Kathiravan, V. & Ashokkumar, S. Bio-fabrication of zinc oxide nanoparticles using leaf extract of curry leaf (Murrayakoenigii) and its antimicrobial activities. Mater. Sci. Semicond. Process. 34, 365–372 (2015).
Noah, A. Z., El Semary, M. A., Youssef, A. M. & El-Safty, M. A. Enhancement of yield point at high pressure high temperature wells by using polymer nanocomposites based on ZnO & CaCO3 nanoparticles. Egypt. J. Petrol. 26, 33–40 (2017).
Mongkholrattanasit, R., Kryštůfek, J., Wiener, J. & Studničková, J. Natural dye from Eucalyptus leaves and application for wool fabric dyeing by using padding techniques. Nat. Dyes. 4, 57–78 (2011).
Al-Marri, A. H. et al. Green synthesis of Pd@graphene nanocomposite: Catalyst for the selective oxidation of alcohols. Arab. J. Chem. 9, 835–845 (2016).
Bessa, L. J., Fazii, P., Giulio, M. D. & Cellini, L. Bacterial isolates from infected wounds and their antibiotic susceptibility pattern: Some remarks about wound infection. Int. Wound J. ISSN. 12(1), 47–52 (2015).
Al-Qadiri, H. M. et al. Studying of the bacterial growth phases using fourier transform infrared spectroscopy and multivariate analysis. J. Rapid Methods Autom. Microbiol. 16, 73 (2008).
Akerlund, T., Nordstrom, K. & Bernander, R. Analysis of cell size and DNA content in exponentially growing and stationary-phase bath cultures of Escherichia coli. J. Bacteriol. 177, 6791 (1995).
Ahmad, W. Preliminary phytochemical, antimicrobial and photochemical study of Calotropis gigantea leaf extract. Curr. Chem. Lett. 9, 105–112 (2020).
Patil, S. M. & Saini, R. Antimicrobial activity of flower extracts of Calotropis gigantea. Int. J. Pharm. Phytopharmacol. Res. 1(4), 142–145 (2012).
Alam, M. A., Habib, M. R., Nikkon, F., Rahman, M. & Karim, M. R. Antimicrobial activity of Akanda (Calotropis gigantea L.) on some pathogenic bacteria. Bangladesh J. Sci. Ind. Res. 43(3), 397–404 (2008).
Kumar, G., Karthik, L. & Rao, K. V. B. Antibacterial activity of aqueous extract of Calotropis gigantealeaves—An in vitro study. Int. J. Pharm. Sci. Rev. Res. 4(2), 141–144 (2010).
Acknowledgements
The authors are thankful to Universiti Sains Malaysia (USM) for providing facilities and financial supports for this research work under Research University Grant (1001/CIPPT/8012338). Furthermore, the technical staffs support from of Advanced Medical and Dental Institute and School of Materials and Mineral Resources Engineering, Universiti Sains Malaysia, Pulau Pinang, Malaysia, in the characterization of the sample is acknowledged.
Funding
The authors are thankful to Universiti Sains Malaysia (USM) for providing facilities and financial supports for this research work under Research University Grant (1001/CIPPT/8012338).
Author information
Authors and Affiliations
Contributions
G.A.G. carried out the green sample preparation, sample characterization and the antibacterial assays, included bacterial preparation, MIC, MBC, time kill-assay and Kirby-Bauer disc diffusion test. N.H.H. assist in the antimicrobial experimental procedures. S.S. is material science expert that advice on nanocomposite physiochemical analysis. R.B.S.M.N.M. is the principal investigator which contribute in the experimental idea and design, writing process and gave final approval of this paper for publication. All authors have given approval to the final version of the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Govindasamy, G.A., Mydin, R.B.S.M.N., Sreekantan, S. et al. Compositions and antimicrobial properties of binary ZnO–CuO nanocomposites encapsulated calcium and carbon from Calotropis gigantea targeted for skin pathogens. Sci Rep 11, 99 (2021). https://doi.org/10.1038/s41598-020-79547-w
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-020-79547-w
This article is cited by
-
Blood proteins self-assembly, staphylococcal enterotoxins-interaction, antibacterial synergistic activities of biogenic carbon/FeSO4/Cu/CuO nanocomposites modified with three antibiotics
BMC Chemistry (2024)
-
Physical characterization, biocompatibility, and antimicrobial activity of polyvinyl alcohol/sodium alginate blend doped with TiO2 nanoparticles for wound dressing applications
Scientific Reports (2024)
-
Immobilized lipase enzyme on green synthesized magnetic nanoparticles using Psidium guava leaves for dye degradation and antimicrobial activities
Scientific Reports (2024)
-
Enhanced Cytotoxic Efficacy of Ocimum basilicum Leaf Extract-Mediated TiO2 Nanocrystals
Journal of Cluster Science (2024)
-
Psidium guajava-mediated green synthesis of Fe-doped ZnO and Co-doped ZnO nanoparticles: a comprehensive study on characterization and biological applications
Bioprocess and Biosystems Engineering (2024)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.