Abstract
Dysregulation of non-coding microRNAs during the course of tumor development, invasion and/or progression to the distant organs, makes them a promising candidate marker for the diagnosis of cancer and associated malignancies. This exploratory study aims at evaluating the usefulness of plasma concentration of circulating mir-146a as a non-invasive biomarker for acute lymphoblastic leukemia (ALL). Total RNA including miRNA was isolated from 110 plasma samples of patients (n = 66), healthy controls (n = 24) and follow up (n = 20) cases and reverse transcribed. Relative concentrations were assessed using real-time quantitative PCR and fold-change was calculated by 2−ΔΔCt method. Finally, relative concentrations were correlated to clinicopathological factors. Patients (n = 66) were analyzed to determine fold expression of miR-146a in plasma samples of ALL. Before chemotherapy, pediatric (n = 42) and adult (n = 24) showed overexpression of miR-146a compared with healthy controls (P < 0.0001). There was no effect of age and gender on mir-146a expression in plasma. mirR-146a expression was independent of clinical and hematological features. Moreover, miR-146a levels in plasma of paired samples (n = 20) after treatment showed significant decrease in expression (P < 0.001). Expression of plasma miR-146a may be utilized as non-invasive marker to diagnose and predict prognosis in pediatric and adult patients with ALL. Moreover predicted targets may be utilized for ALL therapy in future.
Similar content being viewed by others
Introduction
Acute lymphoblastic leukemia (ALL) is characterized by hematological malignancy resulting uncontrolled lymphoid lineage proliferation of the hematopoietic cells. It is a disease of bone marrow that has effect both on children and adults1. The peak incidence of ALL is between two to five years of age with higher ratio in male than female2. However, in adults, the worldwide incidence of ALL is approximately one in 100,000 per year3. A study reported an estimated 6250 new cases of ALL and 1450 deaths in 20154, rendering it the most common cause of death in developed as well as developing countries like Pakistan. The diagnosis of ALL is usually based on the histopathological examination including bone marrow aspiration and biopsy supplementing with immunologic, cytogenetic and molecular characteristics5. Although current standard methods for diagnosis of ALL have greatly improved diagnosis, but their clinical applications are limited due to their unpleasant, invasive and inconvenient nature. It is, indeed, of great concern to develop such diagnostic procedures which overcome these limitations including invasive procedures of bone marrow aspiration and biopsy. Many efforts have been done in the past for developing diagnostic markers that can easily and precisely diagnose specific diseases and its progression. Most of these markers are protein in nature but these are not satisfactory for tumor diagnosis due to their low sensitivity and specificity for detection6. Recently, advanced improvements in diagnosis and treatment strategies have improved pediatric outcome of ALL in developed countries where survival rate has reached to about 90%7. However, in developing countries the rate of survival is significantly reduced, possibly due to population differences, poor economic status, late diagnosis, less availability of advanced therapeutics, toxic effects of chemotherapy and most importantly lack of clinical trials on account of diagnosis and prognosis8. Thus, non-invasive diagnosis and improved prognosis still remained a great challenge for research particularly in Pakistan. The discovery of non-coding RNAs called microRNAs (miRNAs) opened a new horizon to discover novel diagnostic and prognostic markers for several malignant disorders including leukemia9. Studies on expression profiling of miRNA have shown its role in the development of ALL10,11,12. A single miRNA can regulate the expression of more than one gene, playing role in different stages of hematopoiesis and deregulation at any level may lead to leukemia10. miRNAs help to establish accurate classification of leukemia and to determine origin of the tumor of different types of leukemia13. miRNA is highly stable in both fresh and stored samples of plasma, making it the most potential blood-based biomarker of diagnostic and prognostic significance11. Several studies have shown that there are distinct microRNAs for specific cancer types that differentiate between malignant and its adjacent normal tissue6. miR-146a has been reported to play a key role in immune system, inflammation, myeloproliferation and oncogenesis14. As an oncogene its up-regulation may alter hematopoiesis leading to the development of leukemia13,15. There are few reports available in the literature on the role of miR-146a in ALL. A study reported the up-regulation of miR-146a in ALL samples of children16. Another report described its down-regulation in adult AML patient samples17. The miR-146a has shown an important role in hematopoiesis, predominantly in T-cell development affecting proliferation and apoptosis of NB4 cells18. Some abnormal phenotypes in hematopoietic system were found in a mice deficient with miR-146a that indicates its potential role in hematopoiesis19. The overexpression of miR-146a has been reported in stem cell lines including monocytic and lymphocytic lineage after bone marrow transplant17. A study presented microarray results of miRNA profiling on bone marrow biopsy and peripheral blood samples of childhood ALL. After validation through qPCR, up-regulation of miR-146a was revealed in bone marrow biopsy but its overexpression in blood samples was not found20. A study on 49 bone marrow samples reported miR-146a overexpression in childhood cases of ALL21. Most of these studies reported up-regulation of miR-146a in bone marrow samples of ALL. The studies reporting its expression in plasma samples are limiting. The present research was conducted to validate whether miR-146a levels in plasma of ALL patients were overexpressed or otherwise and analyze the plasma miR-146a expression levels after treatment to evaluate its potential as a biomarker for ALL. The altered expression in the current study provides the basis to utilize circulating miR-146 s as a novel predictive marker for diagnosis and prognosis of adult as well as pediatric-ALL.
Methods
Patients and clinical samples
Whole blood samples were collected in EDTA tubes from a total of 110 study subjects including patients (n = 66), controls (n = 24) and follow up cases (n = 20). Patients were diagnosed based on morphology, cytogenetic and immunophenotype and characterized according to WHO classification criteria22. The patients registered at INMOL hospital, Lahore, Pakistan from January, 2013 to November, 2016 were included in the study. The patients who were newly diagnosed were designated as “cases” and those with any kind of infectious diseases were excluded to determine accurate representation of the study. The cases monitored after four cycles of chemotherapy and nominated as “follow up cases” were also included to evaluate remission status after treatment. Complete remission was considered with bone marrow having less than 5 blasts percentage. The control samples were obtained from healthy subjects as “normal control” that has no malignancy and infectious disease. Patient’s history, clinical features, hematological and other laboratory parameters, date of diagnosis and start of treatment were recorded on a pre-designed performa.
Plasma isolation
From whole blood, plasma was isolated within 2–4 h after collection by two step centrifugation at 4 °C, first for 5 min at 2500×g and second for 2 min at 3500×g. The plasma was separated in RNase free 1.5 mL tubes considering recommended procedures for working on cell free miRNA23. Then, aliquots (each of 250 µL) were prepared for isolation of miRNA and down-stream process. The prepared aliquots were stored at − 80 °C till further analysis.
miRNA isolation from plasma
Plasma was thawed on ice and processed to isolate miRNA according to manufacturer’s instruction provided in the miRNeasy serum/plasma kit from Qiagen (Hilden, Germany). The syn-cel-miR-39 (synthetic caenorhabditis elegans mir-39) from miRNeasy serum/plasma spike-In control (Qiagen, Hilden, Germany) was added to each denatured sample as exogenous control for normalization of sample-to-sample variation during the RNAisolation24,25. The elution containing miRNA was stored at − 20 °C without delay for downstream process. The quality and quantity of RNAwas evaluated using NanoDrop by Thermo Scientific (USA). Quantification of RNAincluding miRNA was performed at 260 nm using RNase free water as a blank. The samples with absorbance ≥ 2 were considered for further analysis.
cDNA synthesis from RNA including miRNA
cDNA was synthesized by reversely transcribed isolated RNA including miRNA using master mix from miScript II RT kit from Qiagen (Hilden, Germany) using the protocols followed by the manufacturer. RNA with concentration of 100 ng (used as a template) was added to the master mix containing nucleic mix (10 × miScript) HiSpec buffer (5 × miScript), reverse transcriptase and RNase free water. Reaction was carried out in a total of 20 µL volume using thermo cycler from BioRad®. Reaction conditions for reverse transcription were first incubation of 60 min at 37 °C followed by second incubation of 5 min at 95 °C. The synthesized cDNA was carefully stored in microtubes at – 20 °C for further use.
Quantitative real time polymerase chain reaction (qPCR)
miRNA quantification was performed using relative quantitative method of qPCR. SYBR green dye from Qiagen (Hilden, Germany) was used to measure relative quantification of miRNA from plasma using protocols provided with the kit. Primer assays (10 × miScript) were purchased from Qiagen (Hilden, Germany) that contained forward sequences. Universal primer (available with SYBR green PCR kit) was used as a reverse primer for qPCR assays. The sequences of primers and their accession numbers were listed in Supplementary Table S1. Reactions were run in duplicate using real time thermal cycler, CFX96 from BioRad. Reference gene, hsa-mir-16, was used as a normalizer26 and NTC (no template control) was run as a negative control in each run to control for contamination. Normalized fold change was calculated using Livak 2−ΔΔCt method27 that utilizes ΔCt (CtmiR-146a–CtmiR-16) and ΔΔCt (CtmiR-146a–CtmiR-16)mean of the patients – (CtmiR-146a–CtmiR-16)mean of the controls.
Target prediction analysis of miR-146a
The total 3367 targets of miR-146a were retrieved from miRNA target database target scan (release 7.2)28. These targets were evaluated based on their target score and those with highest score were analyzed with MetaCore and STRING (Version 10.5) to search for targets related to ALL.
Statistical analysis
The data was analyzed using statistical tools like GraphPad prism software (version 7) and SPSS for windows (version 21). For validation of the results, Mann Whitney test, ANOVA (analysis of variance) and student’s t test was applied depending upon different variables. Kruskal Wallis analyses was performed to compare multiple groups. The diagnostic value of miR-146a was evaluated through ROC curves produced by MedCalc (version 15.8). The potential of miR-146a as a prognostic factor was determined using Univariate analysis. Non-parametric spearman’s correlation analyses were performed to evaluate clinical significance of miR-146a in ALL. The P-values obtained were two-tailed that considered significant at 0.05. Targets of miR-146a were determined through target predicting software, targetScan, and network and pathway was generated using gene ontology (GO) analysis including STRING and MetaCore software.
Ethical approval and consent to participate
The study was approved by the ethical review board of the University of the Punjab, Lahore, Pakistan with approval No. 284/15. The study design was constructed according to the guidelines of the declaration of Helsinki to conduct research on human beings. The samples were taken after informed consent form all the study participants.
Consent for publication
The present study does not contain any individual’s personal information.
Results
Characteristics of study subjects
Overall 110 study subjects were included in the study comprising of patients (n = 66), normal controls (n = 24) and follow up cases (n = 20). In patients, there were 66 ALL cases of which 31 from B-ALL and 35 T-ALL. The minimum age of ALL patients enrolled was 4 years and maximum 48 years with a mean age of 18.17 years. There were 42 childhood and 24 adult cases of ALL, of which 53 were males and 13 were females. Detailed demographic and clinical characteristics of the patients were listed (Table 1). The number of healthy controls in this study were 24 of which 16 (66.7%) were male and 8 (3.3)% were female. The mean age (years) of normal healthy controls calculated was 23.708 ± 13.788. The minimum age of healthy controls was 4.0 years and maximum age was 47.0 years. There were 14 children and 10 adults in healthy controls used for this study. Other characteristics of normal (healthy controls) including hematological parameters were shown (Table 2).
Circulating miR-146a is up-regulated in ALL
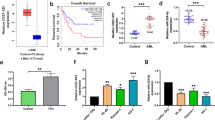
To explore potential role of circulating miR-146a in the development of ALL, we determined its normalized fold expression in plasma samples of patients and controls by relative quantification using real time PCR. Fold change calculated by 2−ΔΔCt method revealed significant up-regulation of miR-146a in patients of ALL (33.46 ± 15.84) B-ALL (34.74 ± 16.19) and T-ALL (32.22 ± 15.66), compared with controls (1.36 ± 1.03) with a P-value of < 0.0001 (Fig. 1A). However, there was no significant difference of expression in ALL subtype, and childhood and adults in ALL, B-ALL, and T-ALL (P > 0.05) (Fig. 2A1–C1). Moreover, no gender differences were observed in all the subtypes of ALL (P > 0.05) (Fig. 2A2–C2). However, when categorized according to WHO risk classification, patients of ALL with high risk showed higher mean expression levels of mir-146a, compared with standard risk patients (P = 0.0469) (Fig. 2A3). The categorization based on risk also showed statistically significant differences in T-ALL (Fig. 2C3) although these differences were not significant in B-ALL (Fig. 2B3). We found that levels of plasma miR-146a in ALL patients were independent of subtype, age and gender. Furthermore, correlation analysis between age and miR-146a was insignificant (P < 0.05) that confirmed its importance as a diagnostic marker for childhood as well as adult ALL (Fig. 3A).
Normalized fold expression of plasma mir-146a. (A) Relative quantification of plasma mir-146a in patients with ALL (n = 84), B-ALL (n = 48), T-ALL (n = 39), and healthy controls (n = 25). The y-axis denotes relative expression of plasma mir-146a normalized to mir-16 as a reference gene. (B) The levels of plasma mir-146a significantly reduced after treatment in patients of ALL (P < 0.0001). (C) Receiver operating curve (ROC) analyses for ALL with an AUC of 1.00 representing 100% sensitivity and 100% specificity.
Association of plasma mir-146a expression with age, gender and risk status. Normalized fold expression of mir-146a in childhood and adult patients in (A1) ALL, (B1) B-ALL, (C1) T-ALL. Scatter plots represented no significant difference in mir-146a expression levels in childhood compared with adults. There were no gender differences in mir-146a expression in plasma of (A2) ALL, (B2) B-ALL, (C2) T-ALL. Fold expression of mir-146a was significantly increased in high risk groups compared with standard risk in (A3) ALL and (C3) T-ALL, however, no significant difference was observed in (B3) B-ALL.
Plasma miR-146a levels are independent of hematological parameters
A remarkable feature of a diagnostic marker is that it should be independent of clinical relevance and show no evident change with clinical and hematological parameters. To identify this aspect, correlation analysis was performed between miR-146a levels and hematological parameters. We could not detect any significant correlation with hemoglobin, RBC’s, WBC’s, lymphocytes, neutrophils, platelets and peripheral blood blasts (P < 0.05) (Fig. 3B–H). The insignificant correlation proved that miR-146a levels in plasma were not affected by hematological parameters and could not influence its significance to use as a diagnostic marker for ALL.
Plasma miR-146a as a diagnostic marker for ALL
To evaluate diagnostic potential of plasma miR-146a in ALL, ROC (receiver operating curve) analyses were performed that showed area under curve (AUC) of 1 for ALL (95% CI 0.960 to 1.00) having 100% sensitivity, 100% specificity (P < 0.0001) (Fig. 1C). For B- and T-ALL, AUC of 1 was observed (95% CI 0.935 to 1.00) showing 100% sensitivity and 100% specificity (P < 0.0001).
Plasma miR-146a as a prognostic marker for ALL
To evaluate prognostic impact of miR-146a in plasma of ALL, expression levels were analyzed before and after complete rounds of chemotherapeutic treatment in paired samples. It was noted that mean expression of miR-146a before chemotherapy was 33.46 ± 15.84, 34.74 ± 16.19 and 32.33 ± 15.66 in patients of ALL, B-ALL and T-ALL, respectively. Interestingly, these levels significantly decreased after treatment with a P value of < 0.0001 (Fig. 1B). Mean fold expression in male patients of ALL was 32.95 ± 15.74 that reduced to 0.8865 ± 0.754 showing significant difference (P < 0.0001) (Fig. 4A). Similarly, plasma miR-146a expression in females with ALL was 35.55 ± 16.68 that decreased to 1.61 ± 1.196 (P = 0.0036) (Fig. 4B). Moreover, miR-146a mean levels in plasma of pediatric and adult samples were 32.4 ± 14.06 and 35.33 ± 18.72, that reduced to 0.8486 ± 0.7688 and 1.337 ± 0.9601, respectively, (P < 0.0001) (Fig. 4C,D). Furthermore, miR-146a expression levels were significantly lowered in after chemotherapy in childhood B-ALL (P = 0.0167), adult B-ALL (P = 0.0018), childhood T-ALL (P < 0.0001) and adult T-ALL (P = 0.0167). In addition, we analyzed pre- and post-treated levels of hematological parameters along with miR-146a expression that also showed significant decrease after treatment (Table 2).
Potential predictive targets of miR-146a
miR-146a targets a number of tumor suppressor genes that have shown their role in the pathogenesis of ALL. We first, selected targets of highest score (≥ 0.90) through targetScan and then analyzed these targets by STRING as shown (Fig. 5A). The genes predicted in the network were involved in cell cycle, proliferation, cell growth, development, DNA replication and repair mechanism and cell death. Further, we constructed a hypothetical pathway of these targets on MetaCore software for predicting novel targets for miR-146a (Fig. 5B). The round shape highlighted with red color showed the up-regulated targets that include N-RAS, RAS, AMPK-alpha, PBX2, ErbB4, TRAF6, LIN28 and NUMB. These are involved in the regulation of gene expression by transducing signals from cytoplasm to the nucleus. N-RAS and RAS present in the cell membrane are transported into the cytoplasm and then bind to ERK1/2. In the cytoplasm, AMPK-alpha subunit is phosphorylated and binds with ERK1/2. Subsequently, ERK1/2 and TRAF6 activates P53, a tumor suppressor gene in the nucleus that negatively regulates Bcl2 causing reduced apoptosis leading to uncontrolled proliferation of leukemic cells. The oncogene, C-Myc has an important role in cell growth, proliferation and apoptosis. LIN28 binds with Oct3/4-SOX2 complex and activates C-Myc, involving STAT5 and STAT3. Similarly, PBX2, ErbB4 and NUMB activate C-Myc by activating Notch1 pathway. Thus, by regulating tumor suppressor and oncogenes, aforementioned targets caused increased proliferation, abnormal cell growth and reduced cell death ultimately leading to the development of ALL.
Target prediction analysis of mir-146a. (A) Network analysis of mir-146a predicted targets by STRING. The targets with score > 0.9 and P < 1.0e−16 were selected to achieve highest confidence (B) Pathway analysis performed using MetaCore showed up-regulated targets genes of mir-146a highlighted with round shape (red). The thickness of lines showed the extent of interaction with the target molecules.
Discussion
There has been an emerging interest in miRNA to explore its potential as a predictive marker for diagnosis and prognosis of several hematological diseases including ALL. In the previous studies, miR-146a has shown a major role in the hematopoiesis, predominantly in T-cell development influencing proliferation and apoptosis of NB4 cells18. Another study developed a mice deficient with miR-146a and found some abnormal phenotypes in hematopoietic system indicating its potential role in hematopoiesis19. Aberrant miR-146a expression has been studied in ALL. A single report described decreased expression of miR-146a in blood and bone marrow of children with T-ALL29. All other reports showed up-regulation of miR-146a in bone marrow. We performed our experiment on plasma samples isolated from whole blood to determine whether miR-146a levels in plasma were overexpressed or otherwise.
In the current work, we, first, found up-regulation of miR-146a in plasma samples of childhood and adult cases of ALL, compared with healthy subjects as normal controls. Previously, overexpression of miR-146a has been reported in stem cell lines including monocytic and lymphocytic lineage after bone marrow transplant17. miR-146a has shown oncogenic role in childhood ALL as it has shown up-regulation in pediatric samples of ALL16. A study presented microarray results of miRNA profiling on bone marrow biopsy and peripheral blood samples of childhood ALL. After validation through qPCR revealed up-regulation of miR-146a in bone marrow biopsy but could not find its overexpression in blood samples30. A study on 49 bone marrow samples reported miR-146a overexpression in childhood cases of ALL21. We were able to determine its expression both in children as well as adults with ALL and its subtypes including B-and T-cell. Another study reported down-regulation of miR-146a expression in adult AML patients17 that enhanced disease status by regulating TRAF6 involved in NF-κB pathway31.
Further, we analyzed clinical relevance of miR-146a with hematological parameters that showed no significant correlation with WBC’s, RBC’s, hemoglobin, platelets, neutrophils and lymphocytes. On the basis of these results, it may be suggested that miR-146a levels were not affected by the clinical features. These results are in agreement with the previous finding that also reported no clinical association with WBC’s, hemoglobin and platelets count in ALL32.
The most striking feature of the current research was determination of miR-146a expression levels after treatment in paired samples of ALL. We were able to find significant reduction in miR-146a expression after chemotherapy demonstrating its prognostic significance in plasma samples of pediatrics and adults. Similar effects of treatment were reported on bone marrow samples by Duyu et al., who described post-treatment decrease in miR-146a expression in childhood ALL30. Association of poor survival with overexpression of miR-146a in adults of ALL has been described33. Moreover, favorable treatment outcome in ALL patients was related to miR-146a down-regulation34. The significant reduction in expression of miR-146a in pre- and post-treatment plasma samples indicated its prognostic significance and it was suggested that plasma miR-146a has a potential to predict treatment response and can be utilized as a prognostic marker of pediatric and adult ALL.
miRNA may act as an oncogenic or tumor suppressor based on the target gene regulated by miRNA13. miR-146a is a dual nature miRNA showing both tumor suppressor and oncogenic properties in different types of leukemia. Studies reported its role as a tumor suppressor by regulating STAT1, an apoptotic factor, and Bcl-XL, an antiapoptotic factor, promoting apoptosis in jurkat cells in ALL cell lines35. It has been reported to target TRAF and IRAK6 that are regulatory genes of immune response36. Previously, the targets for mir-146a i.e., TRAF6, IRAK131, and IRAK237, were reported to be involved in immune system. We found the role of these targets in ALL that may serve as potential targets for treatment of ALL.
Conclusion
We concluded that miR-146a up-regulation in plasma may be utilized as a novel, non-invasive marker for diagnosis of ALL in childhood and adults. Furthermore, treatment response may be monitored through altered miR-146a expression levels that may improve prognosis of pediatric and adult ALL. The non-invasive diagnosis and prognosis may overcome poor overall survival of patients with ALL and good treatment outcome. miR-146a target prediction may also pave a way to understand the mechanisms in the regulation of gene expression establishing more effective targets for ALL therapy. Therefore, plasma miR-146a may be employed as a promising marker for diagnosis, prognosis and treatment of adult as well as pediatric ALL.
Data availability
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
References
Niraj Shah, K. B., Rushworth, S. A. & MacEwan, D. Understanding the role for miRNA in human leukemia. RNA Dis. 2, 1–7 (2015).
Inaba, H., Greaves, M. & Mullighan, C. G. Acute lymphoblastic leukaemia. Lancet 381, 1943–1955 (2013).
Abbasi, S., Maleha, F. & Shobaki, M. Acute lymphoblastic leukemia experience: Epidemiology and outcome of two different regimens. Mediterr. J. Hematol. Infect. Dis. 5, e2013024–e2013024 (2013).
Mannis, G. N. et al. Quantification of acute lymphoblastic leukemia clonotypes in leukapheresed peripheral blood progenitor cells predicts relapse risk after autologous hematopoietic stem cell transplantation. Biol. Blood Marrow Transplant. 22, 1030–1036 (2016).
Chiaretti, S. et al. Gene expression profiling identifies a subset of adult T-cell acute lymphoblastic leukemia with myeloid-like gene features and over-expression of miR-223. Haematologica 95, 1114–1121 (2010).
Wittmann, J. & Jäck, H.-M. Serum microRNAs as powerful cancer biomarkers. Biochim. Biophys. Acta (BBA) 1806, 200–207 (2010).
Hunger, S. P. et al. Improved survival for children and adolescents with acute lymphoblastic leukemia between 1990 and 2005: A report from the children’s oncology group. J. Clin. Oncol. 30, 1663–1669 (2012).
Gupta, S. et al. The magnitude and predictors of abandonment of therapy in paediatric acute leukaemia in middle-income countries: A systematic review and meta-analysis. Eur. J. Cancer 49, 2555–2564 (2013).
Schotte, D., Pieters, R. & Den Boer, M. L. MicroRNAs in acute leukemia: From biological players to clinical contributors. Leukemia 26, 1–12 (2012).
Luan, C., Yang, Z. & Chen, B. The functional role of microRNA in acute lymphoblastic leukemia: Relevance for diagnosis, differential diagnosis, prognosis, and therapy. Onco Targets Ther. 8, 2903–2914 (2015).
Kroh, E. M., Parkin, R. K., Mitchell, P. S. & Tewari, M. Analysis of circulating microRNA biomarkers in plasma and serum using quantitative reverse transcription-PCR (qRT-PCR). Methods 50, 298–301 (2010).
Zhu, Y. D. et al. Distinctive microRNA signature is associated with the diagnosis and prognosis of acute leukemia. Med. Oncol. 29, 2323–2331 (2012).
Di Leva, G. & Croce, C. M. miRNA profiling of cancer. Curr. Opin. Genet. Dev. 23, 3–11 (2013).
Boldin, M. P. et al. miR-146a is a significant brake on autoimmunity, myeloproliferation, and cancer in mice. J. Exp. Med. 208, 1189–1201 (2011).
Ahmad, J. et al. MicroRNA in carcinogenesis & cancer diagnostics: A new paradigm. Indian J. Med. Res. 137, 680–694 (2013).
Hua, Z., Chun, W. & Fang-yuan, C. MicroRNA-146a and hemopoietic disorders. Int. J. Hematol. 94, 224–229 (2011).
Starczynowski, D. T. et al. MicroRNA-146a disrupts hematopoietic differentiation and survival. Exp. Hematol. 39, 167-178.e164 (2011).
Xu, L. et al. MiR-146a expression level as a novel putative prognostic marker for acute promyelocytic leukemia. Dis. Mark. 2014, 150604 (2014).
Zhai, P.-F. et al. The regulatory roles of microRNA-146b-5p and its target platelet-derived growth factor receptor & #x3b1; (PDGFRA) in erythropoiesis and megakaryocytopoiesis. J. Biol. Chem. 289, 22600–22613 (2014).
Kotani, A. et al. miR-128b is a potent glucocorticoid sensitizer in MLL-AF4 acute lymphocytic leukemia cells and exerts cooperative effects with miR-221. Blood 114, 4169–4178 (2009).
Zhang, H. et al. MicroRNA patterns associated with clinical prognostic parameters and CNS relapse prediction in pediatric acute leukemia. PLoS ONE 4, e7826 (2009).
Arber, D. A. et al. The 2016 revision to the World Health Organization classification of myeloid neoplasms and acute leukemia. Blood 127, 2391–2405 (2016).
Hastings, M. L., Palma, J. & Duelli, D. M. Sensitive PCR-based quantitation of cell-free circulating microRNAs. Methods 58, 144–150 (2012).
Kloten, V. et al. Multicenter evaluation of circulating plasma microRNA extraction technologies for the development of clinically feasible reverse transcription quantitative PCR and next-generation sequencing analytical work flows. Clin. Chem. 65, 1132–1140 (2019).
Benning, L. et al. Digital PCR for quantifying circulating microRNAs in acute myocardial infarction and cardiovascular disease. J. Vis. Exp. 137, 57950 (2018).
Shahid, S., Shaheen, J., Shahid, W., Akhtar, M. W. & Sadaf, S. mir-16-5p as a suitable reference gene for normalization of quantitative real time PCR in acute lymphoblastic leukemia. Pak. J. Zool. 51, 747–754 (2019).
Livak, K. J. & Schmittgen, T. D. Analysis of relative gene expression data using real-time quantitative PCR and the 2−ΔΔct method. Methods 25, 402–408 (2001).
Agarwal, V., Bell, G. W., Nam, J.-W. & Bartel, D. P. Predicting effective microRNA target sites in mammalian mRNAs. Elife 4, e05005 (2015).
Xia Feng, J. X. & Xie, K. miR-146 promotes apoptosis of Jurcat cell in children with acute lymphoblastic leukemia by targeting STAT1 expression. Int. J. Clin. Exp. Med. 9, 21280–21287 (2016).
Duyu, M. et al. Prospective evaluation of whole genome microRNA expression profiling in childhood acute lymphoblastic leukemia. BioMed. Res. Int. 2014, 967585 (2014).
Taganov, K. D., Boldin, M. P., Chang, K.-J. & Baltimore, D. NF-κB-dependent induction of microRNA miR-146, an inhibitor targeted to signaling proteins of innate immune responses. Proc. Natl. Acad. Sci. 103, 12481–12486 (2006).
Tavakoli, F. et al. Evaluation of microRNA-146a expression in acute lymphoblastic leukemia. Front. Biol. 11, 53–58 (2016).
Wang, Y. et al. MicroRNAs expression signatures are associated with lineage and survival in acute leukemias. Blood Cells Mol. Dis. 44, 191–197 (2010).
Schotte, D. et al. MicroRNA characterize genetic diversity and drug resistance in pediatric acute lymphoblastic leukemia. Haematologica 96, 703–711 (2011).
Yan, W. et al. The effect of miR-146a on STAT1 expression and apoptosis in acute lymphoblastic leukemia Jurkat cells. Oncol. Lett. 13, 151–154 (2017).
Pichler, K., Schneider, G. & Grassmann, R. MicroRNA miR-146a and further oncogenesis-related cellular microRNAs are dysregulated in HTLV-1-transformed T lymphocytes. Retrovirology 5, 100 (2008).
Hou, J. et al. MicroRNA-146a feedback inhibits RIG-I-dependent type I IFN production in macrophages by targeting TRAF6, IRAK1, and IRAK2. J. Immunol. 183, 2150–2158 (2009).
Acknowledgements
The authors acknowledge the Higher Education Commission (HEC), Pakistan for providing partial funding support to accomplish the present research.
Funding
The present research was partially funded by Higher Education Commission (HEC), Pakistan.
Author information
Authors and Affiliations
Contributions
S.S.* collected the samples, performed the experimental work, prepared the manuscript and statistically analyzed the study. W.S. helped in sample collection and manuscript preparation. J.S. helped in experimental work. M.W.A. and S.S. supervised the research and experimental design All authors reviewed the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Shahid, S., Shahid, W., Shaheen, J. et al. Circulating miR-146a expression as a non-invasive predictive biomarker for acute lymphoblastic leukemia. Sci Rep 11, 22783 (2021). https://doi.org/10.1038/s41598-021-02257-4
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-021-02257-4
This article is cited by
-
MiR-126 and miR-146a as Melatonin-Responsive Biomarkers for Neonatal Brain Ischemia
Journal of Molecular Neuroscience (2023)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.